Abstract
Polypharmacy is commonly defined based on the number of medications taken concurrently using standard cut-offs, but several studies have highlighted the need for a multidimensional assessment. We developed a multidimensional measure of polypharmacy and compared with standard cut-offs. Data were extracted for 2141 respondents of the 2007 Prescription Drug Survey, a sub-study of the Health Retirement Study. Latent classes were identified based on multiple indicators of polypharmacy, including quantity, temporality and risk profile. A four-class model was selected based on fit statistics and clinical interpretability: ‘High risk, long-term’ (Class 1), ‘Low risk, long-term’ (Class 2), ‘High risk, short-term’ (Class 3), and ‘High risk for drug interactions, medium-term, regular’ (Class 4). Classes differed regarding sex, cohabitation, disability and multimorbidity. Participants in the ‘low risk’ class tended to be male, cohabitating, and reported fewer health conditions, compared to ‘high risk’ classes. Polypharmacy classes were compared to standard cut-offs (5+ or 9+ medications) in terms of overlap and mortality risk. The three ‘high risk’ classes overlapped with the groups concurrently taking 5+ and 9+ medications per month. However, the multidimensional measure further differentiated individuals in terms of risk profile and temporality of medication taking, thus offering a richer assessment of polypharmacy.
Similar content being viewed by others
Introduction
Polypharmacy is increasingly prevalent among older adults and is associated with a range of adverse health outcomes1,2. Polypharmacy is commonly understood as the concurrent use of multiple medications by one individual but there is no standard agreed definition and measurement varies across studies3,4. The most common approach is to define polypharmacy based on the number of medications taken, with a threshold of four or more5, five or more6,7,8, ten or more9,10 or other cut-points11,12,13. However, recent studies have highlighted the limitations of these ‘cut-point’ measures and emphasised the need to move towards more multidimensional assessments of polypharmacy. For example, incorporating the clinical appropriateness of prescribed medications or potential adverse drug interactions4,14. Thus, further research is needed to develop more comprehensive assessments of polypharmacy whilst ensuring that derived measures remain practical for clinicians and researchers.
Recent reviews15 have highlighted the following dimensions as key to understanding polypharmacy and its association with adverse outcomes: (1) temporality (i.e., how long the patient has been taking a particular medication); (2) dosage form (e.g., oral, topical); (3) appropriateness of the prescribed medication; (4) anticholinergic properties of the medication; and (5) potential drug interactions.
Temporality and dosage form are well known to be associated with medication adherence15,16. For example, Monégat et al.3 propose combining these indicators to define polypharmacy as continuous (e.g. number of medications prescribed more than twice a year), cumulative (e.g. average number of medications prescribed over quarters of a year) and simultaneous (e.g. total prescriptions per day).
Regarding appropriateness, several tools have been developed for assessing the appropriateness of medications for older adults including the Beers criteria17 and STOPP/START tool (Screening Tool of Older Person’s Prescriptions/Screening Tool to Alert doctors to Right Treatment)18. The Beers criteria is a widely used consensus criteria for medication use in older adults which describes potentially inappropriate drugs for this population based on their high risk of adverse outcomes such as falls and mortality17,19,20. The Beers criteria provide information about drug–drug and drug–disease interactions and highlight anticholinergic medications for their adverse effects linked to impaired cognitive and physical function, and risk of dementia21,22. The STOPP/START criteria is comparable to Beers criteria when in clinical inpatient settings but some studies suggest that adaptations are needed if these are to be applied in broader population settings23.
In summary, some studies support the use of cut-point measures, based on their ability to predict adverse health outcomes, such as frailty, disability, and mortality. However, others argue that limiting the assessment of polypharmacy to ‘quantity’ only is an oversimplification and omits important aspects such as appropriateness or drug interactions. The objectives of this study, therefore, are to: (1) Develop a multidimensional measurement of polypharmacy capturing temporality, dosage form and risk profile, as well as quantity; (2) Compare this multidimensional approach with standard cut-offs based on the number of medications in terms of overlap and predictive ability.
Methods
Sample
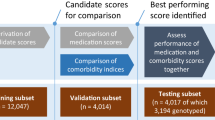
Data were from the 2007 Prescription Drug Survey (PDS), a sub-study of the Health Retirement Study (HRS)24. The HRS has been administered every 2 years since 1992 and provides representative data on adults aged 50 and over living in the United States. We supplemented information on medication use from the PDS with (i) baseline covariates from the 2006 h survey, and (ii) mortality over follow-up from the RAND-HRS Longitudinal File (2008–2016)25,26. Of 3536 respondents to the 2007 PDS (response rate 74%), we excluded participants missing information on current medications, those not taking any medications, and those taking > 25 medications/month. We focused our analysis on individuals aged 50–80 years, since individuals older than 80 constitute a different population, the oldest-old, with differentiated patterns of polypharmacy and higher risk of adverse outcomes related to polypharmacy27 The analytical sample therefore contained 2141 individuals. For Cox proportional hazard models, described below, we further excluded participants missing information on mortality over follow-up reducing the same size for these analyses to 1935.
