Abstract
The sulfoximine insecticide sulfoxaflor is regarded as a potential substitute for neonicotinoids that were recently banned in the EU due to their side effects on bees. Like neonicotinoids, sulfoxaflor acts as a competitive modulator of nicotinic acetylcholine receptors. In agricultural environments, bees are commonly exposed to combinations of pesticides, and neonicotinoids are known to interact synergistically with fungicides. The objective of our study is to assess the acute oral toxicity of sulfoxaflor alone and in combination with a single dose of fluxapyroxad, a succinate dehydrogenase inhibitor (SDHI) fungicide, in three bee species: Apis mellifera, Bombus terrestris and Osmia bicornis. Because synergism may be dose-dependent, we tested a range of sulfoxaflor doses. Synergistic effects were assessed using three different approaches: Bliss criterion of drugs independence, ratio test comparing LD50s and model deviation ratio. Osmia bicornis was the most sensitive species to sulfoxaflor and both O. bicornis and A. mellifera showed significant synergism between the insecticide and the fungicide. For the most part, these synergistic effects were weak and only occurred at early assessment times and intermediate sulfoxaflor doses. The potential ecological relevance of these effects should be confirmed in field and/or cage studies. Overall, our laboratory results demonstrate that sulfoxaflor is somewhat less toxic than the recently banned neonicotinoids imidacloprid, thiamethoxam and clothianidin, but much more toxic than other neonicotinoids (acetamiprid, thiacloprid) still in use in the EU at the time this study was conducted.
Similar content being viewed by others
Introduction
Anthropocene, the current geological epoch, is experiencing dramatic declines in insect abundance and diversity worldwide1,2,3. Among the different insect groups, bees (Hymenoptera: Apoidea: Anthophila), comprising about 20,000 species4, are particularly at risk5,6,7. Bees play a key role in ecosystem functioning 8 and provide an essential ecosystem service in the form of pollination9. The causes of bee decline are various, but agricultural intensification is recognized as one of the main drivers2,10,11. Agricultural intensification not only reduces the availability and quality of floral and nesting resources but also exposes bees to several toxic plant protection products10.
Before being authorized for commercial use, plant protection products undergo a stringent risk assessment process. Therefore, in theory, these products should be safe for bees, as long as they are applied following the producer's recommendations. However, current risk assessment procedures only test single compounds and do not account for the multiple-pesticide scenario to which bees are exposed in agricultural environments12,13. Exposure to multiple chemicals may lead to additive, antagonistic and synergistic interactions14. Importantly, the magnitude of these interactions is dose-dependent14,15. However, tests of synergism at field realistic dosages have only been conducted for a handful of pesticide combinations16,17. In addition, different bee species have different sensitivity to single pesticides18,19,20 and pesticide mixtures21,22 underscoring the need to include bee species other than Apis mellifera in pesticide risk assessment schemes17.
In this study, we assess the acute oral toxicity of the insecticide sulfoxaflor (SUL) at a range of concentrations, alone and in combination with a field-realistic dose of the fungicide fluxapyroxad (FLU), on two social (A. mellifera, Bombus terrestris) and one solitary (Osmia bicornis) bee species.
FLU is a pyrazole-carboxamide fungicide used on cereals and on many insect-pollinated crops, including citrus, pome fruits, and cucurbits23. FLU acts by inhibiting the succinate dehydrogenase (SDHI), a universal component of mitochondria that is highly conserved across living organisms24. With acute oral and contact LD50s of > 110.9 and > 100 µg/bee, respectively, FLU is considered non-toxic to honey bees25. In recent years this compound has experienced a rapid growth in the agricultural pesticide market26. SUL is a sulfoximine-based insecticide and, like neonicotinoids, it acts as an agonist of nicotinic acetylcholine receptors (nAChRs)27,28. This compound is relatively new and is regarded as a likely substitute for the neonicotinoid insecticides recently banned in the EU17,29. Some studies have shown that field-realistic doses of SUL affect egg-laying rates and reproductive success of bumblebees30,31, but not foraging and cognitive performance32. A semi-field study conducted with honey bees showed increased mortality during the exposure phase but no overall effects at the colony level33. Contact exposure to a mixture of SUL and the neonicotinoid insecticide imidacloprid resulted in synergistically increased mortality in honey bees34. By contrast, oral exposure to the same mixture caused significantly lower mortality than SUL alone35. This antagonistic interaction can be explained by the inhibitory effect of imidacloprid on feeding35,36,37,38. To our knowledge, no studies have assessed the toxicity of SUL in combination with a fungicide. Although fungicides are usually considered non-toxic to bees and thus are regularly applied during bloom, several studies have shown they can synergistically interact with neonicotinoid and pyrethroid insecticides39.
Our objective is to establish whether bees exposed to SUL are at risk and if this risk is exacerbated by the simultaneous exposure to a fungicide. We ask the following specific questions: (i) do the two compounds show synergistic effects? (ii) if so, are these effects dose-dependent? (iii) do the three bee species show different sensitivity to SUL and the SUL-FLU mixture? (iv) Are field-relevant SUL exposure levels toxic to these bee species?
