Abstract
We studied the long-term effect of ileal transposition (IT) metabolic surgery on the hepatokines: retinol-binding protein-4 (RBP4), α-2-HS-glycoprotein (aHSG/fetuin-A), and fibroblast growth factor 21 (FGF21), C-reactive protein (CRP) plasma levels, glucose metabolism, body weight, liver histology, as well as total lipids concentration in muscle, liver, and fat tissue of obese Zucker (Crl:ZUC(ORL)-Leprfa) rats. 14 adult males were randomly submitted either to IT or SHAM (control) surgery. Pre-operative hepatokines plasma levels were not significantly different in rats submitted to IT or SHAM protocol. Three months after the procedures the plasma levels of RBP4, aHSG, FGF21, and CRP were significantly lower in IT-operated animals when compared to SHAM-operated group. Three and 12 weeks after the IT and SHAM surgery, the AUCOGTT were significantly lower than AUCOGTT before the surgery. HOMA-IR was lower in rats after IT surgery in comparison to the SHAM-operated rats. Muscle and liver total lipids concentration was reduced after the IT procedure when compared to pre-IT conditions. IT had a significant reductive impact on the body weight in comparison to SHAM surgery in the 4th, 6th, 8th, and 10th week after the surgery. We conclude that IT reduces hepatokines’ plasma concentrations, muscle and liver total lipids concentration but not the inflammatory processes in the liver of Zucker (Crl:ZUC(ORL)-Leprfa) rats.
Similar content being viewed by others
Introduction
Bariatric surgery is the most clinically effective treatment of severe and complex obesity both in terms of weight loss and improvement of weight-related diseases1,2,3. It induces many changes in patients’ lives, including changes in daily physical activity, physical and mental health, eating behaviour and food relations, social relations, sex life and body image4.
Type 2 diabetes mellitus (T2DM) has a very complex pathogenesis. In this study, we focused on T2DM and its associations with selected parameters: retinol-binding protein-4 (RBP4), α-2-HS-glycoprotein (aHSG/fetuin-A), fibroblast growth factor 21 (FGF21), and C-reactive protein (CRP). Plasma concentrations of RBP4, which is considered as an adipocyte-derived marker of T2DM5,6, are two to three-fold higher in overweight and obese subjects in comparison to the control group with normal body weight7. RBP4 plasma levels positively correlate with adipose RBP4 mRNA concentration, intraabdominal fat mass, body mass index (BMI) and insulin resistance7. aHSG/fetuin-A is an insulin receptor inhibitor that is produced in the liver and an important link between obesity and insulin resistance8,9. It is considered as an independent risk factor of T2DM which concentration increases even in prediabetic subjects10. aHSG levels correlate with fatty liver mass and atherogenic lipid profiles both in diabetic and nondiabetic models, regardless of the weight11. FGF21 is a known regulator of glucose and lipid metabolism. It is produced in the liver, pancreas, skeletal muscles and adipose tissue12,13,14,15. FGF21 ameliorates hepatic gluconeogenesis and insulin resistance. It also enhances the lipolysis, while suppressing the liver lipogenesis, and promotes fatty acid oxidation13,16 that is particularly intensified in patients with morbid obesity and T2DM12. Morbid obesity leads to generalized low-grade chronic inflammation, which is reflected by an increased level of C-reactive protein (CRP)17,18. CRP is also considered an independent marker of non-alcoholic fatty liver disease (NAFLD); the monitoring of its plasma level allows assessing the reduction in severe inflammatory activity and steatosis in NAFLD patients after a significant weight loss19. The evident decrease in inflammatory parameters occurred after significant weight loss and was related to the postoperative period, i.e. > 6 months after laparoscopic sleeve gastrectomy20,21. NAFLD is characterized by an excessive fat accumulation in the hepatocytes of people who do not consume alcohol and presents as a wide range of asymptomatic diseases, steatosis necroinflammatory forms of the disease or non-alcoholic steatohepatitis (NASH). The latter one is characterized by varying degrees of steatosis, cytoskeletal damage (hepatocellular ballooning), and lobular inflammation with or without fibrosis22.
The effectiveness of bariatric protocol differs between types of bariatric surgery23. Therefore, the continuous research on the metabolic shifts and their pathophysiological mechanisms after different types of bariatric surgery is still needed. Despite wide research in the animal model, the effects of ileal transposition, type of bariatric surgery, are not fully studied yet but based on the current knowledge24,25,26, we hypothesised that IT would have a beneficial effect on the glucose metabolism and inflammatory processes measured by selected hepatokines. We studied the effects of IT protocol on RBP4, aHSG/fetuin-A, FGF21, and CRP levels, liver histology, HOMA-IR as well as total lipid content in the obese Zucker (Crl:ZUC(ORL)-Leprfa) rats—an animal model of insulin resistance, glucose intolerance, metabolic syndrome, and genetic obesity27.
Results
Post surgery body weight changes
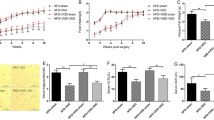
Regardless of the type surgery performed—IT or SHAM—statistically significant differences in the body weight of rats were found and these differences depended on the time after surgery elapsed. Rats that underwent IT surgery had significantly reduced body weight at 4th, 6th, 8th and 10th weeks post operation in comparison to rats after SHAM procedure (p < 0.05, p < 0.001, p < 0.001, p < 0.05, respectively; Fig. 1). None of the selected surgical protocols had an impact on body weight gain after the procedure (p = 0.400). Body weight increase after the IT and sham surgery what is also connected with the fact that all rats were submitted in the age 7 weeks and they were still growing up. We have investigated the correlations between plasma parameters and the body weight. Due to the small size of the study groups, we calculated Spearman's rank-order correlation. The results of the analysis showed no statistically significant correlations between studied parameters.
Comparison of body weight (g) of Zucker (Crl:ZUC(ORL)-Leprfa) rats before surgery and every two weeks after ileal transposition (IT) or control (SHAM) surgery. For readers convenience, the points are connected with dashed lines and additionally significance level for p < 0.05 was marked as * and p < 0.01 as **, respectively.
