Abstract
Seed deterioration, coupled with a decrease in nutrients, is unavoidable following long-term storage, and these seeds are therefore used as livestock fodder. Here, we developed a simple, rapid and efficient method of producing high amounts of antioxidants from deteriorated seeds via melatonin-induced germination. Legume seeds were subjected to high humidity at 55 °C for 12–36 h to obtain aged seeds with a 40% germination rate and severely reduced antioxidant nutrition (total phenolics content, ferric reducing power and 1,1-diphenyl-2-picryhydrazyl (DPPH) radical scavenging capacity). Aged seeds were then treated with 0.1 mM melatonin, resulting in the production of sprouts with a higher total phenolics content (fivefold), greater ferric reducing power (sevenfold) and greater DPPH radical scavenging capacity (twofold) compared to the aged seeds. These findings suggest that melatonin treatment efficiently converted aged seed reserve residues into antioxidant nutrients, providing an alternative use for deteriorated seeds in food production.
Similar content being viewed by others
Introduction
Free radicals produced by the body are neutralized by antioxidants, thereby alleviating oxidative damage1. Proper balance between free radical production and scavenging is therefore necessary for optimum physiological function; therefore, external antioxidants are often used to reduce damage2. For example, certain antioxidants such as polyphenol, which plays an important role in regulating metabolism and alleviating chronic disease3, cannot be biosynthesized by the body and are only acquired through our diet2.
Legumes are an important source of starch, protein and lipids4,5,6,7, as well as containing high levels of polyphenol6 and vitamin C8. For example, soybeans [Glycine max L., Merr.], which are widely used in the food industry, contain a high amount of bioactive molecules such as polyphenol9,10.
Legume seed germination for sprout production was introduced by the Egyptians thousands of years ago11, and involves soaking, draining then leaving the seeds until they germinate and begin to sprout12. Germination drives some of the seed reserves towards the synthesis of new metabolites, leading to profound changes in the accumulation of nutrients in the developing embryo13. Germinated legume seeds in particular produce a large amount of polyphenol in the sprouts6,12,14.
However, seed deterioration is a common natural process in agricultural production, and respiration results in the accumulation of damaged DNA, lipids, and protein15,16 as well as loss of nutrient reserves17,18,19 during ageing or adverse environmental conditions20,21. Seed deterioration in soybean is easily observed both in the field22 and at the storage stage23. Data also suggest that the reduction in antioxidant enzyme (e.g. catalase) activity24 and decrease in non-enzymatic antioxidants (e.g. glutathuione and α-tocopherol) can be monitored in deteriorated seeds due to free radical accumulation25,26,27. Thus, a number of priming methods have been developed for the restoration of aged seeds28. For example, melatonin (N-acetyl-5-methoxy-tryptamine), a well-known animal hormone, has also been discovered in plants and was found to significantly restore vitality in aged seeds29,30,31. Moreover, melatonin was also found to play a signaling role during seed germination under both stressful32,33,34,35 and stress-free conditions36,37.
In this study, four types of deteriorated bean seeds (yellow and black soybean, mung and red bean) were obtained via artificial ageing treatment. Melatonin was then used to restore vitality and promote sprout production, resulting in a large content of antioxidant nutrients. This method uses cheap and readily-available aged legume seeds, and does not require a lot of time or complicated techniques. It can therefore be widely used to address antioxidant nutrient deficiency around the world.