Results
Survival curves differed significantly among treatments in all three species (log-rank tests with survdiff function of the survival R package40: A. mellifera: df = 13, χ2 = 268.66, p < 2·10–16; B. terrestris: df = 13, χ2 = 357.78, p < 2·10–16; O. bicornis: df = 13, χ2 = 427.61, p < 2·10–16; see also Figs. S1-S3). Nevertheless, we found no significant differences between the solvent control and the FLU cumulative survival curves in any of the three bee species (Figs. S1-S3). On the other hand, a visual inspection of the survival curves (Figs. S1-S3) showed that the “toxic threshold” changed depending on the species (≥ 44 ng/bee in A. mellifera, ≥ 88 ng/bee in B. terrestris, ≥ 5.5 ng/bee in O. bicornis). This result is congruent with the different SUL LD50s obtained in the three bee species, showing that O. bicornis was the most sensitive species both when the toxicity endpoint is expressed in ng of SUL per bee and in ng per g of body weight (Table 1). A. mellifera showed higher sensitivity than B. terrestris when the LD50 is expressed in ng per bee but not when expressed in ng per g of body weight (Table 1).
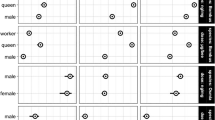
We followed three complementary approaches to assess synergism. Firstly, we compared the observed survival curve of the mixture treatment with the expected survival curve based on the Bliss criterion for drugs independence41. We found that the SUL + FLU combination synergistically decreased O. bicornis survival at 11 and 44 ng of SUL/bee and A. mellifera survival at 44 ng of SUL/bee (Fig. 1). No synergistic effects were observed in B. terrestris (Fig. 1). Secondly, we calculated the Synergism Ratio (SR) to compare the LD50 of SUL alone and in combination with FLU. LD50 values of SUL + FLU were significantly lower than those of SUL alone only in O. bicornis at 3 and 24 h after pesticide exposure (ratio test, Table 1). Third, we applied the toxic unit approach that showed synergism (Model Deviation Ratio MDR > 1.25) in A. mellifera (at 24 h) and in O. bicornis at 3 and 24 h (Table 2). This approach also showed slight antagonism in A. mellifera at 3 h after exposure (Table 2).
Observed (blue lines) and expected (red lines) effects of various doses (in ng bee−1) of sulfoxaflor (SUL) (y-axis) in combination with a single dose of fluxapyrozad (FLU) (1.2 µg/bee) on survival probability in three bee species. Significant synergism based on Bliss criterion of drugs independence (P < 0.05).
Discussion
Neonicotinoids have been extensively used to protect crops and animals from insect pests since the 1990s. However, the development of pest resistance and their recent ban in EU prompted by the detection of side effects on bees, have raised the need to introduce new insecticides into the market as potential substitutes of these compounds42,43. Because SUL is one of the likely successors of neonicotinoids29, it is important to understand its potential effects on bees. The SUL LD50s obtained in our study for the three bee species (5.9–83.5 ng/bee) are somewhat higher than LD50s of neonicotinoids recently banned in the EU in 2018 (clothianidin: 1.17–3.12 ng/bee, thiamethoxam: 5 ng/bee and imidacloprid: 13–30 ng/bee44,45), but much lower than those of other neonicotinoids still in use in the EU at the time this study was conducted (acetamiprid: 14,530–22,200 ng/bee; thiacloprid: 17,320 ng/bee46). In addition, our results demonstrate that SUL may have negative effects even at lower doses if bees are simultaneously exposed to a fungicide (FLU).
Exposure to multiple pesticides is common in agricultural environments12,13,47 and synergism between insecticides and fungicides has been largely documented in bees (review in Carnesecchi et al.16). Several studies have reported synergism between EBI (ergosterol biosynthesis inhibitor) fungicides and insecticides (including neonicotinoids)15,21,45,48,49,50. However, studies showing synergism between SDHI fungicides and insecticides are less frequent likely because, based on their molecular mode of action, an interaction with the xenobiotic detoxification systems of insects is not expected51,52.
Our laboratory study reveals a weak but significant synergistic interaction between a SDHI fungicide (FLU) and an insecticide (SUL) with a similar mode of action to neonicotinoids. At the first two assessment times, co-exposure with FLU significantly reduced SUL LD50s in O. bicornis (synergism ratios: 1.36–1.41). A recent study calculated MDR values for 43 insecticide-fungicide combinations (Table S15 of Carnesecchi et al.16). Most insecticide-EBI fungicide combinations (23 of 30) showed synergism, but synergism was detected in only 2 of 7 insecticide-SDHI fungicide combinations. Our MDR values in A. mellifera (MDRmax = 1.64) and O. bicornis (MDRmax = 1.39) are close to the maximum MDR value obtained for insecticide-SDHI combinations (MDR = 1.45), but 10 times lower than the maximum values obtained for insecticide-EBI fungicide combinations (MDR = 10.00) in Carnesecchi et al.16. While the synergistic toxicity between insecticides and EBI fungicides in bees is likely due to the P450 enzyme inhibition39, the biochemical mechanisms underlying synergism between insecticides and SDHI fungicides are not well understood. Our study was conducted with a FLU-based commercial product, Sercadis. Therefore, a potential contribution of the co-formulants to the observed synergism cannot be excluded17.