Hepatokines levels before and after ileal transposition (IT) and control (SHAM) surgery
Inter-group comparison
Retinol-binding protein-4 (RBP4), α-2-HS-glycoprotein (aHSG/fetuin-A), and fibroblast growth factor 21 (FGF21) plasma concentrations were the same for rats before ileal transposition (pre IT) and control (pre SHAM) surgery (Table 1). C-reactive protein (CRP) plasma concentration was significantly higher in rats selected for IT surgery (pre IT). Since the conditions and diet for both study groups were the same, this result reflects individual differences in the CRP levels in the population of rats used in the experiment (Table 1).
Three months after IT and SHAM (control) procedures, the concentrations of RBP4, aHSG (fetuin-A), and FGF21 measured in plasma were significantly lower in rats after IT surgery when compared to animals after the SHAM procedure (Table 1). Also, CRP plasma levels were significantly lower in rats after IT surgery than in rats after control (SHAM) procedure (Table 1).
Intra-group comparison
Three months after the procedures, RBP4 concentration in rats after IT was significantly lower than in SHAM-operated animals. There were no differences concerning other hepatokines concentrations between the groups at that timepoint (Table 2). This can result from individual differences between animals included in the study.
Oral glucose tolerance test
The glucose levels measured before the operations and 3, 6, 9 and 12 weeks after IT and SHAM (control) surgery are presented in Table 3. Descriptive statistics and results of two-way analysis of variance with all glucose curves together, regardless of time after IT and SHAM surgery showed that glucose concentration significantly depends on time after glucose administration (Table 3, IT: p < 0.001; SHAM: p < 0.001). Intra-IT and intra-SHAM group analysis showed no significant differences in the profiles of glucose curves during OGTT analysed in consecutive weeks after the procedures (Fig. 2, Table 3, IT: p = 0.349, SHAM p = 0.429).
For better understanding, the effects of the surgery at two points in time: 3 weeks and 12 weeks after the procedures were selected and the OGTT results for these point times were compared.
3 weeks after surgery
Descriptive statistics and results of two-way analysis of variance for inter-group comparison between rats at 3 weeks after IT and SHAM surgery showed significant differences in glucose concentration depending on time after the glucose administration (p < 0.001) and differences in the profiles of all studied groups in relation to time after the surgery (p < 0.05). Additionally, a difference in the area under the curve (AUC) was observed: the AUC before the operation was 16,682 (mg/dl)2 whereas the AUC after IT and after SHAM surgery was lower—13,994 (mg/dl)2 and 15,149 (mg/dl)2, respectively (Table 3).
12 weeks after surgery
Statistically significant differences in glucose concentrations in relation to time after glucose administration (p < 0.01) were found at that point. There were no significant changes in the profiles of the glucose curves at 3, 6, 9 and 12 weeks after the surgery (p = 0.111). All glucose curves presented a similar shape and trends in glucose concentrations—increase and decrease—at similar time points. The AUC for rats after IT surgery was 16,188 (mg/dl)2 and 16,682 (mg/dl)2 for rats before the procedures, whereas for rats after the SHAM procedure it was higher—20,579 (mg/dl)2 which proves the beneficial effects of the IT procedure (Table 3).
Multiple comparisons in contrast analysis of glucose concentration measured at consecutive time points during oral glucose tolerance test (OGTT) in blood of Zucker (Crl:ZUC(ORL)-Leprfa) rats 3 weeks post surgery period show no statistical significane differences between IT and SHAM operation. Whereas, the same comparisons of glucose concentration 12 weeks after surgery showed statistically significant differences in glucose concentration after IT and SHAM procedures only for results recorded at 20 (pIT vs. SHAM < 0.05) and 30 min (pIT vs. SHAM < 0.05) of oral glucose tolerance test (OGTT).
Fasting glucose, insulin and HOMA-IR analysis
Fasting glucose concentration in the serum depended on time (p < 0.001) but not on the type of surgery applied in the study (p = 0.481) (Table 4). Twelve weeks after the surgery, fasting glucose concentration was significantly lower in rats after the IT procedure when compared to rats after the SHAM procedure (p < 0.05). Insulin concentration in the serum depended on time (p < 0.05) and on the type of surgery (p < 0.05). Its concentration was significantly lower three (p < 0.05) and lowered 12 weeks after the IT procedure in comparison to insulin concentration measured before the procedure. HOMA-IR depended on the type of surgery (p < 0.01). Three weeks after the surgery, HOMA-IR values were significantly higher in rats after SHAM than in rats after the IT procedure (p < 0.001) (Table 4).
Total lipids content analysis
Total lipids concentration in muscles and liver depended on the type of surgery performed (p < 0.001) (Table 5). Total lipid concentration in muscles and liver of SHAM-operated rats was the same before and after the surgery (p = 0.535 for muscles; p = 0.784 for liver), while for IT-operated rats total lipids concentration was significantly lower after the IT procedure (p < 0.001 for both tissues). Both SHAM and IT procedures did not affect the total lipids concentration in the subcutaneous and visceral fat tissue (p = 0.950, p = 0.370, respectively, Table 5).
Liver histology
Histopathological analysis showed a large number of ballooned hepatocytes within hepatic lobules in the livers of rats after the SHAM procedure and only one liver sample with mild steatosis features. Livers of rats after the IT procedure showed evident hepatocyte ballooning (with only one sample with the reduced ballooning process). Only one liver sample from in the IT operated rats showed mild steatosis features (Table 6, Fig. 3). We did not observe any noticeable lobular inflammation or fibrosis in liver samples from both groups of rats. Taking into account all analyzed samples, there were no significant differences between the tested groups (Figs. 3, 4) however the low number of animals in both studied groups (n = 5) may hinder this result.