Measures
Polypharmacy indicators
We identified eight indicators split over three domains based on the previous literature review4,17 and discussion with expert clinicians and pharmacists. ‘Temporality’ included two indicators: (1) Percentage of prescription medications taken on a regular basis (every day or every week) rather than taken as needed. This categorisation was based on participant self-report in the PDS questionnaire where participants were asked “Of those prescription drugs, how many are ones you take on a regular basis (for example, every day or every week)?” Since most medications were taken regularly, this variable was dichotomised as ‘Up to 75%’ vs. ‘More than 75%’. (2) Duration of medication use, assessed based on participant self-report in the PDS questionnaire where participants were asked how long they had been taking each prescription medication. We summarised this information by counting the number of medications they had been taking for 0–5 months, 6–24 months, and 25 + months. ‘Risk profile’ included three indicators: (3) Number of anticholinergic medications currently taken; (4) number of potentially inappropriate medications currently taken; and (5) number of potential adverse drug interactions. For these measures, we selected the 90% most common medications (219 medications, out of 608 in total) and categorised each medication based on published studies (Tables 7, 2, and 5 from the 2019 Beers criteria17, respectively) and manual coding by an expert pharmacist. Due to small cell counts, all three measures were dichotomised as 0 vs. 1 + medications. Finally, ‘Quantity’ included three indicators derived from participant self-report in the PDS questionnaire: Number of (6) prescription and (7) non-prescription medications used in the last month; and (8) total number of different dosage forms used for current medications (e.g. tablet, liquid, inhalant, other).
Covariates for use in prediction modelling
Age was derived from birth date as the participant’s age on October 1st 2007 (the month when PDS questionnaires were sent out). Sex, ethnicity, education, cohabitation (living with partner vs. not living with partner), chronic health conditions, and Activities of Daily Living (ADLs) were derived from the 2006 h interview. Ethnicity was coded into three categories (White/Caucasian, Black/African American and Other). Education was coded into five categories (less than high-school, GED, high school, some college, above college). GED refers to ‘General Educational Development’, an alternative to the high school diploma. Chronic health conditions were measured as the total number of conditions as well as binary indicators of specific conditions: cancer (malignant tumor excluding skin), lung disease, diabetes, joint disease (arthritis, connective tissue disease), heart problems (myocardial infarction, angina, congestive heart failure, arrhythmia, other heart problems), stroke, high blood pressure, or depression caseness. Depression caseness was quantified as a score of four or greater on the Center for Epidemiologic Studies Depression Scale (CES-D). In addition, we derived a three-category variable to capture multimorbidity (‘no multimorbidity’ = 0–1 conditions; ‘simple multimorbidity’ = 2–3 conditions; and ‘complex multimorbidity’ = 4 + conditions). Previous research has shown that differentiating between simple and complex multimorbidity can better capture the variability in multimorbidity in older adults28,29. ADLs30 included walking across the room, dressing, bathing and showering, eating, and getting in and out of bed. To ease interpretation and for consistency with previous research in older adults31 this variable was dichotomised as 0 (‘No difficulties’) or 1 (‘Reported difficulties in at least one of the tasks’).
Mortality
Date of death was derived from the RAND-HRS Longitudinal File, which incorporates successive biennial HRS interviews between 2008 and 2016. Year and month of death were ascertained from the participant’s exit interview or spouse.
Statistical analyses
The analysis was in two parts. First, to derive a multidimensional measure of polypharmacy we used latent class analysis (LCA) to identify subgroups of participants based on their response patterns across eight polypharmacy indicators. We considered models with 1–10 latent classes, estimated using Mplus 8.232. The optimal number of classes was chosen based on clinical interpretability and model fit [Akaike information criterion (AIC) and sample size adjusted Bayesian information criterion (BIC)]. Second, we used Cox proportional hazards models to assess whether polypharmacy was predictive of mortality over follow-up. We compared polypharmacy defined using cut-points based on the number of prescription medications taken per month, either (i) 5 + vs. fewer or (ii) 9 + vs. fewer; or (iii) membership to polypharmacy latent classes for the chosen LCA model. Class membership was measured using binary indicators (0 vs. 1) for each latent class. We chose the 5 + cut-point because this is most commonly used in past studies15; we additionally considered the 9 + cut-point to reflect growing rates of polypharmacy in recent years. Predictive performance was assessed based on the C-index with adjustment for right-censoring33 using tenfold nested cross-validation with 100 repeats. Cox proportional hazards models were adjusted for age, sex, education, ethnicity, and chronic health conditions, as detailed above. Missing covariate information was imputed using multivariate imputation34. Prediction models were estimated using Scikit-learn35 and Scikit-Survival36 in Python 3.837.