Materials and methods
Seeds
Seeds of yellow soybean (Glycine max (Linn.) Merr.), black soybean (Glycine max var.), mung bean (Vigna radiata (Linn.) Wilczek.) and red bean (Vigna angularis (Willd.) Ohwi et Ohashi) were obtained from a seed distributor in Daqing city, China. They were sown in plastic boxes and placed in a thermostat-controlled seed germination incubator (LD-330, Laiende, China) during the entire germination period. Prior to the germination test, accelerated ageing treatment was conducted in a thermostat-controlled seed ageing cabinet (LH-150S, Shanghaiqixin, China). The seeds were placed in each individual “ageing box” compartment and deionized water was added, and then subjected to 55 °C (low aging treatment) or 62 °C (high aging treatment) for 12 h (yellow and black soybean) or 36 h (mung bean and red bean) to obtain artificially aged seeds with a germination rate of approximately 40% (low aged seeds) or 10% (high aged seeds), respectively. After ageing treatment, the seeds were collected and dried at 35 °C for 24 h then moistened with tap water for 6 h prior to all subsequent treatments.
Seed germination experiments
Seeds were divided into four groups for the germination experiments: a control group (no ageing treatment), aged seeds, and melatonin-treated seeds with and without ageing treatment. Seeds were then soaked in tap water (unaged and aged seeds) or melatonin solution (0.1 mM; unaged and aged seeds) for 8 h, respectively, washed then transferred to plastic boxes containing a layer of fine sand (2 cm depth). Germination tests were carried out in a seed germination incubator (parameters were set as: 25 ± 1 °C and 70% humidity) for five days under dark conditions. Germinated seeds and sprouts were then collected and assayed for total phenolics, ferric reducing power and 1,1-diphenyl-2-picryhydrazyl (DPPH) scavenging capacity as described below. All assays were replicated at least three times to minimize experimental errors, and each replicate involved 100 seeds for each germination test.
Seed germination assay
When the length of the seed radicle was at least 1 mm, the seeds were classified as having germinated. The number of germinated seeds was counted three times (8 h intervals) per day then the germination rate (GR) was calculated as the percentage of seeds that germinated during the first five days after sowing.
Total phenolics assay
Samples (0.5 g) of bean seeds and 5-day-old sprouts were collected from unaged and aged seeds for the total phenolics assay. Total phenolics were measured using Folin–Ciocalteu reagent38 with some modifications39 then absorbance was recorded at 760 nm using a UV–Vis spectrophotometer (Shimadzu) and the results were expressed as gallic acid equivalents.
Antioxidant capacity assay
For the antioxidant capacity assay, seeds were ground into a powder using a Waring blender (ZG-TJ01, Ningbo, China) prior to solvent extraction, and sprouts were ground to a powder in liquid N2 using a mortar and pestle. Approximately 500 mg of seed/sprout powder was then transferred to 0.1 L of 80% (w/v) methanol–water solution, and incubated at room temperature for 4 h in the dark. Extracts were then filtered, the filtrates from each replicate were pooled, and the solvent was discarded under a vacuum at 45 °C using a rotary evaporator. Crude extracts were then stored in a desiccator at 4 °C for subsequent antioxidant capacity analysis using the DPPH radical scavenging method40 and ferric reducing ability of plasma (FRAP) assay41.
The DPPH radical scavenging capacity assay was carried out using 2.95 mL of 60 μM DPPH and 50 μL of extract sample. Absorbance was then read at 517 nm and the readings were compared with the controls, which involved 50 μL of distilled water instead of extract. The % inhibition of DPPH was then calculated using the followed formula: % inhibition = [(Acontrol − Asample)/Acontrol] × 100.
The FRAP solution for the ferric reducing power assay was prepared by diluting 10 mM of tripyridyltriazine and 20 mM of FeCl3 in 100 mM of pH 3.6 sodium acetate buffer at a ratio of 1:1:10. Standards or extracts (both 100 µL) were then mixed with 2.9 mL FRAP reagent and the absorbance at 593 nm was monitored after samples were allowed to react for 30 min at 37 °C. Data were expressed as mmol FeSO4 g−1 sample.
Data analysis
All experiments were conducted in a completely randomized design, with three replicates per treatment. All data were analyzed using Duncan’s multiple range test using SPSS 13.0 software (IBM Cop., Armonk, NY, USA) at p ˂ 0.05.