Discussion
Here we present the effects of the ileal transposition (IT) on retinol-binding protein-4 (RBP4), α-2-HS-glycoprotein (aHSG/fetuin-A), fibroblast growth factor 21 (FGF21), and C-reactive protein (CRP) levels in plasma of obese Zucker (Crl:ZUC(ORL)-Leprfa) rats. We report a significant reduction in the plasma levels of selected hepatokines 3 months after IT surgery when compared to the control group of rats that underwent control (SHAM) procedure. The beneficial effect of IT on hepatokines levels was also observed in the intra-group analysis but this change was not statistically significant for FGF21, aHSG and CRP plasma levels. The individual responses of the animals and quite high standard deviation of measured parameters can probably explain this insignificance of the observed changes. On the other hand, HOMA-IR assessed in serum and total lipids concentration measured in the soleus muscle, liver depended on the type of surgery and, in case of total lipids concentration in the mentioned tissues, on time after the surgery.
To the best of our knowledge, there is no data on the effects of IT surgery on RBP4 levels in plasma. We report the significantly lower RBP4 post operative plasma levels in rats after IT procedure when compared to its plasma levels in rats before IT surgery and to the RBP4 plasma level of rats after control (SHAM) surgery. Decreasing RBP4 plasma level contributed to insulin sensitivity improvement in patients after bariatric surgery28 and reversely, its substantially increased level signalled the systemic insulin resistance development in humans29. In mice, increased levels of plasma RBP4 led to impaired glucose uptake into skeletal muscle and increased glucose production by the liver, whereas lowered plasma RBP4 levels greatly enhanced insulin sensitivity in these tissues29. Our results are in line with the research reporting a decrease in RBP4 plasma concentration 12 months following bariatric surgery in 63 obese women30. Other research reported that RBP4 systemic levels in obese subjects were significantly decreased after substantial body weight reduction associated with different types of bariatric surgery15,31,32 proper dietary plan, physical activity or pharmaceutical interventions33,34. During the IT procedure, the isolated segment of ileum is transposed to the jejunum and the intestinal tract retains physiological length but the distribution of endocrine cells along the gut is altered24,35. In our research, we observed a reduction in the body weight of animals after IT in comparison to animals after SHAM surgery which may explain the reduction in RBP4 plasma levels and confirm the beneficial effects of the IT protocol. Other scientists reported the opposite situation that bariatric surgery did not affect RBP4 plasma levels. Swarbrick et al. reported that 12 months after Roux-en-Y gastric bypass (RYGB) performed on 19 severely obese women, the RBP4 plasma levels were the same when compared to pre-surgical levels, despite marked weight loss and improved insulin resistance36. Other researchers postulated that a substantial reduction in the mass of adipose tissue and plasma lipid parameters influenced the RBP4 plasma levels rather than insulin sensitivity or inflammatory markers30. A decrease in RBP4 levels was only observed after surgically induced weight loss accompanied by relevant reductions in body fat30. Since our experiment was performed on Zucker rats with obesity induced by a mutation in the leptin receptor, we suggest that the body mass reduction obtained by IT procedure was substantial enough to lower RBP4 levels in animals with endogenous obesity.
Similarly to the above-mentioned RBP4, no known studies analyse the effect of IT protocol on the aHSG/fetuin-A concentration in plasma, however, the literature repeatedly describes the impact of other bariatric protocols on this hepatokine concentration. Jüllig et al. observed a much greater decrease in aHSG/fetuin-A concentration after gastric bypass than after sleeve gastrectomy. Authors suggested that excluding the part of the duodenum from the intestinal passage during the gastric bypass affects the activity of the intestinal microbiota that is significantly involved in the regulation of aHSG/fetuin-A concentration. Sleeve gastrectomy only significantly reduced aHSG/fetuin-A levels37. Brix et al. showed a beneficial effect of Roux-en-Y gastric bypass surgery, again surgery with the partial exclusion of the intestinal passage, on the aHSG concentration8. Decreasing aHSG concentration after weight loss is considered a marker of good bariatric effect after surgeries involving gastric bypass8,37. aHSG concentration also positively correlated with changes in waist-to-hip ratio (WHR) and body mass index (BMI) 12 months after gastric bypass10. In our study, we observed a beneficial effect of IT protocol on the aHSG/fetuin-A concentration measured in plasma 12 weeks after surgery when compared to aHSG concentration in plasma of rats that underwent SHAM (control) procedure. Although the obtained results are statistically insignificant, we have observed some further trends: lower, but not significant, aHSG concentration was measured after IT surgery when compared to pre IT values. We hypothesise that it would be possible to reach a statistically significant change by increasing the number of animals included in the study. In our previous study, we showed that the aHSG/fetuin-A plasma levels and the Ahsg mRNA expression in the liver were lower in rats after bariatric surgery, even though the study included animals with diet-induced obesity and studied effects of different bariatric procedure: duodenal-jejunal omega switch (DJOS)15. In the currently presented study, we observed lower sHSG/fetuin-A concentration in rats after IT procedure when compared to concentration level before the IT surgery. On the contrary, comparing the aHSG plasma levels before and after SHAM (control) surgery we observed the increase in the concentration of this hepatokine after the SHAM procedure. SHAM is a surgical intervention without transposition of the ileum and does not have a therapeutic purpose. Since the body weight of the animals that underwent SHAM procedure was increasing during the experiment, the increase in aHSG concentration in the plasma of these animals is understandable.
FGF21 was not analysed in the scientific literature in the context of IT. Liu et al. reported the effects of duodenal-jejunal bypass and sleeve gastrectomy and observed a significant decrease in FGF21 concentration in rats after both types of surgeries16. Pop et al. reported a similar tendency in human subjects after Roux-en-Y gastric bypass surgery38. Although our study did not show statistically significant differences in FGF21 concentrations, as in the case of aHSG (fetuin-A), noticeably lower concentration of this hepatokine is associated with the state after IT surgery, when compared to its concentration in rats before IT and in these that underwent SHAM procedure. Based on these results, we may conclude that the IT procedure leads to the beneficial effects: reducing levels of hepatokines circulating in plasma. The lack of statistically significant differences between pre- and post IT hepatokines levels can result from individual differences between animals included in the study. We based the number of rats subjected to the experiment on Russell's 3R theory stated in 1959: we used the smallest possible group of animals that enabled us to obtain comparable results. But with such a small number of animals, individual changes can have an impact on the test result39. Thus the number of animals included in the study may be also considered the main limitation of our research.