Sensitivity analyses
We conducted two sensitivity analyses to consider whether our findings were influenced by the chosen age criteria (ages 50–80). Firstly, since the Beers criteria were originally intended for use in adults aged 65 and older, we repeated the latent class analysis after excluding younger participants (aged 50–64). Secondly, to consider whether including older participants (aged over 80) would produce different classes, we repeated the analyses for all participants aged 50 and over.
Results
Descriptive summary of analytical sample
The analytical sample contained 2141 respondents, having removed those missing information on current medications (n = 536), younger than 50 years (n = 10) or older than 80 years (n = 815), not taking any medications (n = 30), or taking > 25 medications/month (n = 4). Compared to excluded participants, the analysed sample tended to be younger (mean 74.6 vs. 71.0; p < 0.001) but were similar in terms of sex and ethnicity (see Supplementary Table 2 for details). The sample contained slightly more women than men (n = 1244; 58%) and was predominantly White/Caucasian (n = 1759; 82%). Most participants reported at least one chronic condition (n = 1961; 92%) and 17% reported difficulties with ADLs (n = 367).
Polypharmacy classes
A four-class model was selected based on model interpretability and model fit (AIC and adjusted BIC; see Supplementary Table 1). Below we describe the classes labelled by their distinguishing polypharmacy characteristics (see Table 1 for details):
Class 1 ‘High risk, long-term’ (n = 484)
Individuals in this class tended to have been taking medications for a longer duration (> 2 years), and took a higher number of prescribed medications (8.1 per month, compared to 3.3, 6.9 and 7.7 for other classes). Regarding risk profile, this class was more likely to be taking inappropriate medications than other classes (44% taking at least one, compared to 17–43% in other classes) and had high risk associated with anticholinergic (19%) and drug interactions (20%).
Class 2 ‘Low risk, long-term’ (n = 1331)
Individuals in this class took fewest prescription medications (3.3 per month, compared with 6.9 or more in other classes) and had the lowest risk profile in terms of anticholinergic, inappropriate, drug interactions. Just 4% were taking one or more anticholinergic medications (compared to 18% or more in other classes) and 17% were taking inappropriate medications (compared to 37% or more in other classes). Most medications were taken for long durations (> 25 months).
Class 3 ‘High risk, short-term’ (n = 126)
This class took a high number of both prescription and non-prescription medications each month (6.9 and 1.5, respectively), but tended to take medications on a short-term basis (< 6 months). They had a high risk profile regarding anticholinergic (20% taking at least one) and inappropriate (43% taking at least one) medications and a moderate risk regarding drug interactions.
Class 4 ‘High risk for drug interactions, medium-term, regular’ (n = 120)
Individuals in this class took a high number of prescription medications (7.7 per month), most of which they had been taking regularly for between 6 months and 2 years. They were most likely to have one or more drug interactions (32% compared to 1–20% in other classes) and had a moderate-to-high risk profile regarding anticholinergic and inappropriate medications (18% and 37% taking at least one, respectively).
These classes were similar in terms of age and ethnicity (Table 2). Those in Class 2 (‘Low risk, long-term’) were more likely to be male (44% vs. 39% or less in other classes; p = 0.027) and cohabiting (69% vs. 64% or less in other classes; p = 0.012). There were also differences regarding disability, with those in Class 2 reporting fewer chronic conditions and being less likely to report difficulties with ADLs (11% vs. 22% or more; p < 0.001), compared to other classes. In contrast, those in Class 4 (‘High risk for drug interactions, medium-term, regular’) were most likely to report difficulties with ADLs (35%; p < 0.001).
With regards to health conditions, Class 1 (‘High risk, long-term’) saw the highest levels of overall multimorbidity, with 37% reporting 4 or more conditions (compared to 10–34% in other classes; p < 0.001) and just 9% reporting 0 or 1 conditions (compared to 14–38%; p < 0.001). When we examined specific health conditions independently, we found that individuals in Class 2 (‘Low risk, long-term’) reported the lowest rates for almost all conditions. For example, 15% in Class 2 reported diabetes, compared to 29–41% in other classes (p < 0.001); and 13% reported CES-D depression, compared to 19–28% in other classes (p < 0.001). Among the other classes, rates of almost all specific conditions were highest in Class 4 (‘High risk for drug interactions, medium-term, regular’), whereas Class 3 (‘High risk, short-term’) showed lower percentages for high blood pressure (68%), heart problems (28%) and stroke (10%) when compared with Class 1 and Class 4. Post-hoc pairwise comparisons showed that differences between pairs of classes were statistically significant.
