Abstract
Algal biofuel research aims to make a renewable, carbon–neutral biofuel by using oil-producing microalgae. The freshwater microalga Botryococcus braunii has received much attention due to its ability to accumulate large amounts of petroleum-like hydrocarbons but suffers from slow growth. We performed a large-scale screening of fast-growing strains with 180 strains isolated from 22 ponds located in a wide geographic range from the tropics to cool-temperate. A fast-growing strain, Showa, which recorded the highest productivities of algal hydrocarbons to date, was used as a benchmark. The initial screening was performed by monitoring optical densities in glass tubes and identified 9 wild strains with faster or equivalent growth rates to Showa. The biomass-based assessments showed that biomass and hydrocarbon productivities of these strains were 12–37% and 11–88% higher than that of Showa, respectively. One strain, OIT-678 established a new record of the fastest growth rate in the race B strains with a doubling time of 1.2 days. The OIT-678 had 36% higher biomass productivity, 34% higher hydrocarbon productivity, and 20% higher biomass density than Showa at the same cultivation conditions, suggesting the potential of the new strain to break the record for the highest productivities of hydrocarbons.
Similar content being viewed by others
Introduction
Algal biofuel research aims to make a renewable, carbon–neutral biofuel by using oil-producing microalgae. It initially prospered at 1980th, triggered by oil crisis, and has been reactivated at 2000th by global warming issues1,2,3. At the onset of algal biofuel research in the 1980s, there were large projects to screen natural bioresources, such as the Aquatic Species Program in the USA4, and lists of useful oil-producing microalgae were prepared. Subsequently, improved cultivation and harvesting techniques5,6, and strain selection and genetic engineering of these algae7,8,9,10 have been pursued. A high-throughput screening method for lipid-rich microalgae from environmental samples by fluorescence-activated cell sorting is now available11,12. A lower-cost direct method of isolation for lipid-rich microalgae using a fluorescence microscope and manipulator has also been reported13. This technical progress provides new opportunities to identify cryptic bioresources in nature.
The freshwater, colonial green microalga Botryococcus braunii accumulates large amounts of petroleum-like hydrocarbons in the colony14,15 and has received much attention in algal biofuel research since most oleaginous microalgae accumulate neutral lipids, which are energetically inferior to hydrocarbons16,17. Furthermore, B. braunii accumulates the hydrocarbon oils in an extracellular matrix of the colony, which enables “milking” of the algal oils without killing the algae18,19. Despite these remarkable characteristics, B. braunii has not occupied a leading position in algal biofuel research because of its slow growth rate: typical doubling times are between 3 and 7 days14,15. The typical doubling time of Chlorophyta is 24 h and that of Cyanobacteria is 17 h20. The top 20% of Chlorophyta, Cyanobacteria and other taxa with respect to growth rate have doubling times in the range of 7 to 8 h20. Among oleaginous microalgae, Chlorella vulgaris, Neochloris oleoabundans and Scenedesmus obliquus have short doubling times of 8–9 h21.
To overcome the slow growth of B. braunii, we searched for novel fast-growing strains from natural genetic resources. The Showa strain, known as a fast-growing strain of B. braunii, holds the fastest growth record with a doubling time of 1.4 days22 and the highest record of hydrocarbon productivity (340 mg L−1 d−1) to date23,24. This strain is a wild strain isolated from the pond of a greenhouse in California in the 1980s and was not selected by screening or artificially modified by mutagenesis25. There should be wild strains that grow faster and are more productive than the Showa strain, but, to the best of our knowledge, there has been no large-scale study seeking these strains, a potential of natural genetic resource. A few wild strains and collections of B. braunii have been investigated26,27,28,29,30, but researchers failed to find significantly faster and more productive strains than Showa, probably due to the limited number of strains tested (< 10) and the limited geographic range of locations searched.
This study performed a large-scale screening of fast-growing strains from a total of 180 strains isolated from 22 ponds located in a wide geographic range, from the tropics to a cool-temperate climate. Although B. braunii is widely distributed in freshwater and brackish lakes, reservoirs, or ponds from temperate to tropical environments15, their natural densities are commonly quite low (10–102 colonies per L)31, which makes it difficult to find the alga in natural environments. We have developed a simple method for isolating B. braunii from the natural environments32 and have newly isolated 70 wild strains from natural ponds in tropical Indonesia and temperate Japan31. This study has isolated an additional 110 strains for the large-scale screening.
The objective of this study is to evaluate the potential of the natural genetic resource of B. braunii on an unprecedented scale to answer the following questions with the following approaches: (i) In what kind of natural environment can we expect to find fast-growing strains? We analyzed whether the variations in growth rate between strains are attributable to the differences in the ponds and climate regions where the strains originate. (ii) Are there any wild strains that grow faster than the Showa? We measured biomass and hydrocarbon productivities of nine fast-growing wild strains selected by the screening and compared them to those of Showa and the highest recorded values reported by previous studies for B. braunii.
Results and discussion
Screening of fast-growing wild strains
We have investigated a total of 112 natural ponds, and a total of 180 wild strains were successfully isolated from 22 ponds located in various climate regions (Supplementary Table 1S). The screening of 180 wild strains was performed based on the increasing rate of optical density (OD660) in a 10 mL glass tube. The daily increases in OD660 in glass tubes were generally well fitted by an exponential function, and the coefficient of determination (R2) of the curve fitting was > 0.9 for 70% of the data (n = 590). After removing the 30% of the data with low R2 values, we calculated the doubling time (Dt) from the exponential growth curve in a total of 163 wild strains. Figure 1 shows the histogram of Dt for 163 wild strains: there was a large variation in Dt with the median at 6.0 days from a minimum of 2.7 days to a maximum of 23.4 days. The Showa strain had a Dt of 4.7 days, and 34 wild strains (20%) had a shorter Dt than Showa. We thus successfully identified several wild strains that are potentially faster-growing than Showa. Of these 34 wild strains, 22 strains (65%) are originated from the tropics (Indonesia). When the chemical races of these fast-growing wild strains were estimated from a molecular phylogenetic analysis of 18S rRNA, 83% were estimated as B race, which produces triterpene hydrocarbons.