Although Zucker rats partially simulate human metabolic syndrome (obesity, insulin resistance, dyslipidemia, hyperinsulinemia, and liver), this animal model still has some disadvantages. Because leptin receptor mutations are rare in humans, Zucker rats may not reflect the clinical and pathological circumstances observed in humans. Furthermore, Zucker rats do not naturally develop steatohepatitis, are resistant to liver fibrosis, and require additional interventions to induce the progression of steatosis to NASH. FGF21 is involved in a complex interplay with the adipokines, leptin, and adiponectin. In mice lacking either of these adipokines, the beneficial metabolic effects of FGF21 are diminished40,41. Thus, the impact of FGF21 on the reduction of liver fat concentration in Zucker rats after IT may be also changed.
The decrease in hepatokines plasma levels seems to be a very good prognostic factor of IT surgery. Nevertheless, it should be remembered that Zucker rats used in the experiment were not healthy: leptin receptor mutation evokes many disorders in itself, not only obesity. The response to surgery in organisms with endogenous obesity is hindered when compared to those with diet-induced obesity. For such animals, namely for animals with endogenous obesity, improving the results of hepatokines and thus improving insulin and glucose metabolism may be considered an optimal achievement.
This study also included the analysis of CRP concentration, considered here as a general inflammation indicator. CRP level in rats after IT surgery was significantly lower than in rats after the SHAM procedure. Obesity itself has been recognized as a factor leading to chronic inflammation, which can directly affect CRP42.
When comparing CPR levels before and after the procedures, we noticed that in the group of rats selected for IT surgery, the CRP level was higher before the surgery whereas in the group of rats selected for the SHAM procedure the CRP level was higher after the procedure. Noticeably, the CRP levels in rats selected for both procedures were different even before the procedures, with higher value measures for rats selected for IT surgery. The rats subjected to the study were randomly assigned to the IT and SHAM groups. All animals were kept in the same conditions, having unlimited access to food and water. Animals were not having any signs of acute inflammation or disease. There were no unusual or significant events that occurred during acclimatization time. The 7-week old Zucker (Crl:ZUC(ORL)-Leprfa) rats, upon arrival in the laboratory, were not obese but still, the metabolic profiles of these animals were changed due to the mutation in the leptin receptor. Since both groups were kept in the same conditions and were not subjected to any intervention, it can be assumed that this difference resulted from individual variation. That is why we postulated that the significant differences in the CRP levels before surgery were related to the individual differences resulting from their impaired metabolic processes. We consider this fact as one of the limitations of our study that should be supported by additional analyses.
IT procedure had a significant impact on glucose tolerance in operated rats. Our results show, that time after glucose administration and time after the surgery are significant factors influencing glucose metabolism. Rats after IT procedure showed reduced AUCOGTT at 3 and 12 weeks post IT surgery when compared to rats that underwent SHAM procedure at the same time points. In the same group of rats, we observed that fasting glucose concentration depended on the time after the surgery and it was significantly reduced at 3 and 12 weeks after the IT procedure. HOMA-IR and insulin levels value was significantly lower 3 weeks and declined after 12 weeks after the IT surgery. The positive impact of IT surgery and the ameliorated glucose metabolism was previously reported in Zucker43 and Goto-Kakizaki (GK)44 rats. We argue that IT surgery leading to subtle body weight reduction is also improving tissue-specific glucose metabolism.
We observed significant differences in the body weight between the IT and SHAM operated rats in the 4th, 6th, 8th and 10th week after the procedures. Ikezawa et al. demonstrated that IT surgery notably reduced the eWAT mass of OLETF (Otsuka Long-Evans Tokushima Fatty) diabetic rats45. A study by Yan et al. on GK rats also showed reduced body weight, fat mass, and food intake, improved glucose metabolism, and increased insulin sensitivity after IT procedure46. On the other hand, other IT studies carried out on Zucker rats43,47 and UCD-T2DM rats48 revealed no impact of IT procedure on body weight. In our experiment, we observed reduced total lipid content in the soleus muscle and liver tissues but not in the visceral and subcutaneous fat tissue of rats that underwent IT surgery when compared to the pre-operated animals. The reduced fat tissue and increased glucose tolerance after IT procedure were previously reported in several rodent based animal models such as GK rats49, OLETF diabetic rats45, UCD-T2DM rats48, and Sprague Dawley high-fat diet (HFD)-induced obese (SD-DIO) rats50. In our study at each time point, the body weight of rats after IT was lower than of those after SHAM. Due to the nature of the Zucker (Crl:ZUC(ORL)-Leprfa) rats and the fact that IT surgery does not involve a reduction of the stomach and/or exclusion of any part of the intestines from the food passage, we did not expect any significant reduction in the body weight after the surgery. A significant difference in the body weight of IT operated rats for these time intervals may be due to individual variability and longer recovery time after surgery. The SHAM procedure, during which the incisions are made only in analogous places to minimise interference in the intestines functioning, can lead to faster regeneration and thus to faster return to normal digestion. Both in the first month after surgery and beyond the 10th week, the differences in the body weight of rats are no longer statistically significant. In the initial period, it may be a similar reaction of organisms to the procedure per se, while in the later period adaptation to new anatomical conditions of the gastrointestinal tract. The selection of rats with a leptin receptor mutation is significant, since Zucker (Crl:ZUC(ORL)-Leprfa) strain is considered to represent metabolic syndrome, the weight loss itself seems to be a much bigger challenge than in primarily non-obese organisms.
Our histological analysis showed the same level of liver steatosis and ballooning in animals that underwent IT or SHAM surgery. Obesity and insulin resistance promote hepatic steatosis by increasing free fatty acids (FFA) delivery to the liver. We observed no steatosis or mild steatosis in the liver of analysed rats. First of all, that might be connected to the low number of animals included in the histological analysis (n = 5). Second of all, the biopsy of the liver was performed in the distal part of the left, lateral lobe (using transverse and longitudinal-vertical section). The obesity of the Zucker (Crl:ZUC(ORL)-Leprfa) rats results from the leptin receptor mutation, which in consequence causes polyethiological obesity. Some authors report that the Lepob/Lepob (ob/ob) mice show mild to severe steatosis at 12 weeks of age, but ballooning and lobular inflammation remain absent even after 20 weeks of age51,52. The other studies in the Zucker rat model report that macro/microvesicular steatosis is diffused and located mainly in the periportal area53.