There was considerable overlap between the four polypharmacy classes and the cut-point measure based on 5 + or 9 + concurrent medications. Of those in Class 2 (‘Low risk, long-term’), only 20% were taking 5 + medications per month, compared to 96%, 75%, and 90% of participants in the three ‘high risk’ classes (Classes 1, 3, and 4, respectively); and just 1% were taking 9 + medication (compared to 35%, 28% and 34% in Classes 1, 3 and 4, respectively). Therefore, the four-class polypharmacy measure differentiates not only between low and high risk polypharmacy (Class 2 vs. Classes 1, 3 or 4) but further distinguishes different types of high risk polypharmacy, as we discuss below.
Predicting mortality over follow-up
Our multidimensional measure of polypharmacy and the 5 + and 9 + cut-off measures were similar in their ability to predict mortality over follow-up (2007–2016). The C-index scores for latent class, 5 + and 9 + measures were 0.676, 0.678, and 0.674, respectively, after adjusting for age, sex, education, ethnicity, and chronic health conditions. Notably, polypharmacy was one of the strongest predictors of mortality risk over follow-up, regardless of the measure used. Membership in Class 1 (‘High risk, long-term’) and Class 4 (‘High risk for drug interactions, medium-term, regular’) was strongly associated with increased risk of mortality, compared to Class 2 [Hazard Ratio (HR) for Class 1 = 1.52; 95% CI 1.23, 1.88; HR for Class 4 = 1.85; 95% CI 1.42, 2.40]. Similarly, participants taking 5 + (HR 1.57; 95% CI 1.29, 1.90) or 9 + medications (HR 1.59; 95% CI 1.27, 1.98) experienced substantially higher mortality risks compared to those taking fewer (see Supplementary Table 3 for details).
Sensitivity analyses
We found consistent results when repeating the latent class analysis after (i) excluding younger participants (ages 65 +; Supplementary Table 4) or (ii) including older participants (ages 50 +; Supplementary Table 5). The number of classes, clinical interpretability, and model fit were almost unchanged and the composition of classes in terms of polypharmacy indicators and clinical characteristics was very similar.
Discussion
This study aimed to develop a multidimensional measure of polypharmacy and compare this with a standard cut-point based on taking five or more medications. A four-class model was chosen based on model fit and clinical interpretability, which overlapped with the standard cut-point measure. Our measure distinguished polypharmacy subtypes that differed with regards to quantity, temporality and risk profile. The largest class (Class 2, ‘Low risk, long-term’) tended to be healthier, took fewer medications and had a lower risk profile, compared to other classes. Most individuals in this class were below standard cut-offs for polypharmacy (< 5 medications). By contrast, most individuals in the other three classes all exceeded standard thresholds for polypharmacy. These three classes differ in terms of the potential drivers of this risk and how long have they been taking these medications. For example, Class 1 (‘High risk, long-term’) had the highest percentage of risk associated with anticholinergic and inappropriate medications, while Class 4 (‘High risk for drug interactions, medium-term, regular’) had the highest percentage of risk associated with drug interactions. These findings are in line with Wastesson et al.4 who highlighted the need for more holistic assessments of polypharmacy, including other relevant indicators such as adverse interactions.
Besides risk profile, the other dimension that distinguished classes was duration. Among the three ‘high risk’ classes, individuals in Class 3 (‘High risk, short-term’) tended to take medications for much shorter durations, compared to other classes. The role of duration as a possible differentiating feature links with adherence16 and the on-going debate around deprescribing (the gradual withdrawal from medications with healthcare supervision) which is key to personalized medicine. While research interest in deprescribing has recently grown38, it remains limited in practice by social factors such as specialist or nurse influences39, and more research should be performed to examine whether duration is key for deprescribing guidelines for some specific drugs or not. It is important to note that duration of treatment is not a sufficient criterion for deprescribing and only is suitable when it targets drugs that are potentially inappropriate, for which the risks outweigh the benefits. Therefore, individuals taking medications for longer periods of time could be targeted for deprescribing drugs that are potentially inappropriate. Our measure could be used to identify these individuals, for example, prioritizing individuals classified in Class 1 ‘High risk, long-term’.