To answer the question (i) In what kind of natural environment we can expect to find fast-growing strains, we analyzed the relative importance of strain, chemical race, the pond of origin, and climate where the pond is located as determinants of Dt of a strain using a mixed model analysis. The model analysis showed that 57% of the total variance of Dt data was attributable to the variance between strains (σ2S; Table 1). The σ2S was significantly larger than zero (P < 0.05), indicating a significant genetic variation in Dt between strains. In contrast, the variance component of the pond effect (σ2P) was not significantly different from zero (P > 0.05). This reflects substantial variations in Dt between strains isolated from the same pond. There were no specific ponds where fast-growing strains originated. The least-square means of Dt calculated from the model for each pond had relatively larger error bars compared to the differences of the least-square means between ponds (Fig. 2). Furthermore, the effects of race and climate on Dt were not significant (P > 0.05; Table 2).
Least square means of the doubling time (Dt) of wild strains of Botryococcus braunii in relation to chemical races and location of origin. Least square means were computed from a mixed model with race and climate as fixed effects and strain and pond as random effects. Bars indicate 95% confidence intervals. There were no significant differences (P > 0.05) between (a) Pond, (b) Race, or (c) Climate. See Supplementary Tables 1S for details of the abbreviation and locations of the ponds.
Our analyses did not provide any clear answers to the question (i). Fast-growing strains were found in both tropical and temperate climates, and in every pond, there were substantial variations in intrinsic growth rates between strains (Fig. 2). This implies that natural selection does not operate on the growth characteristics measured under laboratory conditions. Because the culture conditions of the screening are far from the conditions in natural environments, the growth rate measured in the laboratory might be a ‘hidden characteristic’ in nature and is unlikely to be exposed to the process of natural selection. Frequent gene flow may also damp the power of natural selection. Hirano et al. reported no genetic differentiation between tropical and temperate strains31, probably due to dispersal by birds and wind across a large geographic scale.
Biomass and hydrocarbon productivities of fast-growing wild strains
We selected 9 fast-growing strains based on the screening results and determined their biomass and hydrocarbon productivities in two separate tests. Test 1 cultured 4 wild strains plus the Showa strain for 30 days, and Test 2 cultured the other 5 strains plus the Showa strain for 40 days. Figure 3 shows the changes in algal biomass density in the fed-batch cultures. Biomass densities increased over the first two weeks, reached a plateau, and then gradually decreased in some culture bottles. The specific biomass growth rate (μmax) was the highest, and Dt was the shortest during the first 4 days of cultivation, and then the μmax decreased with increasing biomass density. In contrast, biomass productivity (Px) increased with an increase in algal density despite the decrease in the growth rate and reached the maximum at 2–7 days before the peak of algal density.
Biomass growth curves of fast-growing wild strains of Botrycoccus braunii in a fed-batch culture system. Every 2–3 days, 100 mL (20%) of culture was sampled to estimate algal biomass density, and the same amount of new medium was added. (a)–(e): Test 1 for 4 wild strains with a standard strain, Showa. (f)–(k): Test 2 for five wild strains with Showa. There were 3 repetitions (■, ▲, ●) of culture bottles for each strain. The arrow indicates the period of minimum doubling time, the horizontal bar indicates the period of maximum biomass productivity.
Figure 4 shows the minimum Dt and the maximum Px of 9 wild strains compared to the Showa strain. In each culture bottle, the two minimum Dt and the two maximum Px values were used to calculate the averages for each strain. In Test 1, the Dt of Showa was 1.97 days, and all four wild strains had shorter Dt than Showa. The Dt of OIT-678 (1.48 days) was significantly shorter than that of Showa (P < 0.01). In Test 2, another five wild strains had Dt (2.22–2.65 days) similar to that of Showa (2.23 days), and there were no significant differences (P > 0.05). These results demonstrate that the first screening by OD measurements in glass tubes successfully identified fast-growing strains with similar or faster biomass growth rates than the Showa strain. The Px was also significantly higher in fast-growing wild strains than that of Showa (P < 0.05, Fig. 4). Three wild strains (OIT-678, OIT-805b, OIT-685) had an approximately 36% higher Px than Showa (P < 0.05). Thus, the fast-growing strains also had high biomass productivities.
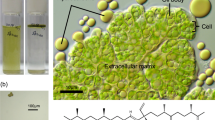
Doubling times and biomass productivity of 9 wild strains and Showa of Botryococcus braunii. (a) The bar indicates average with standard error (n = 6). Numerical values are shown in the bar. The values significantly different from that of Showa are indicated by asterisks: *, P < 0.05; **, P < 0.01 (Dunnett’s post hoc test). (b) Microscopic view of a wild strain, OIT-678 and the Showa strain. The colony was flattened by cover glass, and hydrocarbons (HCs) exuded from extracellular matrices (EMs).
Hydrocarbon content, productivity, and constituent were analyzed for 6 productive wild strains (Table 3). The hydrocarbon content of the Showa strain was 26%, and the wild strains had similar or somewhat higher hydrocarbon contents (26–38%). Hydrocarbon productivities of the wild strains ranged from 40 to 58 mg L−1 d−1, which is 1.1–1.9 times higher than that of Showa. The major constituent hydrocarbons were botryococcenes, C30H50 for OIT-678, C33H56 for OIT-805b, C34H58 for OIT-685, and OIT-775, respectively. Therefore, all these strains were identified as B race.