In agreement with a previous study15, we found synergism to be dose-dependent. Thompson et al.15 showed that synergism increases with increasing fungicide dose. We tested different doses of insecticide with a single fungicide dose and found that synergism increased at intermediate insecticide doses in both O. bicornis (11 and 44 ng/bee) and A. mellifera (44 ng/bee). Lack of synergism at the lower and higher doses tested can be explained by the low toxicity of SUL at the lowest doses and the high toxicity of this compound (almost 100% mortality) at the highest doses . In addition, we found that the magnitude of synergism was dependent on the assessment time (synergism shortly after exposure; Tables 1 and 2). A previous study on O. bicornis yielded a similar result (Figs. 3 and 4 in49). Bees exposed to an insecticide-fungicide mixture experienced a strong decline in survival during the first hours after exposure. However, the longevity of those bees that survived the first hours of exposure to the mixture was similar to that of control bees, reflecting a high level of intra-population variability in sensitivity. In Sgolastra et al.49 this variability was partly explained by differences among individuals in emergence time (bees that took longer to emerge were more sensitive). However, all our bees emerged over two consecutive days and therefore other (unknown) factors should account for the observed intra-populational variability.
Comparison of pesticide sensitivity among the three bee species showed that O. bicornis was consistently more susceptible to SUL and SUL + FLU than the other two bee species, at all assessment times, both when expressed in ng of SUL per bee and in ng per g of bee body weight. This result is in agreement with previous studies and confirms that Osmia bees are more sensitive than honey bees and bumblebees to insecticides that target insect nAChRs18,21,45,53. Differences in P450 enzymes involved in xenobiotic detoxification could explain the different sensitivity to nAChR compounds between the bee families Apidae (A. mellifera and B. terrestris) and Megachilidae (O. bicornis)54,55. Probably owing to their large body size, bumblebees were less sensitive than honey bees, but when accounting for body weight, both species had similar sensitivity.
The LD50 value at 24 h of SUL obtained in our study for A. mellifera (55.4 ng/bee) was lower than the value reported in the dossier for the registration of this compound (146 ng/bee)56,57. This difference can be explained by differences in the age of the bees used (foragers and in-hive bees, respectively). It has been demonstrated that foragers are usually more sensitive to pesticides than in-hive bees50. The LD50 we obtained for B. terrestris (83.5 ng/bee) falls between the two values (27 and 150 ng/bee) reported in EFSA55 for two SUL formulated products. LD50 for Bombus impatiens is lower (19.4 ng/bee)58, probably due to the smaller size of this species.
The occurrence of synergistic effects between SUL and FLU also differed across bee species. We detected synergism in A. mellifera (at 24 h, MDR approach; and at 44 ng/bee, Bliss independence criterion approach) and O. bicornis (at 24 h, MDR and SR approaches; and at 11 and 44 ng/bee Bliss independence criterion approach). On the other hand, no synergism was observed in B. terrestris at any assessment time or SUL concentration. In the latter species, the MDR and SR (~ 1.00) indicate an additive effect between SUL and FLU.
Because SUL has only recently been introduced into the pesticide market, information on the levels of exposure under field conditions is scarce. However, studies conducted for the registration of this compound report SUL levels in the nectar of a variety of crops at various times after application (Appendix F of EPA document56). Based on this information, application of SUL commercial products, Closer and Transform, in the US is allowed up to 3 days before bloom for several crops, including pome fruits, stone fruits, canola and citrus. For other crops such as alfalfa and strawberry, label specifications advice users to notify local beekeepers or to spray when pollinators are least active (2 h prior to sunset or when temperatures are below 10 °C). Importantly, these label restrictions were established considering only honey bee endpoints and therefore do not necessarily protect other bees. Using the SUL levels reported in the EPA document56 and assuming a consumption of 80 mg of nectar with 15% sugar content45,59 we can compare the expected dose that a bee would consume during 1 h of foraging on crops treated with SUL with the LD50 of O. bicornis, the most sensitive of the three species tested in our study. In 5 of the 6 crops measured, including the two with no specific restrictions during bloom, the concentration of SUL in the nectar (≥ 0.073 mg/Kg; Table S1) would cause lethal effects in O. bicornis (Table S1). In peaches treated in pre-bloom, SUL residues would be toxic to O. bicornis even 5–7 days after application. These outcomes, based on theoretical calculations, should be confirmed in field or semi-field empirical studies. In addition to acute exposure and mortality, risk assessment should also consider chronic exposure and account for potential sublethal effects. A recent study shows that chronic exposure to SUL concentrations as low as 5 ng/g of sucrose solution has negative effects on reproduction of bumblebee colonies30.
Our laboratory study confirms that bee species differ in their sensitivity to pesticides and, more importantly, in their response to pesticide combinations. Based on the ratio between the lowest LD50 found for A. mellifera and O. bicornis, SUL is 10.6 times more toxic to O. bicornis per g of body weight. Although the interspecific differences observed in our study may partly be explained by differences in the protocols used for each species, these protocols were designed to mimic field conditions most likely to be encountered by each bee species in the field. In fact, differences among species in life history traits result not only in different sensitivity45,54, but also different exposure routes and levels60,61,62,63, thus hindering extrapolations across species64.