Across polypharmacy classes, individuals were similar in age and ethnicity but differed in terms of sex, cohabitation, disability and health conditions. Those in Class 2 (‘Low risk, long-term’) were more likely to be male and cohabiting, report fewer chronic conditions and difficulties with activities of daily living. These findings are consistent with previous studies showing no differences in polypharmacy by ethnicity40 and higher rates of polypharmacy among women41,42 or separated or never married individuals43. In addition, we found significant differences in disability and health conditions among the ‘high risk’ classes. For disability, Class 4 (‘High risk for drug interactions, medium-term, regular’) was most likely to report difficulties with ADLs, compared to other classes. While for multimorbidity, although all ‘high risk’ classes were more likely to have two or more co-existing health conditions, individuals in Class 1 (‘High risk, long-term’) were more likely to have complex multimorbidity (4 or more conditions). These results are in line with previous research that has found that individuals with polypharmacy are more likely to have difficulties with ADLs and more likely to have two or more chronic health conditions44,45. Moreover, the link between high risk polypharmacy (Class 1) and complex multimorbidity (4 or more conditions) is of particular clinical relevance, since these individuals are more likely to have higher healthcare needs and may be using multiple healthcare providers. It should be noted that most previous research uses polypharmacy standard cut-offs, making it difficult to draw direct comparisons. Further research exploring differences between the ‘high risk’ classes should be performed in other populations. For example, recent research showing sex differences in polypharmacy are specific to older adults aged 50–80, but this pattern seems to reverse among those over 80, the oldest old45.
With regards to the comparison of the multidimensional and the cut-point measures of polypharmacy, the three ‘high risk’ classes (Classes 1, 3, and 4) overlapped substantially with the groups taking 5 + and 9 + medications per month, whereas most members of Class 2 (‘Low risk, long term’) were taking fewer than five medications per month. When we examined the predictive value of the proposed multidimensional measure, we found that it predicted mortality even after adjusting for demographic characteristics and co-existing health conditions. This suggests that although multimorbidity is known to be inherently associated with polypharmacy46, polypharmacy has an independent role in its association with mortality. However, it is notable that our multidimensional measure and cut-off measure (5 + and 9 +) were similar in terms of their ability to predict mortality over follow-up. This suggests that both our theoretically derived multidimensional measure or standard cut-offs can be used for mortality risk stratification purposes. As our results highlight, researchers without detailed information on polypharmacy may apply standard cut-offs, but these definitions may overlook differences in the risk profile of these individuals for other health outcomes, such as hospital admission or need for long-term care.
This is the first study to our knowledge to provide evidence of a multidimensional measure of polypharmacy using data from a large population study and its main strengths can be summarized in the following three points. Firstly, these findings further our understanding of the polypharmacy construct in older adults by providing empirical evidence to the on-going debate around the need for multidimensional approaches that go beyond quantity. By using a large population study and dimensions that can be extracted from other population data sources and electronic health records, our measure can be replicated and compared in other settings. We expect that this study will facilitate cross-cohort comparisons and further work in this direction. Finally, this measure has the potential to be used in translational medicine in the development of new screening tools to identify individuals in need of clinical reviews of their medication regimen. For example, it could be embedded in pipelines in hospital settings to support clinical decision making. However, further research will have to follow up to ensure its direct clinical applicability.
However, several limitations must also be acknowledged. First, data were self-reported which may introduce potential biases, such as recall or social desirability. Future studies should replicate our findings using objective measures, for example, extracted from electronic health records. Secondly, our data were drawn from a population aged 50 years and over and while polypharmacy is common in older adults who are more likely to have two or more chronic health conditions (i.e. multimorbidity), research has highlighted socially deprived individuals are likely to develop multimorbidity earlier in life47 and could also be taking multiple medications. Thus, further studies with younger populations or even specific sub-populations traditionally neglected in large population studies (e.g. those with mental health disorders) should be considered. While the Beers criteria were originally intended for populations over 65 years old, results from our sensitivity analyses suggest they may be useful for younger populations, as some past studies have suggested48. Thirdly, our data were drawn from the United States and these analyses should be replicated in other countries to allow comparison of prescription guidelines. For example, the Beers criteria are not often used outside the USA, in part due to differences between national drug formularies (i.e. differences in approved and utilized drugs) between countries. Therefore, future studies should therefore investigate the potential transferability of Beers criteria for cohorts over 65 or outside the USA.
Fourth, although we focused on indicators likely to be available in routine clinical datasets to ensure replicability and potential for implementation, we did not include other complex measures of medication regimen such as the medication regimen complexity index or the drug burden index or considered the potential benign impact of some medication12. Other measures to address alternative dimensions of polypharmacy include the medication regimen complexity index (MRCI)49, the drug burden index (DBI)50, and the medication appropriateness index (MAI)51,52. However, these cannot be directly compared to our measure as some are broader (MRCI) and others are more specific (DBI and MAI). The MRCI is a 65-item instrument with different complexity levels which are based on weighted averages of the number of drugs, dosage frequency, administration instructions, and prescribed dosage forms. Although previous research shows that it is a good predictor of hospitalization, hospital readmission, and medication adherence53, recent reports have highlighted that the MRCI is a long and complex instrument that does not outperform patient medication counts to predict medication-related hospital admission54. The DBI, which also has been found to be associated with functioning outcomes in older adults55, is a more specific measure that captures overall exposure to medications with anticholinergic and sedative properties that implements the principle of dose response to determine the effect of medication exposure. However, this measure does not account for other relevant factors for research in older adults (e.g., multimorbidity56). The MAI has shown to be also a valuable research tool for measuring potentially inappropriate prescribing in the elderly, and especially sensitive for those with higher levels of inappropriateness. However, it does not address some aspects of suboptimal prescribing (i.e., polypharmacy or underuse of medically necessary medications) and its evaluation requires an expert clinician, can be time consuming, and it may be subject to reliability issues when more than one evaluator is used57. Future studies should explore the potential convergent or divergent validity of these scales against our proposed measure and compare its applicability with clinical data sources.