A novel fast-growing strain, OIT-678
The OIT-678 is the only wild strain with a significantly faster growth rate and higher biomass productivity than the Showa strain (Fig. 4). Therefore, we assessed the potential of this strain with additional experiments. To assess the maximal growth rate of the strain, we performed a fed-batch cultivation with a high flow rate (40% replacement every two days). Even in this high-flow rate condition, both OIT-678 and Showa increased biomass density for 10 days. The OIT-678 showed a significantly lower doubling time (t-test: ∣t∣ = 2.1, df = 38, P < 0.05) and a higher specific growth rate (∣t∣ = 2.06, df = 38, P < 0.05) than Showa. The Dt of OIT-678 and Showa were 1.23 days and 1.37 days, respectively (Fig. 5a). Although the difference in Dt between the two strains appears small, the outcome in biomass production is quite large. When starting cultivation with the same initial amount of biomass, the biomass of OIT-678 will be twice as much as that of Showa after 12 days. The biomass productivity of OIT-678 was 1.4 times larger than that of Showa, as mentioned earlier (Fig. 4).
Growth rate, biomass density, and colony size of a novel fast-growing strain, Botryococcus braunii OIT-678, compared with the Showa strain. (a,b) Fast-growing potential was assessed by a 500 mL fed-batch culture (4 replicates for each strain), in which 40% of the culture volume is replaced by new culture medium every 2 days. Until the 10th day of cultivation, biomass growth rates of two consecutive sampling points were calculated, and their averages were compared between strains. (c,d) Potential for a high-density culture was assessed by using a nutrient-rich medium, WFAM. First, 1 g L−1 of algae were inoculated and cultivated in two 500 mL bottles for each strain with AF-6 culture media for 24 days (Phase I). Algal density was monitored by OD. At the end of Phase I, 83 mL of 6 × WFAM were prepared in new 500 mL bottles, and 415 mL of algal culture was transferred to the bottle and cultivated (Phase II). From the 60th to the 75th day, 5% of the algal culture was sampled four times (red arrows), and the same amount of 6 × WFAM was added. This gradually increased the concentration of the media up to 2 × WFAM (Phase III). Cultivation was continued until the 87th day of cultivation (Phase IV). (e) Colony size measured on the 75th day (star). The box plot indicates the range of 10–90% of the data with bars and the range of 25–75% of the data with a box. Wilcoxon signed-rank test results are shown by letters in the boxes, and significant differences between the samples are indicated by different letters (P < 0.001). (f,g) Appearance of typical colonies: microscopic view (left) and macroscopic view (right).
We also assessed the ability for long-term, high-density cultivation of the strain by using a nutrient-rich media, WFAM (Supplementary Table 2S). WFAM contains higher amounts of nitrate (841 mg L−1 NO3−) and phosphate (94 mg L−1 PO43−) than AF-6 (309 mg L−1 NO3− and 9.7 mg L−1 PO43−). We started the cultivation by using AF-6 as the culture media and used two 500 mL bottles per strain in batch culture mode (Phase I, Fig. 5c,d). The initial biomass density was adjusted to 1 g L−1. After the algal density plateaued on the 24th day of cultivation, the culture media was replaced with WFAM. After replacement, algal density increased again for both strains (Phase II, Fig. 5c,d) and surpassed the plateau of Phase I, suggesting that the increases in algal biomass density were limited by the amounts of nutrients available in Phase I.
A second plateau in the algal density appeared between the 50th and 60th days of cultivation. We then sampled 5% of the algal culture four times (red arrows, Phase III in Fig. 5c,d) and added the same amount of 6 × WFAM. This gradually increased the concentration of the media up to 2 × WFAM until the 75th day of cultivation (Phase III). However, the algal density did not increase for 2 weeks following 75 days of cultivation (Phase IV in Fig. 5c,d), suggesting that the increases in algal biomass density in this phase were light-limited.
The maximal algal biomass density was 6.3 g L−1 for OIT-678 and 5.2 g L−1 for Showa. OIT-678 had a sixfold larger colony size than Showa (Fig. 5e–g). The increase in colony size induces an aggregated distribution of biomass in the culture and decreases the optical density at a given biomass density23. In agreement with this expectation, the ratio of OD to biomass density was smaller in OIT-678 (= 0.38) than in Showa (= 0.52; Fig. 5c,d), indicating a lower light attenuation in the culture of OIT-678. Thus, the OIT-678 can make a higher-density culture than Showa, which is at least partly due to the ability to reduce light attenuation in the culture by forming larger colonies.
Top records of fast growth rate and high productivity
We performed literature reviews on the growth rate and biomass productivity of B. braunii to find the fastest growth rates and the highest productivities (Table 4). The OIT-678 strain established a new record of Dt = 1.23 days in B. braunii race B, where the previous record was 1.4 days of Showa22. The Dt of OIT-678 falls short of the 1.11 days reported in the strain CCAP807/1, race A27, and the 0.52 days of an unknown strain33. The strain CCAP807/1 showed the Dt of 1.11 days under a continuous light condition (Php = 24:0, Table 4), which should be better than our culture condition (Php = 14:10). In addition, although the race A and race B strains are classified as the same species, their hydrocarbon structures and biosynthetic processes are largely different14, and they showed a high divergence value of 18S rRNA sequences at almost the species level34. Therefore, our finding of a novel fast-growing strain in race B is a significant advance.