Results
Effects of melatonin on aged seed germination
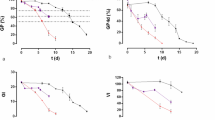
Treatment with melatonin slightly inhibited or delayed the germination of unaged seeds compared with the untreated control (Fig. 1A). Ageing treatment significantly impaired seed germination (Fig. 1B,C), with 5-day germination rates (GR) of 36–45 and 9–12% in the low and high aged beans, respectively (Fig. 1B,C; p ˂ 0.05). However, ageing treatment-impaired germination was profoundly reversed by melatonin, and compared with the aged seeds, treatment with 0.1 mM melatonin significantly increased the GR by approximately 123 and 36% in the low and high aged mung bean seeds, respectively (Fig. 1B,C; p ˂ 0.05). Similar changes were also observed in the yellow soybean, red bean and black soybean seeds (Fig. 1B,C; p ˂ 0.05).
Effects of melatonin on aged seed germination. Recovery effects of melatonin on the germination rate (GR) in unaged (A), low aged (B) and high aged (C) bean seeds imbibed for 5 days. Bars represent standard deviations of the mean (n = 3). Means within each bean type followed by the same letter are not significantly different (p ˂ 0.05). MT, melatonin; Yellow, yellow soybean; Red, red bean; Black, black soybean; Mung, mung bean.
Total phenolics and antioxidant capacity in the seeds and sprouts
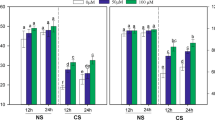
The total phenolics (TP) content was compared among unaged (control) seeds, aged seeds and sprouts germinated from unaged and aged seeds (Table 1). As shown in Table 1, the aged seeds exhibited a lower TP compared with the unaged seeds, with a decrease of approximately 68, 61, 65 and 59% in the yellow soybean, black soybean, red bean and mung bean, respectively (Table 1; p ˂ 0.05). However, melatonin-mediated germination profoundly enhanced TP accumulation in the sprouts (Table 1), with an increase in the yellow soybean sprouts of approximately 2.1- and 11-fold compared with the unaged and aged seeds, respectively (Table 1; p ˂ 0.05). Similar changes were also observed in the other bean seeds (Table 1). Overall, the TP contents of both the seeds and sprouts were in the order of yellow soybean < mung bean < red bean < black soybean (Table 1). In addition, germination significantly increased the TP content, and this was further enhanced by melatonin treatment in germinated seeds and sprouts compared with the unaged seeds (Table 1; p ˂ 0.05), with significant increases of approximately 20, 12, 14 and 15% in germinated seeds and sprouts from yellow soybean, black soybean, red bean or mung bean, respectively (Table 1; p ˂ 0.05).
Aging treatment also significantly reduced the ferric reducing power of the unaged (control) seeds (Table 2) by approximately 57, 55, 62 and 60% in the aged yellow soybean, black soybean, red bean and mung bean, respectively (Table 2; p ˂ 0.05). However, the ferric reducing power was greatly enhanced after sprouting, increasing by approximately 5.5- and 1.9-fold in the black soybean compared to the aged and unaged seeds, respectively (Table 2; p ˂ 0.05). Overall, the ferric reducing power of both the seeds and sprouts was also in the order of yellow soybean < mung bean < red bean < black soybean (Table 1). In addition, germination significantly increased the ferric reducing power, and this was further enhanced by melatonin treatment in germinated seeds and sprouts compared with unaged seeds (Table 1; p ˂ 0.05), with significant increases of approximately 17, 9, 7 and 12% in germinated seeds and sprouts from yellow soybean, black soybean, red bean or mung bean, respectively (Table 1; p ˂ 0.05).