Fifth, polypharmacy is variable over time58,59 and several concepts of polypharmacy require longitudinal data to explore transitions between drugs, the duration of use, total quantity of drugs used or pauses in treatment. Although we considered treatment duration estimated by the participants, data remain limited to drugs that were used during the study period, and the quality of adherence to therapy cannot be assessed. Future longitudinal studies should be performed to address some of these questions. Finally, we used the most recent Beers criteria for assessing appropriateness and drug interactions17, however these criterion change over time so future research will need to include updates.
To sum up, our multidimensional measure provided a more nuanced characterisation of polypharmacy that allowed us to group individuals in a richer and more meaningful way than when using standard cut-offs. The multidimensional measure and standard cut-offs were similar in terms of mortality risk stratification, but for studies addressing outcomes besides mortality, we believe that the multidimensional measure can provide novel insights. Despite differences in data collection between sites or countries, many of the included indicators could be extracted from comparable population datasets, or potentially, from electronic health records supported by future algorithm developments such as natural language processing. While further research is needed to understand the clinical applicability of a multidimensional measure of polypharmacy, it may also be possible to develop novel artificial intelligence pipelines to detect individuals in need of clinical review of their medication regimen.
Data availability
Data was extracted from the Health Retirement Study which is a publicly available dataset. More details in https://hrs.isr.umich.edu/data-products/access-to-public-data.
References
Guthrie, B., Makubate, B., Hernandez-Santiago, V. & Dreischulte, T. The rising tide of polypharmacy and drug–drug interactions: Population database analysis 1995–2010. BMC Med. 13, 74 (2015).
Rawle, M. J., Richards, M., Davis, D. & Kuh, D. The prevalence and determinants of polypharmacy at age 69: A British birth cohort study. BMC Geriatr. 18, 118–130 (2018).
Monégat, M. Polypharmacy: Definitions, measurement and stakes involved. Review of the literature and measurement tests. Inst. Rech. Doc. En Économie Santé 204, 8 (2014).
Wastesson, J. W., Morin, L., Tan, E. C. K. & Johnell, K. An update on the clinical consequences of polypharmacy in older adults: A narrative review. Expert Opin. Drug Saf. 17, 1185–1196 (2018).
Rankin, A. et al. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst. Rev. 9, 1–211 (2018).
Nobili, A. et al. Association between clusters of diseases and polypharmacy in hospitalized elderly patients: Results from the REPOSI study. Eur. J. Intern. Med. 22, 597–602 (2011).
Richardson, K., Ananou, A., Lafortune, L., Brayne, C. & Matthews, F. E. Variation over time in the association between polypharmacy and mortality in the older population. Drugs Aging 28, 547–560 (2011).
Slabaugh, S. L., Maio, V., Templin, M. & Abouzaid, S. Prevalence and risk of polypharmacy among the elderly in an outpatient setting. Drugs Aging 27, 1019–1028 (2010).
Morin, L. et al. Choosing wisely? Measuring the burden of medications in older adults near the end of life: Nationwide, longitudinal cohort study. Am. J. Med. 130, 927-936.e9 (2017).
Rieckert, A. et al. Polypharmacy in older patients with chronic diseases: A cross-sectional analysis of factors associated with excessive polypharmacy. BMC Fam. Pract. 19, 113 (2018).
Jyrkkä, J., Enlund, H., Korhonen, M. J., Sulkava, R. & Hartikainen, S. Polypharmacy status as an indicator of mortality in an elderly population. Drugs Aging 26, 1039–1048 (2009).
Libby, A. M. et al. Patient-level medication regimen complexity across populations with chronic disease. Clin. Ther. 35, 385-398.e1 (2013).
Veronese, N. et al. Polypharmacy is associated with higher frailty risk in older people: An 8-year longitudinal cohort study. J. Am. Med. Dir. Assoc. 18, 624–628 (2017).
Guillot, J., Maumus-Robert, S. & Bezin, J. Polypharmacy: A general review of definitions, descriptions and determinants. Therapies 75, 407–416 (2019).
Masnoon, N., Shakib, S., Kalisch-Ellett, L. & Caughey, G. E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 17, 1–12 (2017).