Material and methods
Bee populations and experimental procedures
For honey bees and bumblebees we followed, with some exceptions (see below), OECD standard toxicity protocols65,66. Standard protocols are not available for Osmia spp. Therefore, for O. bicornis we followed methods developed in previous studies45,49. The use of different protocols partly hinders the direct comparison across species. However, applying a unique protocol is impractical because the three bee species drastically differ in life history traits, in their response to laboratory conditions and in feeding behaviour60. For this reason, we decided to apply the most suitable methodology for each species.
We used three healthy, queen-right honey bee colonies (A. mellifera ligustica) located at the CREA-AA (Council for Agricultural Research and Economics–Agricultural and Environment Research Center), Bologna, Italy. These hives were managed following the guidelines for organic beekeeping (exclusively oxalic acid), with no treatment conducted in the 6 months preceding the study. In July 2019, we placed funnel traps in front of the hives to collect forager bees67. Following a previous study45, we chose to work with forager bees, instead of in-hive bees65 because they are more likely to be directly exposed to contaminated nectar and because a recent study showed that foragers are more sensitive to pesticides than in-hive bees50. Bees, anaesthetized for ∼30 min with a mixture of 60% CO2 and 40% synthetic air, were transferred in groups of 20 individuals to cardboard cages (9.5 × 6.5 × 5 cm). After a starvation period of ∼1 h, 200 μL of the test solution (sucrose 50% w/w) was provided to each group of bees using a common feeder (Eppendorf tube’s cap), assuming that, through trophallaxis, all individuals would ingest a similar dose (ca. 20 μL)65. The test solution was completely consumed within 1 h of exposure in all cages. Following the exposure phase, cages were maintained in an incubator in complete darkness at 25 ± 2 °C and 50–70% relative humidity for the duration of the test (96 h). We provided each cage with a 5 mL syringe filled with sucrose syrup for ad libitum feeding until the end of the experiment.
Six bumblebee colonies (B. terrestris) were purchased from BioPlanet s.c.a. (Cesena, Italy). Colonies contained 60–80 workers, brood in all stages of development and a laying queen. In October 2019, adult workers were collected under red light laboratory conditions and individually transferred to Nicot cages (7.1 × 2.0 cm). Very small (< 0.14 g) and very large (> 0.42 g) individuals were excluded66. Newly-emerged bees, recognisable by the greyish pubescence, and old bees, recognisable by sparse hairiness in the abdomen, were also excluded. Bees were acclimatised to the test conditions overnight (12–24 h) with ad libitum access to a sucrose solution. Throughout the test, they were maintained at 25 ± 2 °C and 50–70% relative humidity in continuous darkness. Prior to pesticide exposure, bees were starved for 3 h. Because Bombus do not perform trophallaxis and to avoid hierarchy fights among queen-less workers66, we used an individual feeding method whereby the test solution was offered through a 1 mL syringe inserted into the Nicot cage. Each individual was provided with 20 μL of test solution (sucrose 42% w/w) for an exposure period of 3 h. Feeders were visually inspected after the exposure phase, and only bees that consumed 100% of the test solution were used in the statistical analyses. Following the exposure phase, bees were maintained individually in the Nicot cages and fed ad libitum through a 2.5 mL syringe filled with sucrose syrup. To avoid confinement side effects, the Nicot cages of each treatment were placed side by side on a tray, so that workers could perceive their mutual presence.
Osmia bicornis individuals were obtained from a parent population reared in a pesticide-free area of the Kazimierz Landscape Park (Poland) and wintered from October 2018 at 3 °C at the Department of Agricultural and Food Sciences, University of Bologna, Italy. In May 2019, female cocoons were incubated at 23 °C until emergence and then transferred to a Plexiglas flight cage (50 × 50 × 50 cm) to allow them to deposit the meconium. We only used bees that emerged over two consecutive days during the peak of the emergence period (days 6–7)38,49,68. One day after emergence, these unmated, meconium-free females were individually housed in small plastic cylinders (width: 3.5 cm; height: 5.5 cm) with a transparent plastic lid. Since Osmia do not perform trophallaxis, we fed each bee individually with the ‘petal method’45, a modification of the ‘flower method’69. The test solution (20 μL) was pipetted into a tiny plastic ampoule (internal diameter 2 mm, external diameter 3 mm, height 5 mm) attached to a natural petal (Euryops, Asteraceae) inserted into a holder (diameter 1 cm, height 1 cm) made with salt paste. After ca. 3 h of exposure, bees that consumed 100% of the test solution were placed in groups of 3–5 individuals in larger plastic cages (width: 5.5–8 cm; height: 7 cm) with a transparent lid through which a 2.5 ml syringe filled with sucrose solution (sucrose 33% w/w) was inserted. These cages were kept in the laboratory at 23 ± 2 °C and 50–70% relative humidity under natural light.