In our experiment, we observed mild steatosis, moderate ballooning, and lack of inflammation. It is generally accepted that the genetic mutation alone is not sufficient for worseing of hepatic conditions and a second stimulus is needed. The second stimulus usually takes the form of a chemical challenge (including lipopolysaccharide, CCL4, and TAA administration) or dietary measures (including methionine- and choline-deficient MCD diet and HFD)54,55. Given that, the second factor stimulating degenerative processes in the liver, such as steatosis and inflammation, might be oxidative stress. In our other study, we observed an increase of TAC and TOS in IT-operated animals comparing to SHAM-operated and control ones. This result might support the evidence that the observed, after IT procedure, reduced oxidative stress may substantially ameliorate insulin resistance and neutralize the negative consequences of obesity.
We found very little differences between samples from rats that underwent SHAM (control surgery) and rats that underwent the IT procedure. This may result from the characteristics of the IT procedure: transplantation of a selected fragment of the ileum to the proximal part of the jejunum does not affect the intestinal absorption surface morphology but can significantly affect the absorption processes both in the interponate and in the remaining parts of the intestine56,57. In our previous study, we reported significant changes in the morphology of intestines after IT procedure: increased villus length, decreased crypt depth, changes in the thickness of the mucosamuscularis-serosa (MMS)24. The previous paper also reported that rats after IT procedure, when compared to SHAM operated rats, expressed cellular hyperplasia with the increased mitochondrial density in the transposed ileum segment and microvillus degeneration in jejunum regions. The main limitation of our previous study was a lack of biochemical and molecular analysis, which would give information on whether those morphological changes are beneficial or deleterious for IT animals. Knowing that the amount of nutrients consumed by rats is rather the same (both before and after the procedure in both groups), we assume that the amount of absorbed substances does not differ between IT and SHAM operated rats. Studies on diet-induced obese rats that underwent duodenal-jejunal omega switch (DJOS) showed an improvement in the steatosis grade in rats maintained on a high fat diet before and after DJOS surgery58. However, DJOS treatment limited the surface for nutrients absorption as this procedure excludes the duodenum from the intestinal passage. It seems that ileal transposition, which does not affect the length of the intestinal passage, is therefore not relevant for fatty liver prevention and treatment. Perhaps longer observation would allow drawing more precise conclusions.
Conclusions
Ileal transposition (IT) reduces hepatokines’ plasma concentrations but does not improve the liver glucostatic function of the Zucker (Crl:ZUC(ORL)-Leprfa) rats.
Materials and methods
Animals, diet, and study design
The study was conducted according to the Guide for the Care and Use of Laboratory Animals (Directive 2010/63/EU) and with the Local Ethics Committee approval of all experimental procedures (58/2014). Local Ethics Committee at the Medical University of Silesia in Katowice approved all experimental protocols. Fourteen male, obese, 12-week-old Zucker rats (Crl:ZUC(ORL)-Leprfa) (Charles River Breeding Laboratories, Wilmington, Mass, USA) were used in the experiment. The animals were kept under controlled conditions: 12:12 light/dark cycle, 70 ± 1% humidity, unlimited access to water and standard rat food (24% protein, 4.9% fat, 7% crude ashes, 4.7% crude fibre, lysine13.6 g/kg, methionine 4.5 g/kg, calcium 12 g/kg, phosphorus 8.3 g/kg; Provimi Kliba AG, Switzerland).
After one week of acclimatisation, the animals were randomly assigned to two experimental groups: SHAM—control surgery group (n = 7) and IT—ileal transposition surgery group (n = 7) and underwent respective surgery.
Ileal transposition and SHAM surgery
The surgery procedures were performed as previously described by Grüeneberger et al. and Stygar et al.43,59. Briefly, 2% isoflurane with 2 l/min oxygen flow under spontaneous breathing was used to induce and maintain anaesthesia; analgesia was performed with xylazine (5 mg/kg b.w., ip; Xylapan, Vetoquinol Biovet, Poland) and antibiotic prophylaxis with gentamicin (5 mg/kg b.w., im, KRKA, Poland). After an abdominal midline incision (length 4–5 cm) was performed, the Bauhin’s valve was identified. Then 50% of the distal ileum was located and transected. The ileal continuity was restored by an end-to-end extramucosal anastomose using PDS 6/0 (Ethicon Endo-Surgery Inc. USA) and the transposed segment was excluded. Then, the ligament of Treitz was identified and the jejunum was divided 5 cm aborally. The transposed segment of ileum was inserted in an isoperistaltic fashion and two end-to-end anastomoses were performed (Fig. 5). For control (SHAM) surgery, transections were performed at all three analogous points, anastomoses were completed correspondingly, nevertheless without IT (Fig. 5). Fascia and skin closures were performed with a continuous suture using Monocryl 4/0 and Vicryl 4/0. After the surgery, all rats were kept on a liquid diet for 24 h (Nutrison, Nutricia, Poland).
Scheme of ileal transposition (IT) and control (SHAM) surgery performed in Zucker (Crl:ZUC(ORL)-Leprfa) rats. Detailed description of each type of surgery can be found in the “Materials and method” section.
Blood samples collection, hepatokines, CRP, insulin, and fasting glucose analysis
Under anesthesia and analgesia described previously, blood for hepatokines, CRP, insulin, and fasting glucose analysis was collected from the abdominal aorta into tubes containing 10 μl of EDTA (Sigma-Aldrich, St. Louis, Mo, USA) just before and 12 weeks after the surgery. Then it was centrifuged at 4000 rpm for 10 min at 4 °C and plasma samples were collected and stored at − 80 °C until analysis.