Griffith, S. A review of the factors associated with patient compliance and the taking of prescribed medicines. Br. J. Gen. Pract. 40(332), 114–116 (1990).
2019 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 67, 674–694 (2019).
Boland, B., Guignard, B., Dalleur, O. & Lang, P.-O. Application of STOPP/START and Beers criteria: Compared analysis on identification and relevance of potentially inappropriate prescriptions. Eur. Geriatr. Med. 7, 416–423 (2016).
Ali, S., Peterson, G. M., Bereznicki, L. R. & Salahudeen, M. S. Association between anticholinergic drug burden and mortality in older people: A systematic review. Eur. J. Clin. Pharmacol. 76, 1–17 (2019).
do Nascimento, M. M. G. et al. Potentially inappropriate medications: Predictor for mortality in a cohort of community-dwelling older adults. Eur. J. Clin. Pharmacol. 73, 615–621 (2017).
Fox, C. et al. Effect of medications with anti-cholinergic properties on cognitive function, delirium, physical function and mortality: A systematic review. Age Ageing 43, 604–615 (2014).
Gray, S. L. et al. Cumulative use of strong anticholinergics and incident dementia: A prospective cohort study. JAMA Intern. Med. 175, 401–407 (2015).
Hill-Taylor, B. et al. Effectiveness of the STOPP/START (Screening Tool of Older Persons’ potentially inappropriate Prescriptions/Screening Tool to Alert doctors to the Right Treatment) criteria: Systematic review and meta-analysis of randomized controlled studies. J. Clin. Pharm. Ther. 41, 158–169 (2016).
Sonnega, A. et al. Cohort Profile: The Health and Retirement Study (HRS). Int. J. Epidemiol. 43, 576–585 (2014).
RAND. RAND HRS Longitudinal File 2016. (Produced by the RAND Center for the Study of Aging, with funding from the National Institute on Aging and the Social Security Administration., 2019).
Health and Retirement Study. RAND HRS Longitudinal File 2016 public use dataset. (Produced and distributed by the University of Michigan with funding from the National Institute on Aging (grant number NIA U01AG009740). 2019).
Wauters, M. et al. Mortality, hospitalisation, institutionalisation in community-dwelling oldest old: The impact of medication. Arch. Gerontol. Geriatr. 65, 9–16 (2016).
Storeng, S. H., Vinjerui, K. H., Sund, E. R. & Krokstad, S. Associations between complex multimorbidity, activities of daily living and mortality among older Norwegians. A prospective cohort study: The HUNT Study, Norway. BMC Geriatr. 20, 21 (2020).
Harrison, C., Britt, H., Miller, G. & Henderson, J. Examining different measures of multimorbidity, using a large prospective cross-sectional study in Australian general practice. BMJ Open 4, e004694 (2014).
Katz, S., Ford, A. B., Moskowitz, R. W., Jackson, B. A. & Jaffe, M. W. Studies of illness in the aged: The index of ADL: A standardized measure of biological and psychosocial function. JAMA 185, 914–919 (1963).
Zaninotto, P., Batty, G. D., Allerhand, M. & Deary, I. J. Cognitive function trajectories and their determinants in older people: 8 years of follow-up in the English Longitudinal Study of Ageing. J. Epidemiol. Community Health 72, 685–694 (2018).
Muthen, L. & Muthen, B. Mplus User’s Guide. (Muthén & Muthén, 2017).
Uno, H., Cai, T., Pencina, M. J., D’Agostino, R. B. & Wei, L. J. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat. Med. 30, 1105–1117 (2011).
Buck, S. F. A method of estimation of missing values in multivariate data suitable for use with an electronic computer. J. R. Stat. Soc. Ser. B Methodol. 22, 302–306 (1960).
Pedregosa, F. et al. Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
Pölsterl, S., Navab, N. & Katouzian, A. fast training of support vector machines for survival analysis. In Machine Learning and Knowledge Discovery in Databases (eds. Appice, A. et al.) 243–259 (Springer International Publishing, 2015). https://doi.org/10.1007/978-3-319-23525-7_15.
Van Rossum, G. & Drake, F. L. Python 3 Reference Manual. (CreateSpace, 2009).
Ulley, J., Harrop, D., Ali, A., Alton, S. & Davis, S. F. Deprescribing interventions and their impact on medication adherence in community-dwelling older adults with polypharmacy: A systematic review. BMC Geriatr. 19, 15 (2019).
Ailabouni, N. J., Nishtala, P. S., Mangin, D. & Tordoff, J. M. Challenges and enablers of deprescribing: A general practitioner perspective. PLoS ONE 11, e0151066 (2016).
Assari, S. & Bazargan, M. Race/Ethnicity, socioeconomic status, and polypharmacy among older Americans. Pharmacy 7, 41 (2019).