Treatments
For each bee species, we tested six doses of SUL in a geometric series (factor of 2), ranging from 2.75 to 176 ng/bee (concentration in the syrup solution: 0.14 to 8.8 mg/L) and a control (0 ng/bee). Each exposure to SUL was tested alone and in combination with a single dose of FLU (1.2 µg/bee).
SUL (purity 98%) was purchased from LGC (LGC Standards, Middlesex, UK) . The stock solution with a concentration of 0.88 mg SUL/mL acetone was used to prepare the test solutions. To obtain a range of appropriate concentrations based on the desired exposure level, the stock solution was first diluted in acetone until we reached final concentrations ranging from 0.014 to 0.88 mg/mL. These solutions were added to the feeding solution at a ratio of 10 μL/mL.
We used the commercial formulation of FLU (Sercadis, BAFS, Cesano Maderno, Italy). The tested concentration corresponds to the field application rate of Sercadis (300 g of a.i./L of commercial product) in orchards (20 mL/hL). To obtain this concentration, we prepared a stock solution with a FLU concentration of 3 g/L by dissolving Sercadis in distilled water. The stock solution was then diluted with the sugar solution at a ratio of 20 μL/mL to achieve the final concentration (60 mg/L).
Following the exposure phase, bees were fed ad libitum with sucrose solution. The final concentration of acetone in the feeding solution was adjusted to 1% (v/v) by adding pure acetone to all treatments not including SUL.
Body weight
To minimise stress from manipulation, test bees were not weighed. Instead, 30 individuals of each species were randomly selected and weighed to obtain an average fresh body weight per species. Mean (± SD) body weights were 0.081 ± 0.015, 0.263 ± 0.049 and 0.092 ± 0.008 g in A. mellifera, B. terrestris and O. bicornis, respectively.
Data analysis
Mortality was assessed 3 h after the end of the exposure phase and then checked every 24 h for 4 days. With these data we built Kaplan–Meier (K–M) survival curves for each species and compound concentration. Bees that were alive after 96 h were included as right-censored data. Then, a log-rank omnibus test was conducted to determine whether there were overall differences between treatments (survdiff function of the survival R package40 with \(\rho =0\)). Next, pairwise comparison of survival curves between treatments were carried out by means of pairwise tests (pairwise_survdiff function of the survminer R package70, with Holm multi-comparison corrections and \(\rho =0\)).
We followed three complementary approaches to test for synergism. The first approach was based on Bliss drugs independence criterion41. Bliss independence was tested for every single SUL + FLU combination with the FHtestrcc function of the FHtest R package71. We determined the expected survival curve, computed as:
where \(S\left(t\right)\) is the probability for an individual to have survived longer than t .
Thus, if Bliss independence held, we would expect no differences between the expected and the observed survival curves (i.e. our null hypothesis \({H}_{0};\)41):
However, assuming that there could be a synergistic effect between the two components such that it would decrease survival of bees exposed to SUL + FLU, our alternative hypothesis \({H}_{1}\), was that the observed survival would be lower than the expected one:
Note that the definition of Bliss independence in Demidenko & Miller41 paper differs from our Eq. (2) because their study analysed increased survival following drug administration, whereas we analysed decreased survival following exposure to toxicants (E. Demidenko, pers. comm.).
Tests for each species and SUL concentration were then calculated to determine whether \({H}_{0}\) could be rejected. Since a visual inspection of survival curves indicated that differences between observed and expected curves were higher at early and/or middle time points, we selected a value of \(\rho =1\), equivalent to the Peto and Peto log-rank test (see manual of the aforementioned FHtest R package for detailed information).
The second approach relied on a ratio test based on LD50 values. For each bee species, the LD50 values and their 95% confidence limits of SUL and SUL + FLU for each assessment time (3, 24, 48, 72 and 96 h) were determined after probit regression analysis using IBM SPSS Statistics 22.0.0.0 software (package for Windows, 64-bit edition, Chicago, USA). Then, a ratio test comparing the ratio of the LD50 of SUL and SUL + FLU respectively was performed. This test produces a synergism or antagonism ratio with the associated 95% confidence interval. Synergism occurs when the ratio is higher than 1 and the two confidence intervals do not overlap “1”72.
Finally, for our third approach we calculated the model deviation ratio (MDR) to determine if the SUL + FLU interaction caused synergistic (MDR > 1.25), additive (0.83 < MDR < 1.25), or antagonistic (MDR < 0.83) effects (refined thresholds for MDR in 16). MDR measures the deviations from the assumption of additivity following the concentration addition (CA) model73. MDR values are the ratio between the expected toxic unit (TU) (by definition = 1) and the observed TU for a binary mixture. To estimate MDR, we calculated the TU of each individual pesticide (SUL, FLU) and of the binary mixture (SUL + FLU). TUFLU = 0.0108 (obtained by dividing the FLU ingested dose (1.2 µg/bee) by its acute oral LD50 in Apis mellifera (110.9 µg/bee;25). TUSUL is the ratio between the LD50 of SUL alone and in combination in each assessment time. TUSUL+FLU = TUSUL + TUFLU; MDR = 1/TUSUL+FLU.
Data availability
Data available in the Supplementary information.
References
Hallmann, C. A. et al. More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12, e0185809 (2017).