All concentrations were measured in duplicates. Hepatokines, insulin and CRP concentrations were measured using ELISA kits (Cloud-Clone Corp., USA). More specifically: retinol-binding protein-4 (RBP4)—kit no. SEA929Ra with detection range of 0.312–20 ng/ml and minimum detectable dose of < 0.151 ng/ml; α-2-HS-glycoprotein (aHSG/fetuin-A)—kit no. SEA178Ra, with detection range of 6.25–400 ng/ml and minimum detectable dose of < 2.55 ng/ml; fibroblast growth factor 21 (FGF21)—kit no. CEC918Ra with detection range of 12.5–200 pg/ml and minimum detectable dose of < 4.9 pg/ml); insulin—kit no. CEA448Ra with detection range of 123.5–10,000 pg/ml and minimum detectable dose of < 49.3 pg/ml; CRP—kit no. SEA821Ra with detection range of 0.78–50 ng/ml and minimum detectable dose of < 0.34 ng/ml. Fasting glucose concentration was determined using the glucose oxidase method (Sigma-Aldrich Kit, USA).
HOMA-IR
HOMA-IR was then calculated as fasting glucose (mmol/l) × fasting insulin (μU/ml)/22.5. A homeostatic model assessment of insulin resistance (HOMA-IR) was calculated according to Matthews et al.60.
Oral glucose tolerance test
The oral glucose tolerance test (OGTT) was performed before the surgery and every 3 weeks: in the 3rd, 6th, 9th and 12th week after the operation. As the gastric emptying in the initial postoperative period may be hindered and the intestinal passage may be slowed down, the animals fasted for 12 h (with unrestricted access to water) before the OGTT to avoid the effect of potentially residual food on the test result.
For OGTT purposes, the anaesthesia was induced using 2% isoflurane and 2 l/min oxygen flow to facilitate the orogastric tube placement (central venous catheter, Arrow International Inc., Reading, Penn, USA). Analgesia was performed with xylazine (5 mg/kg b.w., ip; Xylapan, Vetoquinol Biovet, Poland). The OGTT was initiated by infusing a 40% glucose solution (Braun Glucose 40%) at 1.5 g/kg dosage via gastric tube. Blood glucose concentration was determined at 0 (fasting), 10, 20, 30, 60, 90, and 120 min of glucose administration, using the glucose oxidase method (Sigma-Aldrich Kit, USA). Blood was collected form the lateral tail vein.
Body weight measurement
The body weight of rats was measured regularly every 2 weeks, starting from the day of the surgery. It was measured in the most similar conditions possible: at the same time, between 7 and 9 am, before feeding the animals.
Total lipids contents analysis
Samples (70 mg) of the soleus muscle, liver, subcutaneous and visceral fat were collected from the dissected organs and homogenized with a knife homogenizer in Eppendorf tubes (2 ml). Homogenization was carried out in 1 ml of hexane/isopropanol extraction mixture (3:2 v/v) with the addition of butylated hydroxytoluene.
Total lipids were extracted according to the modified procedure of Kozubek et al.61. The homogenates were shaken (800 rpm, 20 min, room temperature) and then centrifuged (10,000g, 5 min). The obtained supernatant was transferred to a new test tube and treated with 0.5 ml Na2SO4 and again centrifuged (10,000g, 5 min. The upper lipid-containing phase was collected from the solution and evaporated on a thermoblock at 34 °C in a nitrogen stream. The nitrogen atmosphere and the addition of butylated hydroxytoluene were used to protect lipids from the peroxidation process caused by atmospheric oxygen. The obtained dry total lipid extracts were weighed on an analytical balance with an accuracy of 0.1 mg61.
Liver histology
Histopathological examination
The liver samples were fixed in 10% buffered formalin, processed and embedded in paraffin. Five μm thick tissue sections were cut. Histopathological preparations were made using haematoxylin–eosin staining (H&E). H&E-stained liver sections were evaluated for ballooning hepatocytes (0—none, 1—few balloon cells, 2—many cells/prominent ballooning) and for hepatic steatosis (0 for < 5%, 1 for 5–33%, 2 for > 33–66%, and 3 for > 66%).
Statistical analysis
The statistical methodology was previously presented by Stygar et al.15. Statistical analysis was performed using STATISTICA 12.5 PL (StatSoft, Cracow, Poland). All tests were two-tailed. Interval data were expressed as mean value ± standard deviation in the case of normal distribution. Distribution of variables was evaluated by the Shapiro–Wilk test and the quantile–quantile plot. The following tests were used to verify hypotheses: parametric test for two independent or dependent samples (the Student’s t-test). Homogeneity of variances was assessed by the Fisher-Snedecore test.
For OGTT data, distribution of variables was evaluated by the Shapiro–Wilk test and quantile–quantile plot, homogeneity of variances was assessed by the Levene's test. The interval data were expressed as a mean value ± standard deviation in the case of normal distribution. For the comparison of data, multivariate repeated-measures ANOVA with contrast analysis at post-hoc were used. Sphericity was tested with the Mauchly's tests. Statistical significance was set at a p-value < 0.05 and all tests were two-tailed. On all glucose plots, the curve for the pre-operation values was marked in black and the same scale was used to facilitate interpretation. The area under the curve was calculated for all OGTT curves.
Data from the histopathological assessment of Zucker (Crl:ZUC(ORL)-Leprfa) rats liver samples are expressed as median with range. Comparisons between medians were done with the nonparametric Mann–Whitney U test.
Ethical standards
Institutional and national guidelines for the care and use of animals were followed (directive 2010/63/EU). All animal experimental protocols were approved by the Local Ethics Committee (58/2014).
Data availability
The original data is available after contact with the corresponding author.
References
Sjöström, L. et al. Bariatric surgery and long-term cardiovascular events. JAMA 307(1), 56–65 (2012).
Stygar, D. et al. Metabolic surgery in Zucker rats influenced miRNA, caveolin-1 expression and lipid metabolism. Open Life Sci. 10(1), 451–460 (2015).
Stygar, D. M. et al. The impact of DJOS surgery, a high fat diet and a control diet on the enzymes of glucose metabolism in the liver and muscles of Sprague-Dawley rats. Front. Physiol. 10, 571 (2019).