Khichi et al. reported Dt = 0.52 days in a flat panel photobioreactor with an unknown strain of B. braunii33. This Dt record is extraordinarily fast compared to previous studies. However, they estimated the Dt based on indirect measurements of algal biomass using the OD. The OD of an algal culture can change greatly with the growth of contaminant bacterium as well as changes in the colony size of B. braunii23. Further studies, including race identification and direct measurements of biomass growth and productivity, are needed to confirm the record as the fastest B. braunii strain.
In terms of biomass and hydrocarbon productivity, our wild strains (Px = 182 mg L−1 d−1 and PHC = 58 mg L−1 d−1; Fig. 4 and Table 3) are not record-breaking. This is because productivity largely changed due to cultivation methods, and our method may not be optimal. Khatri et al. reported an exceptionally large productivity values (Px = 1500 mg L−1 d−1, PHC = 340 mg L−1 d−1) by an ultra-high density cultivation (Xmax = 20 g L−1) of the Showa strain (Table 4)23, which are some of the highest productivities for algal oil ever reported. In strains belonging to race A, Song et al. also reported a record value of Px = 1300 mg L−1 d−1 with a high-density culture (7.8 g L−1)35. Thus, the high-density culturing appears to be a promising method to improve the volumetric productivities of biomass and hydrocarbons of B. braunii. We determined the biomass and hydrocarbon productivities of our wild strains in relatively low densities (0.5–2.0 g L−1; Fig. 3). Therefore, there is a great potential for improvement of the productivity of our strains by adopting the high-density cultivation methods.
For high-density cultivations, strains with large-sized colonies might be suitable due to the increased permeability of light. Since light tends to be the most limiting resource for algal growth in high-density cultures, strains with efficient light capture characteristics should be useful. The increase in average colony size in culture is expected to increase the average amount of light received by the surface of a colony because of the reduction of self-shading among colonies23. Although the increase in colony size should decrease the amounts of light transmitted into the inner parts of a colony, cells placed in the inner parts of a colony are old and may have a limited physiological capacity to utilize strong light36. The OIT-678 strain formed a higher-density culture with a larger-sized colony than Showa (Fig. 5) and is therefore expected to have the potential to achieve higher productivities than Showa in dense-culturing methods.
Comparisons to other oleaginous microalgae
The growth rates of the Showa strain and OIT-678 are still much slower than those of other fast-growing microalgae. The oleaginous microalgae Chlorella vulgaris, Neochloris oleoabundans, and Scenedesmus obliquus have short doubling times of 8–9 h21. Because of its colony-forming habit, B. braunii invests resources to construct and maintain the extracellular matrix of the colony, and the self-shading among cells within the colony appears to be unavoidable. The synthesis of energetically expensive hydrocarbons may also restrict the potential for fast growth14.
Despite of the slow growth, the biomass and oil productivities of B. braunii are comparable to other fast-growing microalgae. The oil productivities of 30 microalgal species, which were assessed in 250 mL flasks under continuous illumination and bubbled with CO2-enriched air, showed that the averages (maximums) of the biomass productivity, the lipid content, and the lipid productivity are 190 mg L−1 d−1 (370 mg L−1 d−1), 23% (40%), and 40 mg L−1 d−1 (61 mg L−1 d−1), respectively37. Our novel wild strains of B. braunii had comparable productivities (Table 3). Furthermore, a high-density and continuous cultivation of the Showa strain23 achieved a hydrocarbon productivity of 340 mg L−1 d−1, and OIT-678 has the potential to exceed that productivity. This record is one of the highest productivities of algal oil to date. According to a comprehensive review38 published in 2011, the highest lipid productivities of microalgae under phototrophic conditions were 142 mg L−1 d−1 by Nannochloropsis oculate39 and 133 mg L−1 d−1 by Neochloris oleoabundans40. Subsequently, Ho et al. reported a high lipid productivity of 140 mg L−1 d−1 for Scenedesmus obliquus41. Further high lipid productivities were then reported for Chlorella vulgaris (1425 mg L−1 d−1)42 and C. protothecoides (590 mg L−1 d−1)43. Thus, the Chlorella species may hold the current record for highest records of lipid productivity of microalgae under phototrophic cultivation.
However, as these records of the Chlorella species are instantaneous values measured in batch cultivations, the average productivities under continuous cultivations should be lower than this value. Most oleaginous microalgae, including Chlorella and Scenedesmus species, require nutrient depletion to initiate lipid accumulation41,42,43. Because the nutrient depletion restricts further biomass growth in the culture, an initial period of cultivation for biomass accumulation is required prior to lipid production. Consequently, even if high lipid productivity is noted during the lipid accumulation phase, the average productivity over the total period, including the period for biomass growth, should decrease44. In contrast, B. braunii accumulates hydrocarbons mainly during the exponential and early linear growth stages45,46,47,62 (i.e., this alga can produce hydrocarbons and grow biomass simultaneously). In addition, the hydrocarbons produced by B. braunii have superior fuel properties to the lipids (triacylglycerol) produced by the oleaginous microalgae such as the Chlorella and Scenedesmus species in terms of high thermal values and compatibility with the existing petroleum infrastructure. Therefore, B. braunii can be regarded as one of the most promising species for the production of algal biofuel, and our novel fast-growing strains are expected to increase the feasibility of biofuel production.
The promises and challenges of creating a biorefinery from Botryococcus braunii
Improvements to cultivation methods such as mixotrophic48 and attached cultivation49, as well as the high-density cultivation23, have great potential to increase the biomass and hydrocarbon productivity of B. braunii. Furthermore, biological research is being developed on the genetic transformation50, genome sequencing51, and bacterial symbionts52,53 of B. braunii. Although many biotechnological and engineering advances have been made in the course of biomass production and biomass processing techniques to yield biofuels from B. braunii, the high production costs are still one of the major issues for the commercialization of algal biofuel production54.