Bjerrum, L., Søgaard, J., Hallas, J. & Kragstrup, J. Polypharmacy: Correlations with sex, age and drug regimen. Eur. J. Clin. Pharmacol. 54, 197–202 (1998).
Jörgensen, T., Johansson, S., Kennerfalk, A., Wallander, M.-A. & Svärdsudd, K. Prescription drug use, diagnoses, and healthcare utilization among the elderly. Ann. Pharmacother. 35, 1004–1009 (2001).
Kutsal, Y. G. et al. Polypharmacy in the elderly: A multicenter study. J. Am. Med. Dir. Assoc. 10, 486–490 (2009).
Marengoni, A. et al. Aging with multimorbidity: A systematic review of the literature. Ageing Res. Rev. 10, 430–439 (2011).
Midão, L., Giardini, A., Menditto, E., Kardas, P. & Costa, E. Polypharmacy prevalence among older adults based on the survey of health, ageing and retirement in Europe. Arch. Gerontol. Geriatr. 78, 213–220 (2018).
Muth, C. et al. Evidence supporting the best clinical management of patients with multimorbidity and polypharmacy: A systematic guideline review and expert consensus. J. Intern. Med. 285, 272–288 (2019).
Barnett, K. et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 380, 37–43 (2012).
Maerz, A. H. et al. The Beers criteria: Not just for geriatrics anymore? Analysis of Beers criteria medications in nongeriatric trauma patients and their association with falls. J. Trauma Acute Care Surg. 87, 147–152 (2019).
George, J., Phun, Y.-T., Bailey, M. J., Kong, D. C. & Stewart, K. Development and validation of the medication regimen complexity index. Ann. Pharmacother. 38, 1369–1376 (2004).
Hilmer, S. N. et al. A drug burden index to define the functional burden of medications in older people. Arch. Intern. Med. 167, 781–787 (2007).
Hanlon, J. T. et al. A method for assessing drug therapy appropriateness☆. J. Clin. Epidemiol. 45, 1045–1051 (1992).
Samsa, G. P. et al. A summated score for the medication appropriateness index: Development and assessment of clinimetric properties including content validity. J. Clin. Epidemiol. 47, 891–896 (1994).
Alves-Conceição, V. et al. Medication regimen complexity measured by MRCI: A systematic review to identify health outcomes. Ann. Pharmacother. 52, 1117–1134 (2018).
Curtain, C. M. et al. Medication regimen complexity index prediction of adverse drug reaction-related hospital admissions. Ann. Pharmacother. 54, 996–1000 (2020).
Hilmer, S. N. et al. Drug burden index score and functional decline in older people. Am. J. Med. 122, 1142-1149.e2 (2009).
Kouladjian, L., Gnjidic, D., Chen, T. F., Mangoni, A. A. & Hilmer, S. N. Drug Burden Index in older adults: Theoretical and practical issues. Clin. Interv. Aging 9, 1503–1515 (2014).
Hanlon, J. T. & Schmader, K. E. The medication appropriateness index at 20: Where it started, where it has been and where it may be going. Drugs Aging 30,11 893-900 (2013).
Wastesson, J. W., Morin, L., Laroche, M.-L. & Johnell, K. How chronic is polypharmacy in old age? A longitudinal nationwide cohort study. J. Am. Geriatr. Soc. 67, 455–462 (2019).
Sirois, C. et al. Mixed Bag “Polypharmacy”: Methodological pitfalls and challenges of this exposure definition. Curr. Epidemiol. Rep. 6, 390–401 (2019).
Funding
RB is funded in part by Grant MR/R016372/1 for the King’s College London MRC Skills Development Fellowship programme funded by the UK Medical Research Council (MRC) and by grant IS-BRC-1215-20018 for the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. EC is funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. The HRS (Health and Retirement Study) is sponsored by the National Institute on Aging (Grant number NIA U01AG009740) and is conducted by the University of Michigan.
Author information
Authors and Affiliations
Contributions
E.C. and R.B. conceived the idea for this study and designed it. E.C. undertook statistical analyses. All authors contributed to the interpretation of the data. A.F. and O.D. provided clinical input. O.D. categorized each medication and manually coded them. E.C. and R.B. drafted the article and all authors contributed to its critical revision and provided final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carr, E., Federman, A., Dzahini, O. et al. A multidimensional measure of polypharmacy for older adults using the Health and Retirement Study. Sci Rep 11, 8783 (2021). https://doi.org/10.1038/s41598-021-86331-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86331-x
This article is cited by
-
Polypharmacy and medication regimen complexity in transfusion-dependent thalassaemia patients: a cross- sectional study
International Journal of Clinical Pharmacy (2024)
-
Classifying Polypharmacy According to Pharmacotherapeutic and Clinical Risks in Older Adults: A Latent Class Analysis in Quebec, Canada
Drugs & Aging (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.