Similarly, the sprouts exhibited a greater DPPH radical scavenging capacity (DRSC) compared to the aged and unaged (control) seeds (Table 3). Black soybean extracts exhibited the greatest inhibitory effects compared to the remaining three beans, both the seeds and sprouts (Table 3). However, ageing treatment significantly reduced the DRSC, by approximately 54, 48, 46 and 50% in the aged yellow soybean, black soybean, red bean and mung bean, respectively, compared with the unaged seeds (Table 3; p ˂ 0.05). However, this ageing-impaired DRSC was significantly reversed by melatonin-induced seed sprouting (Table 3), with an increase of 246, 140, 155 and 191% in sprouts of yellow soybean, black soybean, red bean and mung bean, respectively, compared to respective aged seeds (Table 3; p ˂ 0.05). In addition, germination increased the DRSC, and this was further enhanced by melatonin treatment in germinated seeds and sprouts compared with unaged seeds (Table 1; p ˂ 0.05), with increases of approximately 11, 5, 5 and 7% in germinated seeds and sprouts from yellow soybean, black soybean, red bean or mung bean, respectively (Table 1; p ˂ 0.05).
Discussion
Germination has a profound effect on antioxidant biosynthesis in seeds12,13. In this study, the germination rates of the control bean seeds (yellow and black soybeans, red and mung beans) decreased from an average of 90 to 40% (low aged seeds), but even as low as 10% (high aged seeds) after artificial ageing treatment (Fig. 1; p ˂ 0.05). Ageing treatment therefore profoundly inhibited seed germination, as in seeds of other crops42. Moreover, aging treatment also caused a severe decrease (more than 50%) in the phenolics content and antioxidant capacity (ferric reducing power and DPPH radical scavenging capacity) even in low aged bean seeds (Tables 1, 2, 3), which is partly in line with previous reports showing a rapid decrease in the GSH and vitamin E content in aged crop seeds26,27. These findings also suggest that the amount of antioxidant nutrients gained from aged seeds via direct consumption is very low.
As shown in Fig. 1, 0.1 mM melatonin delayed or slightly inhibited the germination of unaged bean seeds, which is in agreement with a previous report showing inhibition of stevia seed germination following treatment with a high concentration (more than 0.1 mM) of melatonin36. However, the ageing-induced loss in germination ability was significantly reversed by treatment with melatonin (Fig. 1; p ˂ 0.05), a healthy and non-toxic antioxidant43. This is in accordance with previous reports whereby melatonin was found to improve germination in aged maize seeds29,30. Melatonin, which is an important requirement of the human diet44, is found in a number of edible seeds45,46, and can also be synthesized in germinated legume seeds and sprouts47. Melatonin is therefore a safe reagent for improving germination in aged seeds.
As shown in Tables 1, 2, 3, germination increased the total phenolics accumulation and antioxidant capacity and this was further enhanced by melatonin in sprouts from unaged bean seeds. Interestingly, melatonin treatment also caused increases in the total phenolics content, ferric reducing power and DPPH radical scavenging capacity in sprouts germinated from aged seeds, with increases of at least five-, seven- and two-fold compared to aged seeds, respectively (p ˂ 0.05). Germination of aged bean seeds via melatonin restoration therefore has a significant effect on antioxidant nutrient accumulation, which is partly in accordance with a previous report, whereby melatonin improved germination and enhanced the antioxidant capacity of the ascorbate–glutathione system in aged oat seeds31. In general, aged seeds are unable to germinate and produce healthy seedlings due to reserve shortages18; thus, these seeds are used as animal fodder due to their low nutrient (e.g. protein and starch) content17,18,19,26,27,48. However, as shown here, melatonin-induced sprouting resulted in the conversion of reserve residues into antioxidant nutrients, increasing the overall health benefits of the previously aged seeds49,50. In addition, the taste of the resulting bean sprouts is more favorable than that of the aged seeds11. Melatonin-induced sprouting of aged seeds therefore results in a functional food product, highlighting the potential applicability of this new method in food production. However, despite our findings, some problems remain. For example, the germination rate of severely aged seeds (e.g. aged seeds with a 10% germination rate) could not be restored to a high level by melatonin, suggesting that this method can only be used with less aged bean seeds.