In order to reduce the production costs, other potential applications such as wastewater treatment, CO2 mitigation, and the manufacture of high-value products should be coupled with biofuel production2. Wastewater treatment by B. braunii removes nutrients55 and heavy metals56 and may also be effective for pharmaceutical products remediation as recently reported in other microalgae57. Substantial amounts of high-value chemicals aside from hydrocarbons are also identified in B. braunii24,58. These potential applications can be combined with hydrocarbon production in a biorefinery from B. braunii.
When the biorefinery is scaled up to industrial levels in the future, the environmental sustainability concerns of the system cannot be ignored. In particular, due to the irreversibility of energy systems, exergy-based measures should be incorporated into traditional life cycle assessments to analyze the sustainability of the biorefinery from thermodynamic, economic, and environmental perspectives59.
Conclusions
This study performed a large-scale screening of the natural genetic resource of Botryococcus braunii on an unprecedented scale with 180 strains isolated from tropical to temperate climates and identified 9 fast-growing strains that have growth rates faster or similar to Showa, a standard fast-growing strain. Their biomass productivities were 12–37% higher than that of Showa. One strain, OIT-678, established a new record doubling time (1.2 days) as the fastest race B strain. Further studies are important to test whether the newly-isolated fast-growing strains outperform the highest productivities for hydrocarbons recorded by the formerly fastest strain Showa.
Methods
Isolation of wild strains
Since 2015 we have investigated a total of 112 natural ponds, including 50 ponds in tropical Indonesia and 9, 34, and 19 ponds in subtropical, warm-temperate, and cool-temperate Japan, respectively31,32. Surface water was collected using a phytoplankton net with 100 μm mesh to capture B. braunii, and a single colony was isolated by micropipette and incubated in a glass tube with a culture medium (See Kawamura et al.32 for a detailed protocol). A total of 180 wild strains were successfully isolated from 22 ponds located in various climate regions (Supplementary Table 1S). The 18S ribosomal sequences were determined for 103 strains, and chemical races (A, B, L, S races)34 were estimated based on the molecular phylogenetic tree31.
Screening of fast-growing wild strains
Screening of 180 wild strains was performed based on the growth rate observed in a 10 mL glass tube. The Showa strain was used as a benchmark. The algae were pre-cultured for 2 weeks before screening using AF-6 as the culture media (Supplementary Table 1S) with 100 μmol m−2 s−1 photosynthetic active radiation (PAR) of 12 h of illumination per day in a 2% CO2 incubator at a temperature of 26 ℃. This environmental conditions for cultivation were selected based on the optimal culture conditions for the Showa strain22, except for the light intensity (100 μmol m−2 s−1), which was lower than the optimal (300–1250 μmol m−2 s−1). This is because in our incubator equipment (See Kawamura et al.32 for details), strong light illuminations increased temperature (> 30 ℃) and restricted algal growth. The pre-cultured algal cells were transferred into three glass tubes with new culture media by adjusting algal density to be 0.1 of OD660. The optical densities (ODs) of the three glass tubes were then measured at 2–3 day intervals for 10–14 days. An exponential curve was fitted to the daily increase in OD, and the specific growth rate and the doubling time were calculated by using Eqs. (1) and (2), respectively.
where μmax = Specific growth rate (d−1) and Dt = Doubling time (d).
Biomass productivity of fast-growing wild strains
Biomass and hydrocarbon productivities were evaluated for a total of 9 wild strains selected by the screening. The selected strains were cultivated in 500 mL glass bottles with AF-6 media by illuminating 100 μmol m−2 s−1 PAR for 14 h per day with 2% CO2 bubbling in an incubator at a constant temperature of 26 ℃. Three culture bottles were used for each strain as a repetition, and the Showa strain was also cultured as a benchmark. Twenty percent of the culture media (100 mL) was sampled every 2 or 3 days, and the culture bottles were replenished with new culture media. At the start of the fed-batch cultivation, 0.1 g L−1 algal cells were inoculated. Cultivations were performed for 3–4 weeks. Algal cells were harvested with a 5 μm nylon mesh, dried at 70 ℃ for one week, and algal densities (mg L−1) were determined. The biomass productivity (Px) and the specific growth rate (umax) of two consecutive sampling time intervals were calculated by using Eqs. (3) and (4), respectively:
where Px = Biomass productivity (mg L−1 d−1) and Xt = Algal density at the tth days of cultivation (mg L−1).
Hydrocarbon analysis
About 200 mg of the dried algal cells were transferred to a glass tube containing 20 mL of acetone to extract the lipid components, disrupted by ultrasonication for 15 min, and centrifuged at 1000 × g for 10 min. The supernatant was transferred to a new glass tube. This process of acetone extraction was repeated 2–3 times until the supernatants became colorless. A chloroform/methanol mixture (25 mL, 2:1, v/v) was then added to the residual biomass, shaken overnight, and filtrated with ADVANTEC 5A filter paper with a vacuum. The filter paper was washed two times in the chloroform/methanol mixture. The chloroform/methanol filtrates and the acetone extracts were combined and concentrated in a rotatory evaporator. The residual lipid component was dissolved in 20 mL n-hexane and subjected to silica gel column chromatography on Wakogel C-300 (Wako Pure Chemical Industries, Ltd., Japan) with n-hexane as the mobile phase. All eluates before a yellow band of carotenes were collected, evaporated to remove the solvent, dried under vacuum, and then weighed to determine the amounts of hydrocarbons present22.