Sánchez-Bayo, F. & Wyckhuys, K. A. G. Worldwide decline of the entomofauna: a review of its drivers. Biol. Conserv. 232, 8–27 (2019).
Wagner, D. L. Insect declines in the Anthropocene. Annu. Rev. Entomol. 65, 457–480 (2020).
Michener, C. D. The Bees of the World. (The Johns Hopkins University Press, 2007).
Biesmeijer, J. C. et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science (80-. ). 313, 351–354 (2006).
Nieto, A. et al. European Red List of Bees. IUCN Global Species Programm (Publication Office of the European Union, 2014). https://doi.org/10.2779/77003.
Zattara, E. E. & Aizen, M. A. Worldwide occurrence records suggest a global decline in bee species richness. One Earth 4, 114–123 (2021).
Ollerton, J., Winfree, R. & Tarrant, S. How many flowering plants are pollinated by animals?. Oikos 120, 321–326 (2011).
Klein, A. M. et al. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 274, 303–313 (2007).
Goulson, D., Nicholls, E., Botías, C. & Rotheray, E. L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science (80-. ). 347, 1255957 (2015).
Kremen, C., Williams, N. M. & Thorp, R. W. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. U. S. A. 99, 16812–16816 (2002).
Mullin, C. A. et al. High levels of miticides and agrochemicals in north American apiaries: implications for honey bee health. PLoS ONE 5, e9754 (2010).
Tosi, S., Costa, C., Vesco, U., Quaglia, G. & Guido, G. A 3-year survey of Italian honey bee-collected pollen reveals widespread contamination by agricultural pesticides. Sci. Total Environ. 615, 208–218 (2018).
Cedergreen, N. Quantifying synergy: A systematic review of mixture toxicity studies within environmental toxicology. PLoS ONE 9, e96580 (2014).
Thompson, H. M., Fryday, S. L., Harkin, S. & Milner, S. Potential impacts of synergism in honeybees (Apis mellifera) of exposure to neonicotinoids and sprayed fungicides in crops. Apidologie 45, 545–553 (2014).
Carnesecchi, E. et al. Investigating combined toxicity of binary mixtures in bees: Meta-analysis of laboratory tests, modelling, mechanistic basis and implications for risk assessment. Environ. Int. 133, 105256 (2019).
Sgolastra, F. et al. Bees and pesticide regulation: Lessons from the neonicotinoid experience. Biol. Conserv. 241, 108356 (2020).
Arena, M. & Sgolastra, F. A meta-analysis comparing the sensitivity of bees to pesticides. Ecotoxicology 23, 324–334 (2014).
Uhl, P., Awanbor, O., Schulz, R. S. & Brühl, C. A. Osmia bicornis is rarely an adequate regulatory surrogate species. Comparing its acute sensitivity towards multiple insecticides with regulatory Apis mellifera endpoints . bioRxiv 366237 (2018). https://doi.org/10.1101/366237.
Heard, M. S. et al. Comparative toxicity of pesticides and environmental contaminants in bees: Are honey bees a useful proxy for wild bee species?. Sci. Total Environ. 578, 357–365 (2017).
Biddinger, D. J. et al. Comparative toxicities and synergism of apple orchard pesticides to Apis mellifera (L.) and Osmia cornifrons (Radoszkowski). PLoS One 8, e72587 (2013).
Robinson, A. et al. Comparing bee species responses to chemical mixtures: Common response patterns?. PLoS ONE 12, e0176289 (2017).
FAO. Fluxapyroxad (256). 659–926 (2015). Available at: http://www.fao.org/fileadmin/templates/agphome/documents/Pests_Pesticides/JMPR/Evaluation12/Fluxapyroxad.pdf.
Bénit, P. et al. Evolutionarily conserved susceptibility of the mitochondrial respiratory chain to SDHI pesticides and its consequence on the impact of SDHIs on human cultured cells. PLoS ONE 14, e0224132 (2019).
EFSA. Conclusion on the peer review of the pesticide risk assessment of the active substance fluxapyroxad (BAS 700 F). EFSA J. 10, 2522 (2012).
Sierotzki, H. & Scalliet, G. A review of current knowledge of resistance aspects for the next-generation succinate dehydrogenase inhibitor fungicides. Phytopathology 103, 880–887 (2013).
Zhu, Y. et al. Discovery and characterization of sulfoxaflor, a novel insecticide targeting sap-feeding pests. J. Agric. Food Chem. 59, 2950–2957 (2011).
Sparks, T. C. et al. Sulfoxaflor and the sulfoximine insecticides: Chemistry, mode of action and basis for efficacy on resistant insects. Pestic. Biochem. Physiol. 107, 1–7 (2013).
Brown, M. J. F. et al. A horizon scan of future threats and opportunities for pollinators and pollination. PeerJ 4, e2249 (2016).
Siviter, H., Brown, M. J. F. & Leadbeater, E. Sulfoxaflor exposure reduces bumblebee reproductive success. Nature 561, 109–112 (2018).
Siviter, H., Horner, J., Brown, M. J. F. & Leadbeater, E. Sulfoxaflor exposure reduces egg laying in bumblebees Bombus terrestris. J. Appl. Ecol. 57, 160–169 (2019).