Numerous data suggest that melatonin promotes seed germination29,30,31,32,33,34,35,36,37. For example, our previous study showed that melatonin priming recovered germination of aged maize seeds by increasing antioxidant enzyme activities29. Furthermore, Su et al.30 and Yan et al.31 addressed the possible mechanisms at a transcriptomic and proteomic levels, respectively, confirming the effect of melatonin. Compared with aged seeds, melatonin can also improve the germination ability of unaged seeds under both stressful33,34,35 and stress-free conditions36,37, improving antioxidant enzyme (e.g. SOD and CAT) activities32,33, but also enhancing non-enzymatic antioxidant (e.g. total phenolics and ascorbate acid) accumulation33,35,36. In this study, compared with aged seeds, melatonin also promoted phenolics nutrient accumulation in unaged bean seeds (Table 1). Antioxidant nutrient accumulation can be partly attributed to melatonin-mediated protein biosynthesis32,33,34 and starch degradation32 in the germinated seeds, suggesting that antioxidant nutrient synthesis is required for melatonin-mediated germination in both aged and unaged seeds.
Overall, the results of this study suggest that melatonin can profoundly improve the germination rate and promote sprout production in aged legume seeds. Compared with the aged seeds, melatonin-induced sprouting resulted in rapid conversion of reserve residues into highly valuable bioactive antioxidant nutrients (e.g. phenolics). Since melatonin is an easily acquired natural antioxidant, this method provides an alternative approach for the utilization of deteriorated seed resources.
References
Lobo, V., Patil, A., Phatak, A. & Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmac. Rev. 4, 118–126 (2010).
Chang, S., Alasalvar, C. & Shahidi, F. Review of dried fruits: Phytochemicals, antioxidant efficacies, and health benefits. J. Funct. Foods 21, 113–132 (2016).
Cory, H., Passarelli, S., Szeto, J., Tamez, M. & Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. https://doi.org/10.3389/fnut.2018.00087 (2018).
Tang, D., Dong, Y., Ren, H., Li, L. & He, C. A review of phytochemistry, metabolite changes, and medicinal uses of the common food mung bean and its sprouts (Vigna radiata). Chem. Cent. J. 8, 4. https://doi.org/10.1186/1752-153X-8-4 (2014).
Xu, B., Yuan, S. & Chang, S. Comparative analyses of phenolic composition, antioxidant capacity, and color of cool season legumes and other selected food legumes. J. Food Sci. 72, 167–177 (2007).
Lópezamorós, M., Hernández, T. & Estrella, I. Effect of germination on legume phenolic compounds and their antioxidant activity. J. Food Compos. Anal. 19, 277–283 (2006).
Trugo, L. et al. Influence of malting on selected components of soya bean, black bean, chickpea and barley. Food Chem. 65, 85–90 (1999).
Xue, Z. et al. Bioactive compounds and antioxidant activity of mung bean (Vigna radiata L.) soybean (Glycine max L.) and black bean (Phaseolus vulgaris L.) during the germination process. Czech J. Food Sci. 34, 68–78 (2016).
Kocira, S. et al. Modeling biometric traits, yield and nutritional and antioxidant properties of seeds of three soybean cultivars through the application of biostimulant containing seaweed and amino acids. Front. Plant Sci. https://doi.org/10.3389/fpls.2018.00388 (2018).
Essa, T. Effect of salinity stress on growth and nutrient composition of three soybean (Glycine max L. Merrill) cultivars. J. Agron. Crop Sci. 188, 86–93 (2002).
Abdallah, M. Seed sprouts, a pharaoh’s heritage to improve food quality. Arab. Univ. J. Agric. Sci. 16, 469–478 (2008).
Dueñas, M., Hernández, T., Estrella, I. & Fernández, D. Germination as a process to increase the polyphenol content and antioxidant activity of lupin seeds (Lupinus angustifolius L.). Food Chem. 117, 599–607 (2009).