Hydrocarbon compositions were analyzed by gas chromatography/electron impact mass spectroscopy on a Shimadzu QP-2010 Ultra GC/EIMS system (Shimadzu) with a 30 m × 0.25 mm × 0.25 µm Rtx-5 column (GL Sciences). The column temperature was held at 50 °C for 1 min after sample injection, raised to 220 °C at a rate of 10 °C min−1 and then to 260 °C at a rate of 2 °C min−1 and finally held at 260 °C for 32 min. Splitless injections with a sampling time of 30 s were carried out. Injection temperature was set at 260 °C and injection volume was 2 µl. The ion source was kept at 200 ºC and the temperature of interface was 250 ºC. Identification of hydrocarbons was based on mass spectra and comparisons of retention times with triterpene hydrocarbons previously identified from the B race of B. braunii.
Additional experiments on a novel fast-growing strain, OIT-678
To assess the potential for fast growth of a wild strain (OIT-678), we performed an additional fed-batch cultivation with a high flow rate. The OIT-678 and Showa strains were each cultivated in four 500 mL bottles under the above-mentioned culture conditions for 10 days. During the cultivation, 40% of the culture volume (200 mL) was sampled every 2 days and replenished with new culture media. Specific growth rates were calculated based on the changes in algal density between two consecutive sampling times. A long-term batch culture with nutrient-rich media (WFAM; Supplementary Table 2S) was also performed. Algal density was monitored by OD660, and in a stationary phase, 5% of the culture volume (25 mL) was sampled four times to determine the maximal attainable densities. Colony sizes were also measured; microscopic pictures were taken immediately after squashing colonies under a cover glass, and the area (μm2) of a colony was measured using Image J software.
Statistical analyses
We analyzed the relative importance of strain, race, the pond of origin, and climate where the pond is located as determinants of doubling time (Dt) of a strain using the model of Eq. (5):
where Yijklm is the overall mean Dt estimated in glass tube m of stain k of race i, isolated from pond l in climate j; α is the overall mean; Ri is the fixed effect of race i; Cj is the fixed effect of climate j; S(i)k is the random effect of strain k nested in race i; P(j)l is the random effect of pond l nested in climate j; εijklm is the random residual error. The observed variance (σ2Y) of Dt can be decomposed into the variance of the race effect (σ2R), the variance of the climate effect (σ2C), the variance between the strains within a race (σ2S), the variance between the ponds within climate (σ2P), and the residual error variance (σ2E). The σ2E includes the variance between replicated glass tubes within a strain and the error in measurements:
Variance components were estimated based on the restricted maximum likelihood (REML) method. The REML method is considered a suitable procedure to estimate variance components for unbalanced data60. The least square means of Dt were computed for each strain, race, pond, and climate.
Average specific growth rate, doubling time, and biomass production rate of fast-growing wild strains measured by 500 mL bottle cultivations were compared to those of the Showa strain as a control by using ANOVA followed by Dunnett’s post hoc test. Because colony size data were not normally distributed, the non-parametric Wilcoxon signed-rank test was used instead of ANOVA. All statistical analysis was conducted using JMP software version 8.0 (SAS Institute, Inc., Cary, NC).
References
Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 25, 294–306 (2007).
Shuba, E. S. & Kifle, D. Microalgae to biofuels: ‘Promising’ alternative and renewable energy, review. Renew. Sustain. Energy Rev. 81, 743–755 (2018).
Wijffels, R. H. & Barbosa, M. J. An outlook on microalgal biofuels. Science 329, 796–799 (2010).
Sheehan, J., Dunahay, T., Benemann, J., Roessler, P. Look Back at the U.S. Department of Energy’s Aquatic Species Program: Biodiesel from Algae; Close-Out Report. Golden, CO: National Renewable Energy Lab. https://doi.org/10.2172/15003040 (1998).
Suparmaniam, U. et al. Insights into the microalgae cultivation technology and harvesting process for biofuel production: A review. Renew. Sustain. Energy Rev. 115, 109361 (2019).
Johnson, T. J. et al. Photobioreactor cultivation strategies for microalgae and cyanobacteria. Biotechnol. Prog. 34, 811–827 (2018).
Banerjee, S., Banerjee, S., Ghosh, A. K. & Das, D. Maneuvering the genetic and metabolic pathway for improving biofuel production in algae: Present status and future prospective. Renew. Sustain. Energy Rev. 133, 110155 (2020).
Ghosh, A. et al. Progress toward isolation of strains and genetically engineered strains of microalgae for production of biofuel and other value added chemicals: A review. Energy Conv. Manage. 113, 104–118 (2016).
Slocombe, S. et al. Unlocking nature’s treasure-chest: screening for oleaginous algae. Sci. Rep. 5, 9844 (2015).
Nascimento, I. A. et al. Screening microalgae strains for biodiesel production: Lipid productivity and estimation of fuel quality based on fatty acids profiles as selective criteria. Bioenerg. Res. 6, 1–13 (2013).
Pereira, H. et al. Microplate-based high throughput screening procedure for the isolation of lipid-rich marine microalgae. Biotechnol. Biofuels 4, 61 (2011).
Katayama, T. et al. Isolation of lipid-rich marine microalgae by flow cytometric screening with Nile Red staining. Aquacult. Int. 27, 509–518 (2019).
Huang, A. T., Goh, J. L., Ahmadzadeh, H. & Murry, M. A. A rapid sampling technique for isolating highly productive lipid-rich algae strains from environmental samples. Biofuel Res. J. 21, 920–926 (2019).
Banerjee, A., Sharma, R., Chisti, Y. & Banerjee, U. C. Botryococcus braunii: A renewable source of hydrocarbons and other chemicals. Crit. Rev. Biotechnol. 22, 245–279 (2002).