Coulman, K. D. et al. Patient experiences of outcomes of bariatric surgery: A systematic review and qualitative synthesis. Obes. Rev. 18(5), 547–559 (2017).
Cohen, R., Caravatto, P. P. & Petry, T. Z. Innovative metabolic operations. Surg. Obes. Relat. Dis. 12(6), 1247–1255 (2016).
Yang, Q. et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436(7049), 356–362 (2005).
Graham, T. E. et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N. Engl. J. Med. 354(24), 2552–2563 (2006).
Brix, J. M. et al. Elevated fetuin-A concentrations in morbid obesity decrease after dramatic weight loss. J. Clin. Endocrinol. Metab. 95(11), 4877–4881 (2010).
Himmetoglu, S. et al. Serum levels of fetuin-A and 8-hydroxydeoxyguanosine in morbidly obese subjects. Exp. Clin. Endocrinol. Diabetes 121(08), 505–508 (2013).
Huang, H. H. et al. Does bariatric surgery influence plasma levels of fetuin-A and leukocyte cell-derived chemotaxin-2 in patients with type 2 diabetes mellitus?. Peer J. 6, e4884 (2018).
Ou, H. Y. et al. Increased fetuin-A concentrations in impaired glucose tolerance with or without nonalcoholic fatty liver disease, but not impaired fasting glucose. J. Clin. Endocrinol. Metabol. 97(12), 4717–4723 (2012).
Ter Horst, K. W. et al. The FGF21 response to fructose predicts metabolic health and persists after bariatric surgery in obese humans. Mol. Metabol. 6(11), 1493–1502 (2017).
Waluga, M. et al. Fibroblast growth factor-21 and omentin-1 hepatic mRNA expression and serum levels in morbidly obese women with non-alcoholic fatty liver disease. J. Physiol. Pharmacol. 68(3), 363–374 (2017).
Kukla, M. et al. Serum FGF21 and RBP4 levels in patients with chronic hepatitis C. Scand. J. Gastroenterol. 47(8–9), 1037–1047 (2012).
Stygar, D. et al. Changes in liver gene expression and plasma concentration of RBP4, fetuin-A, and FGF21 in Sprague-Dawley rats subjected to different dietary interventions and bariatric surgery. BioMed Res. Int. 2018, 3472190. https://doi.org/10.1155/2018/3472190 (2018).
Liu, Q. et al. Improved FGF21 sensitivity and restored FGF21 signaling pathway in high-fat diet/streptozotocin-induced diabetic rats after duodenal-jejunal bypass and sleeve gastrectomy. Front. Endocrinol. 10, 566 (2019).
Ellulu, M. S. et al. Obesity and inflammation: The linking mechanism and the complications. Arch. Med. Sci. 13(4), 851 (2017).
Sproston, N. R. & Ashworth, J. J. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 9, 754 (2018).
Ziemianski, P. et al. High-sensitivity C-reactive protein as a new predictor of the course of nonalcoholic fatty liver disease in patients after bariatric surgery. Pol. Arch. Internal. Med. 129(7–8), 556–558 (2019).
Aasbrenn, M., Farup, P. G. & Videm, V. Changes in C-reactive protein, neopterin and lactoferrin differ after conservative and surgical weight loss in individuals with morbid obesity. Sci. Rep. 9(1), 1–8 (2019).
Stephens, J. W. et al. Temporal effects of laparoscopic sleeve gastrectomy on adipokines, inflammation, and oxidative stress in patients with impaired glucose homeostasis. Sur. Obes. Relat. Dis. 15(12), 2011–2017 (2019).
Brown, G. T. & Kleiner, D. E. Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metabolism 65(8), 1080–1086 (2016).
Lin, Z. & Qu, S. Legend of weight loss: A crosstalk between the bariatric surgery and the brain. Obes. Surg. 30(5), 1988–2002 (2020).
Sawczyn, T. et al. Ileal transposition (IT) surgery changing the ultrastructure of the transposed segment as well as jejunum. Histomorphometric and electron microscopy analysis. Obes. Surg. 28(5), 1232–1239 (2018).
Sawczyn, T. et al. The influence of high fat diet on plasma incretins and insulin concentrations in Sprague-Dawley rats with diet-induced obesity and glucose intolerance undergoing ileal transposition. Peptides 115, 75–84 (2019).
Skrzep-Poloczek, B. et al. Impact of ileal transposition surgical intervention on antioxidant status measured in liver tissue of obese Zucker rats (Crl:ZUC-Leprfa). Oxid. Med. Cell. Longevity 2018, 7342451 (2018).
Leonard, B. L. et al. Insulin resistance in the Zucker diabetic fatty rat: A metabolic characterisation of obese and lean phenotypes. Acta Diabetol. 42(4), 162–170 (2005).
Mitterberger, M. C. et al. Adipokine profile and insulin sensitivity in formerly obese women subjected to bariatric surgery or diet-induced long-term caloric restriction. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 65(9), 915–923 (2010).
Wolf, G. Serum retinol-binding protein: A link between obesity, insulin resistance, and type 2 diabetes. Nutr. Rev. 65(5), 251–256 (2007).
Broch, M. et al. Association of retinol-binding protein-4 (RBP4) with lipid parameters in obese women. Obes. Surg. 20(9), 1258–1264 (2010).
Gómez-Ambrosi, J. et al. Serum retinol-binding protein 4 is not increased in obesity or obesity-associated type 2 diabetes mellitus, but is reduced after relevant reductions in body fat following gastric bypass. Clin. Endocrinol. 69(2), 208–215 (2008).
Haider, D. G. et al. Serum retinol-binding protein 4 is reduced after weight loss in morbidly obese subjects. J. Clin. Endocrinol. Metab. 92(3), 1168–1171 (2007).
Balagopal, P. et al. Reduction of elevated serum retinol binding protein in obese children by lifestyle intervention: association with subclinical inflammation. J. Clin. Endocrinol. Metab. 92(5), 1971–1974 (2007).
Reinehr, T., Stoffel-Wagner, B. & Roth, C. L. Retinol-binding protein 4 and its relation to insulin resistance in obese children before and after weight loss. J. Clin. Endocrinol. Metab. 93(6), 2287–2293 (2008).