Gan, R. et al. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review. Trends Food Sci. Technol. 59, 1–14 (2017).
Cevallos-Casals, B. & Cisneros-Zevallos, L. Impact of germination on phenolic content and antioxidant activity of 13 edible seed species. Food Chem. 119, 1485–1490 (2010).
Sun, W. & Leopold, A. The Maillard reaction and oxidative stress during aging of soybean seeds. Physiol. Plant. 94, 94–104 (1995).
Stewart, R. & Bewley, J. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 65, 245–248 (1980).
Oliveira, D. et al. The deterioration of Moringa oleifera Lam. Seeds in the course of storage involves reserve degradation. Acta Physiol. Plant. https://doi.org/10.1007/s11738-017-2572-9 (2017).
Mohammadi, H., Soltani, A., Sadeghipour, H. & Zeinali, E. Effects of seed aging on subsequent seed reserve utilization and seedling growth in soybean. Int. J. Plant Prod. 5, 65–70 (2012).
Ravikumar, R., Ananthakrishnan, G., Girija, S. & Ganapathi, A. Seed viability and biochemical changes associated with accelerated ageing in Dendrocalamus Strictus seeds. Biol. Plant. 45, 153–156 (2002).
McGee, D. Environmental factors associated with preharvest deterioration of seeds. In Physiological–Pathological Interactions Affecting Seed Deterioration Vol. 12 (ed. West, S. H.) 53–63 (The Crop Science Society of America Inc, 1986).
Anderson, J. & Baker, J. Deterioration of seeds during aging. Phytopathology 73, 321–325 (1983).
Bhatia, V., Yadav, S., Jumrani, K. & Guruprasad, K. Field deterioration of soybean seed: Role of oxidative stress and antioxidant defense mechanism. J. Plant Biol. 32, 179–190 (2010).
Shelar, V., Shaikh, R. & Nikam, A. Soybean seed quality during storage: A review. Agric. Rev. 29, 125–131 (2008).
Kibinza, S. et al. Catalase is a key enzyme in seed recovery from ageing during priming. Plant Sci. 181, 309–315 (2011).
Kurek, K., Plitta-Michalak, B. & Ratajczak, E. Reactive oxygen species as potential drivers of the seed aging process. Plants 8, 174. https://doi.org/10.3390/plants8060174 (2019).
Sattler, S., Gilliland, L., Magallanes-Lundback, M., Pollard, M. & DellaPenna, D. Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 16, 1419–1432 (2004).
Hsu, J. & Sung, J. Antioxidant role of glutathione associated with accelerated aging and hydration of triploid watermelon seeds. Physiol. Plant. 100, 967–974 (1997).
Varier, A., Vari, A. & Dadlani, M. The subcellular basis of seed priming. Curr. Sci. 99, 450–456 (2010).
Deng, B., Yang, K., Zhang, Y. & Li, Z. Can antioxidant’s reactive oxygen species (ROS) scavenging capacity contribute to aged seed recovery? Contrasting effect of melatonin, ascorbate and glutathione on germination ability of aged maize seeds. Free Radic. Res. 51, 765–771 (2017).
Su, X. et al. Physiology and transcriptome analyses reveal a protective effect of the radical scavenger melatonin in aging maize seeds. Free Radic. Res. 52, 1094–1109 (2018).
Yan, H., Jia, S. & Mao, P. Melatonin priming alleviates aging-induced germination inhibition by regulating β-oxidation, protein translation, and antioxidant metabolism in oat (Avena sativa L.) seeds. Int. J. Mol. Sci. 21, 1898. https://doi.org/10.3390/ijms21051898 (2020).
Cao, Q. et al. Seed priming with melatonin improves the seed germination of waxy maize under chilling stress via promoting the antioxidant system and starch metabolism. Sci. Rep. 9, 15044. https://doi.org/10.1038/s41598-019-51122-y (2019).