Metzger, P. & Largeau, C. Botryococcus braunii: a rich source for hydrocarbons and related ether lipids. Appl. Microbiol. Biotechnol. 66, 486–496 (2005).
Lee, S. Y., Kim, H. M. & Cheon, S. Metabolic engineering for the production of hydrocarbon fuels. Curr. Opin. Biotechnol. 33, 15–22 (2015).
Yoshida, M., Tanabe, Y., Yonezawa, N. & Watanabe, M. M. Energy innovation potential of oleaginous microalgae. Biofuels 3, 761–781 (2012).
Jackson, B. A., Bahri, P. A. & Moheimani, N. R. Repetitive non-destructive milking of hydrocarbons from Botryococcus braunii. Renew. Sustain. Energy Rev. 79, 1229–1240 (2017).
Griehl, C., Kleinert, C., Griehl, C. & Bieler, S. Design of a continuous milking bioreactor for non-destructive hydrocarbon extraction from Botryococcus braunii. J. Appl. Phycol. 27, 1833–1843 (2015).
Griffiths, M. J. & Harrison, S. T. L. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 21, 493–507 (2009).
Singh, S. P. & Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 50, 431–444 (2015).
Yoshimura, T., Okada, S. & Honda, M. Culture of the hydrocarbon producing microalga Botryococcus braunii strain Showa: Optimal CO2, salinity, temperature, and irradiance conditions. Bioresour. Technol. 133, 232–239 (2013).
Khatri, W., Hendrix, R., Niehaus, T., Chappell, J. & Curtis, W. R. Hydrocarbon production in high density Botryococcus braunii race B continuous culture. Biotechnol. Bioeng. 111, 493–503 (2014).
Lozoya-Gloria, E., Morales-de la Cruz, X. & Ozawa-Uyeda, T. A. The Colonial Microalgae Botryococcus braunii as Biorefinery. In Microalgae: From Physiology to Application (ed. Vítová, M.) 88206 (IntechOpen, 2019).
Nonomura, M. Botryococcus braunii var. showa (Chlorophyceae) from Berkeley, California, United States of America. Jpn. J. Phycol. 36, 285–291 (1988).
Gouveia, J. D. et al. Botryococcus braunii strains compared for biomass productivity, hydrocarbon and carbohydrate content. J. Biotechnol. 248, 77–86 (2017).
Moutel, B. et al. Development of a screening procedure for the characterization of Botryococcus braunii strains for biofuel application. Process Biochem. 51, 1855–1865 (2016).
Lee, C.-H., Chae, H.-S., Lee, S.-H. & Kim, H. S. Growth characteristics and lipid content of three Korean isolates of Botryococcus braunii (Trebouxiophyceae). J. Ecol. Environ. 38, 67–74 (2015).
Eroglu, E., Okada, S. & Melis, A. Hydrocarbon productivities in different Botryococcus strains: Comparative methods in product quantification. J. Appl. Phycol. 23, 763–775 (2011).
Li, Y. & Qin, J. G. Comparison of growth and lipid content in three Botryococcus braunii strains. J. Appl. Phycol. 17, 551–556 (2005).
Hirano, K. et al. Detection of the oil-producing microalga Botryococcus braunii in natural freshwater environments by targeting the hydrocarbon biosynthesis gene SSL-3. Sci. Rep. 9, 16974 (2019).
Kawamura, K., Hirano, K., Ardianor & Nuguroho, R. A. The oil-producing microalga Botryococcus braunii: A method for isolation from the natural environments and perspectives on roles of ecological studies in algal biofuel production. J. Ecosyst. Ecogr. 10, 274 (2020).
Khichi, S. S., Anis, A. & Ghosh, S. Mathematical modeling of light energy flux balance in flat panel photobioreactor for Botryococcus braunii growth, CO2 biofixation and lipid production under varying light regimes. Biochem. Eng. J. 134, 44–56 (2018).
Kawachi, M., Tanoi, T., Demura, M., Kaya, K. & Watanabe, M. M. Relationship between hydrocarbons and molecular phylogeny of Botryococcus braunii. Algal Res. 1, 114–119 (2012).
Song, L., et al. Micronutrient requirements for growth and hydrocarbon production in the oil producing green alga Botryococcus braunii (Chlorophyta). Plos One, e41459 (2012).
Van den Berg, T. E., Chukhutsina, V. U., van Amerongen, H., Croce, R. & van Oort, B. Light acclimation of the colonial green alga Botryococcus braunii strain Showa. Plant Physiol. 179, 1132–1143 (2019).
Rodolfi, L. et al. Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 102, 100–112 (2009).
Chen, C. Y., Yeh, K. L., Aisyah, R., Lee, D. J. & Chang, J. S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour. Technol. 102, 71–81 (2011).
Chiu, S. Y. et al. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour. Technol. 100, 833–838 (2009).
Li, Y., Horsman, M., Wang, B., Wu, N. & Lan, C. Q. Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl. Microbiol. Biotechnol. 81, 629–636 (2009).
Ho, S. H., Chen, C. Y. & Chang, J. S. Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 113, 244–252 (2012).
Pribyl, P., Cepak, V. & Zachleder, V. Production of lipids in 10 strains of Chlorella and Parachlorella, and enhanced lipid productivity in Chlorella vulgaris. Appl. Microbiol. Biotechnol. 94, 549–561 (2012).
Sun, Z., Zhou, Z. G., Gerken, H., Chen, F. & Liu, J. Screening and characterization of oleaginous Chlorella strains and exploration of photoautotrophic Chlorella protothecoides for oil production. Bioresour. Technol. 184, 53–62 (2012).