Strader, A. D. et al. Weight loss through ileal transposition is accompanied by increased ileal hormone secretion and synthesis in rats. Am. J. Physiol.-Endocrinol. Metab. 288(2), E447–E453 (2005).
Swarbrick, M. M. et al. Longitudinal changes in pancreatic and adipocyte hormones following Roux-en-Y gastric bypass surgery. Diabetologia 51(10), 1901–1911 (2008).
Jüllig, M. et al. Lower fetuin-A, retinol binding protein 4 and several metabolites after gastric bypass compared to sleeve gastrectomy in patients with type 2 diabetes. PLoS ONE 9(5), e96489 (2014).
Pop, L. M. et al. Roux-en-Y gastric bypass compared with equivalent diet restriction: Mechanistic insights into diabetes remission. Diabetes Obes. Metab. 20(7), 1710–1721 (2018).
Russell, W. M. & Burch, R. L. The Principles of Humane Experimental Technique (Methuen, 1959).
Lin, Z. et al. Adiponectin mediates the metabolic effects of FGF21 on glucose homeostasis and insulin sensitivity in mice. Cell Metab. 17(5), 779–789 (2013).
Véniant, M. M. et al. FGF21 promotes metabolic homeostasis via white adipose and leptin in mice. PLoS ONE 7(7), e40164 (2012).
Choi, J., Joseph, L. & Pilote, L. Obesity and C-reactive protein in various populations: A systematic review and meta-analysis. Obes. Rev. 14(3), 232–244 (2013).
Grueneberger, J. M. et al. Long segment ileal transposition leads to early amelioration of glucose control in the diabetic obese Zucker rat. Videosurg. Other Miniinvasive Technol. 8(2), 130 (2013).
Zhu, H. et al. Ileal transposition rapidly improves glucose tolerance and gradually improves insulin resistance in non-obese type 2 diabetic rats. Gastroenterol. Rep. 6(4), 291–297 (2018).
Ikezawa, F. et al. Effects of ileal interposition on glucose metabolism in obese rats with diabetes. Surgery 151(6), 822–830 (2012).
Yan, K. et al. Ileal transposition surgery decreases fat mass and improves glucose metabolism in diabetic GK rats: Possible involvement of FGF21. Front. Physiol. 9, 191 (2018).
Culnan, D. M. et al. Ileal interposition improves glucose tolerance and insulin sensitivity in the obese Zucker rat. Am. J. Physiol. Gastrointest. Liver. Physiol. 299(3), G751–G760 (2010).
Cummings, B. P. et al. Ileal interposition surgery improves glucose and lipid metabolism and delays diabetes onset in the UCD-T2DM rat. Gastroenterology 138(7), 2437–2446 (2010).
Yan, Z. et al. Myocardial insulin signaling and glucose transport are up-regulated in Goto-Kakizaki type 2 diabetic rats after ileal transposition. Obes. Surg. 22(3), 493–501 (2012).
Kohli, R. et al. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am. J. Physiol. Gastrointest. Liver Physiol. 299(3), G652–G660 (2010).
Kristiansen, M. N. et al. Obese diet-induced mouse models of nonalcoholic steatohepatitis-tracking disease by liver biopsy. World J. Hepatol. 8(16), 673–684 (2016).
Trak-Smayra, V. et al. Pathology of the liver in obese and diabetic ob/ob and db/db mice fed a standard or high-calorie diet. Int. J. Exp. Pathol. 92(6), 413–421 (2011).
Kucera, O. & Cervinkova, Z. Experimental models of non-alcoholic fatty liver disease in rats. World J. Gastroenterol. 20(26), 8364–8376 (2014).
Sanches, S. C. et al. Nonalcoholic steatohepatitis: A search for factual animal models. Biomed. Res. Int. 2015, 574832 (2015).
Hansen, H. H. et al. Mouse models of nonalcoholic steatohepatitis in preclinical drug development. Drug Discov. Today 22(11), 1707–1718 (2017).
Jurowich, C. F. et al. Ileal interposition in rats with experimental type 2 like diabetes improves glycemic control independently of glucose absorption. J. Diabetes Res. 2015, 490365 (2015).
Jurowich, C. F. et al. Duodenal-jejunal bypass improves glycemia and decreases SGLT1-mediated glucose absorption in rats with streptozotocin-induced type 2 diabetes. Ann. Surg. 258(1), 89–97 (2013).
Stygar, D. et al. The effects of duodenojejunal omega switch in combination with high-fat diet and control diet on incretins, body weight, and glucose tolerance in Sprague-Dawley rats. Obes. Surg. 28(3), 748–759 (2018).
Stygar, D. et al. Ileal transposition in rats influenced glucose metabolism and HSP70 levels. Open Life Sci. 10(1), 278–284 (2015).
Matthews, D. R. et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7), 412–419 (1985).
Kozubek, A., Sikorski, A. F. & Szopa, J. Molekularna Organizacja Komórki, II. Lipidy, Liposomy, Błony Biologiczne (Wydawnictwo Uniwersytetu Wrocławskiego, 1996) (in Polish).
Acknowledgements
This work was supported by the Medical University of Silesia (KNW-2-010/N/3/N). The authors would like to thank Anna Dulska for Figure 4 graphic design. Authors would like to express their special gratitude to Scott Richards for scientific English language correction.
Author information
Authors and Affiliations
Contributions
D.S. conceived the idea of the experiment; D.S., A.D. and W.K. maintained animals; T.S. conducted surgery; D.S., J.A., M.K., M.I., K.W.K. and J.J. worked on the manuscript; D.S. and E.C. analysed data and performed statistical analysis; D.S. carried out the analysis, A.G. conducted histopathological examinations; Ł.M. and N.M. performed histological analysis. All authors approved the final version of the submitted and published text.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stygar, D., Sawczyn, T., Dulska, A. et al. Ileal transposition helps to regulate plasma hepatokine levels in obese Zucker (Crl:ZUC(ORL)-Leprfa) rats. Sci Rep 11, 7774 (2021). https://doi.org/10.1038/s41598-021-87293-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-87293-w
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.