Siviter, H. et al. No evidence for negative impacts of acute sulfoxaflor exposure on bee olfactory conditioning or working memory. PeerJ 7, e7208 (2019).
Cheng, Y. et al. A semi-field study to evaluate effects of sulfoxaflor on honey bee (Apis mellifera). Bull. Insectol. 71, 225–233 (2018).
Zhu, Y. C., Yao, J., Adamczyk, J. & Luttrell, R. Synergistic toxicity and physiological impact of imidacloprid alone and binary mixtures with seven representative pesticides on honey bee (Apis mellifera). PLoS ONE 12, e0176837 (2017).
Zhu, Y. C., Yao, J., Adamczyk, J. & Luttrell, R. Feeding toxicity and impact of imidacloprid formulation and mixtures with six representative pesticides at residue concentrations on honey bee physiology (Apis mellifera). PLoS ONE 12, e0178421 (2017).
Thompson, H. M., Wilkins, S., Harkin, S., Milner, S. & Walters, K. F. A. Neonicotinoids and bumblebees (Bombus terrestris): effects on nectar consumption in individual workers. Pest Manag. Sci. 71, 946–950 (2015).
Cresswell, J. E. et al. Differential sensitivity of honey bees and bumble bees to a dietary insecticide (imidacloprid). Zoology 115, 365–371 (2012).
Azpiazu, C. et al. Chronic oral exposure to field-realistic pesticide combinations via pollen and nectar: effects on feeding and thermal performance in a solitary bee. Sci. Rep. 9, 13770 (2019).
Berenbaum, M. R. & Johnson, R. M. Xenobiotic detoxification pathways in honey bees. Curr. Opin. Insect Sci. 10, 51–58 (2015).
Therneau, T. M. Package ‘ survival ’. (2020).
Demidenko, E. & Miller, T. W. Statistical determination of synergy based on Bliss definition of drugs independence. PLoS ONE 14, 1–22 (2019).
Casida, J. E. Neonicotinoids and other insect nicotinic receptor competitive modulators: progress and prospects. Annu. Rev. Entomol. 63, 125–144 (2018).
Matsuda, K., Ihara, M. & Sattelle, D. B. Neonicotinoid insecticides: molecular targets, resistance, and toxicity. Annu. Rev. Pharmacol. Toxicol. 60, 241–255 (2020).
Sanchez-Bayo, F. & Goka, K. Pesticide residues and bees-a risk assessment. PLoS ONE 9, e94482 (2014).
Sgolastra, F. et al. Synergistic mortality between a neonicotinoid insecticide and an ergosterol-biosynthesis-inhibiting fungicide in three bee species. Pest Manag. Sci. 73, 1236–1243 (2017).
Lewis, K. A., Tzilivakis, J., Warner, D. J. & Green, A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. An Int. J. 22, 1050–1064 (2016).
Lambert, O. et al. Widespread occurrence of chemical residues in beehive matrices from apiaries located in different landscapes of western France. PLoS ONE 8, e67007 (2013).
Iwasa, T., Motoyama, N., Ambrose, J. T. & Roe, R. M. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee. Apis mellifera. Crop Prot. 23, 371–378 (2004).
Sgolastra, F. et al. Combined exposure to sublethal concentrations of an insecticide and a fungicide affect feeding, ovary development and longevity in a solitary bee. Proc. R. Soc. B Biol. Sci. 285, 20180887 (2018).
Tosi, S. & Nieh, J. C. Lethal and sublethal synergistic effects of a new systemic pesticide, flupyradifurone (Sivantow), on honeybees. Proc. R. Soc. B Biol. Sci. 286, 20190433 (2019).
Johnson, R. M., Dahlgren, L., Siegfried, B. D. & Ellis, M. D. Acaricide, Fungicide and Drug Interactions in Honey Bees (Apis mellifera). PLoS ONE 8, e54092 (2013).
Tsvetkov, N. et al. Chronic exposure to neonicotinoids reduces honey bee health near corn crops. Science (80-. ). 356, 1395–1397 (2017).
Uhl, P., Awanbor, O., Schulz, R. S. & Brühl, C. A. Osmia bicornis is rarely an adequate regulatory surrogate species. Comparing its acute sensitivity towards multiple insecticides with regulatory Apis mellifera endpoints. PLoS One 14, e0201081 (2019).
Beadle, K. et al. Genomic insights into neonicotinoid sensitivity in the solitary bee Osmia bicornis. 1–19 (2019).
Hayward, A. et al. The leafcutter bee, Megachile rotundata, is more sensitive to N-cyanoamidine neonicotinoid and butenolide insecticides than other managed bees. Nat. Ecol. Evol. 3, 1521–1524 (2019).
EPA. Ecological Risk Assessment for the Registration Review of Sulfoxaflor. United States Environ. Prot. Agency (2019).
EFSA. Peer review of the pesticide risk assessment for the active substance sulfoxaflor in light of confirmatory data submitted. EFSA J. 17, e05633 (2019).