Kawamura, K. et al. Determining the optimal cultivation strategy for microalgae for biodiesel production using flow cytometric monitoring and mathematical modeling. Biomass Bioener. 117, 24–31 (2018).
Okada, S., Murakami, M. & Yamaguchi, K. Hydrocarbon production by the Yayoi, a new strain of the green microalga Botryococcus braunii. Appl. Biochem. Biotechnol. 67, 79–86 (1997).
Okada, S., Devarenne, T. P., Murakami, M., Abe, H. & Chappell, J. Characterization of botryococcene synthase enzyme activity, a squalene synthase-like activity from the green microalga Botryococcus braunii, Race B. Arch. Biochem. Biophys. 422, 110–118 (2004).
Metzger, P., Allard, B., Casadevall, E., Berkaloff, C. & Coute, A. Structure and chemistry of a new chemical race of Botryococcus braunii (Chlorophyceae) that produces lycopadiene, a tetraterpenoid hydrocarbon. J. Phycol. 26, 258–266 (1990).
Zhang, H., Wang, W., Li, Y., Yang, W. & Shen, G. Mixotrophic cultivation of Botryococcus braunii. Biomass Bioener. 35, 1710–1715 (2011).
Chen, P. et al. The growth, lipid and hydrocarbon production of Botryococcus braunii with attached cultivation. Bioresour. Technol. 138, 95–100 (2013).
Berrios, H., Zapata, M. & Rivas, M. A method for genetic transformation of Botryococcus braunii using a cellulase pretreatment. J. Appl. Phycol. 28, 201–208 (2016).
Browne, D. R. et al. Draft Nuclear Genome Sequence of the Liquid Hydrocarbon-Accumulating Green Microalga Botryococcus braunii Race B (Showa). Genome Announc. 5, e00215-e217 (2017).
Tanabe, Y. et al. A novel alphaproteobacterial ectosymbiont promotes the growth of the hydrocarbon-rich green alga Botryococcus braunii. Sci. Rep. 5, 10467 (2015).
Blifernez-Klassen, O. et al. Phytoplankton consortia as a blueprint for mutually beneficial eukaryote-bacteria ecosystems based on the biocoenosis of Botryococcus consortia. Sci. Rep. 11, 1726 (2021).
Shiho, M. et al. Business evaluation of a green microalgae Botryococcus braunii oil production system. Proc. Environ. Sci. 15, 90–109 (2012).
Cabanelas, I. T. D. et al. From waste to energy: Microalgae production in wastewater and glycerol. Appl. Energy 109, 283–290 (2013).
Areco, M. M., Haug, E. & Curutchet, G. Studies on bioremediation of Zn and acid waters using Botryococcus braunii. J. Environ. Chem. Eng. 6, 3849–3859 (2018).
Xie, P. et al. Revealing the role of adsorption in ciprofloxacin and sulfadiazine elimination routes in microalgae. Water Res. 172, 115475 (2020).
Cheng, P. et al. High-value chemicals from Botryococcus braunii and their current applications: A review. Bioresour. Technol. 291, 121911 (2019).
Rosen, M. A. Environmental sustainability tools in the biofuel industry. Biofuel Res. J. 17, 751–752 (2018).
Dieters, M. J., White, T. L., Littell, R. C. & Hedge, G. R. Application of approximate variances of variance-components and their ratios in genetic tests. Theor. Appl. Genet. 91, 15–24 (1995).
Pérez-Mora, L. S., Matsudo, M. C., Cezare-Gomes, E. A. & Carvalho, J. An investigation into producing Botryococcus braunii in a tubular photobioreactor. J. Chem. Technol. Biotechnol. 91, 3053–3060 (2016).
Casadevall, E. et al. Studies on batch and continuous cultures of Botryococcus braunii: Hydrocarbon production in relation to physiological state, cell ultrastructure, and phosphate nutrition. Biotechnol. Bioeng. 27, 286–295 (1985).
Tran, H. L., Kwon, J. S., Kim, Z. H., Oh, Y. & Lee, C. G. Statistical optimization of culture media for growth and lipid production of Botryococcus braunii LB572. Biotechnol. Bioprocess Eng. 15, 277–284 (2010).
Yeesang, C. & Cheirsilp, B. Low-cost production of green microalga Botryococcus braunii biomass with high lipid content through mixotrophic and photoautotrophic cultivation. Appl. Biochem. Biotechnol. 174, 116–129 (2014).
Acknowledgements
This study was supported by a fund from JSPS KAKENHI to K. K. (Nos. 26660179, 20K06215). We appreciate Drs. Naru Takayama, Yukio Komai of OIT, Hendrik Segah, Sulmin Gumiri, Dontes R Siburian Zakiah, Noorkumala Sari Nisa, Andri Cornelius of UPR, and Widha Prahastika of UNMUL for their helpful support with the fieldwork.
Author information
Authors and Affiliations
Contributions
K.K. conceived the research, performed algal cultivations, and drafted the manuscript. S.N. and K.H. performed screening. K.K., K.H., A., R.A.N. conducted fieldwork. S.O. performed hydrocarbon analysis. All authors contributed to the final manuscript editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kawamura, K., Nishikawa, S., Hirano, K. et al. Large-scale screening of natural genetic resource in the hydrocarbon-producing microalga Botrycoccus braunii identified novel fast-growing strains. Sci Rep 11, 7368 (2021). https://doi.org/10.1038/s41598-021-86760-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86760-8
This article is cited by
-
Nitrogen and phosphorus stress as a tool to induce lipid production in microalgae
Microbial Cell Factories (2023)
-
Findings on droplet breakup behavior of the preheated microalgae oil jet for efficiency improvement in diesel engines
Biomass Conversion and Biorefinery (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.