Khan, M. et al. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crops Prod. 140, 111597. https://doi.org/10.1016/j.indcrop.2019.111597 (2019).
Li, J., Zhao, C., Zhang, M., Yuan, F. & Chen, M. Exogenous melatonin improves seed germination in Limonium bicolor under salt stress. Plant Signal. Behav. 14, 11. https://doi.org/10.1080/15592324.2019.1659705 (2019).
Jiang, X., Li, H. & Song, X. Seed priming with melatonin effects on seed germination and seedling growth in maize under salinity stress. Pak. J. Bot. 48(4), 1345–1352 (2016).
Simlat, M. et al. Melatonin significantly influences seed germination and seedling growth of Stevia rebaudiana Bertoni. PeerJ. 6, e5009. https://doi.org/10.7717/peerj.5009 (2018).
Kołodziejczyk, I., Dzitko, K., Szewczyk, R. & Posmyk, M. Exogenous melatonin expediently modifies proteome of maize (Zea mays L.) embryo during seed germination. Acta Physiol. Plant. 38, 146. https://doi.org/10.1007/s11738-016-2166-y (2016).
Singleton, V. & Rossi, J. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Vitic. 16, 144–158 (1965).
Guo, X., Li, T., Tang, K. & Liu, R. Effect of germination on phytochemical profiles and antioxidant activity of mung bean sprouts (Vigna radiate). J. Agric. Food Chem. 60, 11050–11055 (2012).
Brand-Williams, W., Cuvelier, M. & Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 28, 25–30 (1995).
Benzie, I. & Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 239, 70–76 (1996).
Das, S., Nayak, M., Patra, B., Ramakrishnan, B. & Krishnan, P. Characterization of seeds of selected wild species of rice (Oryza) stored under high temperature and humidity conditions. Indian J. Biochem. Biophys. 47, 178–184 (2010).
Arnao, M. & Hernández-Ruiz, J. Functions of melatonin in plants: a review. J. Pineal Res. 59, 133–150 (2015).
Domingos, A., Hersdorff, H. & Bressan, J. Melatonin intake and potential chronobiological effects on human health. Crit. Rev. Food Sci. Nutr. 59, 133–140 (2019).
Aguilera, Y. et al. Estimation of scavenging capacity of melatonin and other antioxidants: Contribution and evaluation in germinated seeds. Food Chem. 170, 203–211 (2015).
Manchester, L. et al. High levels of melatonin in the seeds of edible plants: Possible function in germ tissue protection. Life Sci. 67, 3023–3029 (2000).
Saleh, H., Hassan, A., Mansour, E., Fahmy, H. & EI-Bedawey, A. Melatonin, phenolics content and antioxidant activity of germinated selected legumes and their fractions. J. Saudi Soc. Agric. Sci. 18, 294–301 (2019).
Park, B. et al. Survey on seed decay during their germination of some forages from their aged seeds. J. Biol. Sci. 13, 432–435 (2013).
Zhang, Y. et al. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 20, 21138–21156 (2015).
Holst, B. & Williamson, G. Nutrients and phytochemicals: From bioavailability to bioefficacy beyond antioxidants. Curr. Opin. Biotechnol. 19, 73–82 (2008).
Acknowledgements
This study was supported by grants from the National Key Research and Development Project (No. 2018YFD1000704) and Postdoctoral Science Foundation Funded General Project of Heilongjiang Province (No. LBH-Z19195).
Author information
Authors and Affiliations
Contributions
S.Y.: investigation, project administration, writing—review & editing. X.Z., H.Y. & L.Y.: investigation, methodology, validation, project administration, data curation, formal analysis, funding acquisition; resource; Y.Z.: conceptualization, roles/writing—original draft and supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, S., Zhu, X., Yang, H. et al. A simple new method for aged seed utilization based on melatonin-mediated germination and antioxidant nutrient production. Sci Rep 11, 5937 (2021). https://doi.org/10.1038/s41598-021-85541-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-85541-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.