Mundy-Heisz, K. A., Prosser, R. S. & Raine, N. E. Acute oral toxicity and risks of exposure to the neonicotinoid thiamethoxam, and other classes of systemic insecticide, for the Common Eastern Bumblebee (Bombus impatiens). bioRxiv (2020). https://doi.org/10.1101/2020.01.27.921510.
EFSA. European Food Safety Authority. Guidance on the risk assessment of plant protection products on bees (Apis mellifera , Bombus spp. and solitary bees). EFSA J. 11, 3295 (2013).
Sgolastra, F. et al. Pesticide exposure assessment paradigm for solitary bees. Environ. Entomol. 48, 22–35 (2019).
Gradish, A. E. et al. Comparison of pesticide exposure in honey bees (Hymenoptera: Apidae) and bumble bees (Hymenoptera: Apidae): implications for risk assessments. Environ. Entomol. 48, 12–21 (2019).
Chan, D. S. W., Prosser, R. S., Rodríguez-Gil, J. L. & Raine, N. E. Assessment of risk to hoary squash bees (Peponapis pruinosa) and other ground-nesting bees from systemic insecticides in agricultural soil. Sci. Rep. 9, 1–13 (2019).
Boyle, N. K. & Pitts-Singer, T. L. Assessing blue orchard bee (Osmia lignaria) propagation and pollination services in the presence of honey bees (Apis mellifera) in Utah tart cherries. PeerJ 7, e7639 (2019).
Franklin, E. L. & Raine, N. E. Moving beyond honeybee-centric pesticide risk assessments to protect all pollinators. Nat. Ecol. Evol. 3, 1373–1375 (2019).
OECD. Test No. 213: Honeybees, Acute Oral Toxicity Test. (OECD Guidelines for the Testing of Chemicals, Section 2, 1998). https://doi.org/10.1787/9789264070165-en.
OECD. Test No. 247: Bumblebee, Acute Oral Toxicity Test. (OECD Guidelines for the Testing of Chemicals, Section 2, 2017). https://doi.org/10.1787/9789264284128-en.
Medrzycki, P. et al. Standard methods for toxicology research in Apis mellifera. J. Apic. Res. 52, 1–60 (2013).
Brandt, A. et al. Immunosuppression response to the neonicotinoid insecticide thiacloprid in females and males of the red mason bee Osmia bicornis L. Sci. Rep. 10, 4670 (2020).
Ladurner, E., Bosch, J., Maini, S. & Kemp, W. P. A method to feed individual bees (Hymenoptera: Apiformes) known amounts of pesticides. Apidologie 34, 597–602 (2003).
Kassambara, A., Kosinski, M., Biecek, P. & Fabian, S. Package ‘survminer’. The Comprehensive R Archive Network (2020).
Oller, R. & Langohr, K. FHtest : An R Package for the Comparison of Survival Curves with Censored Data . J. Stat. Softw. 81, (2017).
Robertson, J. L., Russell, R. M., Preisler, H. K. & Savin, N. E. Bioassays with Arthropods. (CRC Press, 2007).
Jonker, M. J., Svendsen, C., Bedaux, J. J. M., Bongers, M. & Kammenga, J. E. Significance testing of synergistic/antagonistic, dose level-dependent, or dose ratio-dependent effects in mixture dose-response analysis. Environ. Toxicol. Chem. 24, 2701–2713 (2005).
Acknowledgements
This study was supported by a grant from the University of Bologna to FS (Grant RFO2019_SGOLASTRA) and a fellowship from the French Association POLLINIS (www.pollinis.org) to CA. Funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. We appreciate the technical assistance of E. Cacciatori, G. Zanetti, T. Brandolini, F. Monti and V. Caringi. We thank E. Demidenko for statistical advice and two anonymous reviewers for their constructive comments.
Author information
Authors and Affiliations
Contributions
C.A., F.S., J.B. and R.M-H. wrote the manuscript. R. M.-H., C.A. and F.S. analyzed the data. C.A., F.S., P. M. and L.B. conducted the experiments. F.S. and J.B. supervised the study. D.T. provided the bee population. All authors read, reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Azpiazu, C., Bosch, J., Bortolotti, L. et al. Toxicity of the insecticide sulfoxaflor alone and in combination with the fungicide fluxapyroxad in three bee species. Sci Rep 11, 6821 (2021). https://doi.org/10.1038/s41598-021-86036-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-86036-1
This article is cited by
-
Participatory hackathon to determine ecological relevant endpoints for a neurotoxin to aquatic and benthic invertebrates
Environmental Science and Pollution Research (2024)
-
Neither sulfoxaflor, Crithidia bombi, nor their combination impact bumble bee colony development or field bean pollination
Scientific Reports (2023)
-
Low concentrations of acetamiprid, deltamethrin, and sulfoxaflor, three commonly used insecticides, adversely affect ant queen survival and egg laying
Scientific Reports (2023)
-
Sulfoxaflor influences the biochemical and histological changes on honeybees (Apis mellifera L.)
Ecotoxicology (2023)
-
Biochemical and gene expression alterations due to individual exposure of atrazine, dichlorvos, and imidacloprid and their combination in zebrafish
Environmental Science and Pollution Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.