Abstract
Resveratrol is a phytochemical with anti-angiogenic, anti-inflammatory, and antioxidant properties. The present study has evaluated the effect of resveratrol on the expression of vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β) and matrix metalloproteinase-9 (MMP-9) as factors related to endometriosis progression. Thirteen eutopic (EuESCs) and 8 ectopic (EESCs) endometrial stromal cells from women with endometriosis and 11 control endometrial stromal cells (CESCs) were treated with resveratrol (100 µM) for 6, 24 and 48 h. The gene and protein expression levels of VEGF, TGF-β, and MMP-9 were measured using real-time PCR and ELISA methods, respectively. Results showed that the basal gene and protein expression of VEGF and MMP-9 were higher in EESCs compared to EuESCs and CESCs (P < 0.01 to < 0.001 and P < 0.05 to < 0.01 respectively). Also, resveratrol treatment decreased the gene and protein expression of VEGF and MMP-9 in EuESCs, EESCs and CESCs (P < 0.05 to < 0.01 and P < 0.05 to < 0.01 respectively) and gene and protein expression of TGF-β in EESCs and EuESCs (P < 0.05 to < 0.01). The effect of resveratrol in reduction of VEGF gene expression was statistically more noticeable in EESCs compared to EuESCs and CESCs (P < 0.05). According to the findings, resveratrol may ameliorate endometriosis progression through reducing the expression of VEGF, TGF-β, and MMP-9 in endometrial stromal cells (ESCs).
Similar content being viewed by others
Introduction
Phytochemicals are a large group of biologically active compounds found in plants1. Intake of dietary phytochemicals has been associated with health benefits and disease prevention2.
Resveratrol is a polyphenolic phytochemical belongs to the stilbenoid class3. Its main sources are grapes, berries, peanuts, and some other plants4. In recent decades, resveratrol is considered because of its beneficiary effects on oxidative stress5, inflammation3, tumor progression6, aging and angiogenesis7.
Endometriosis (EM), one of the most prevalent gynecological disorders, is characterized by the growth of endometrial stroma and glands outside the uterine cavity8. Its main symptoms are chronic pelvic pain and infertility9. To date, several theories have tried to explain the pathogenesis of the disease; among them, Sampson's retrograde menstruation theory is more reputable10. This theory stated that translocation of the endometrial cells into the peritoneal cavity through fallopian tubes leads to their adhesion, angiogenesis, and growth in the peritoneum and formation of the ectopic lesions10,11. So, according to this theory, growth and angiogenesis factors play vital roles in disease progression.
Vascular endothelial growth factor (VEGF) is shown to play a crucial role in angiogenesis in peritoneal endometriosis. It is secreted by the eutopic endometrium, ectopic endometriotic tissue, and peritoneal fluid (PF) macrophages12,13. Previous studies reported the higher concentration of VEGF in PF from patients with endometriosis compared to non-endometriotic controls and its correlation with disease stages14,15.
Transforming growth factor-β (TGF-β) is one of the most potent growth factors and monocytes chemoattractants. It can induce fibrosis and angiogenesis in ectopic implants and causes endometriosis progression16,17. The PF of women with stage III and IV of endometriosis has higher levels of TGF-β compared to women with milder endometriosis, and a significant decrease in concentrations was achieved after treatment with a gonadotropin-releasing hormone (GnRH) agonist18.
Matrix metalloproteinase-9 (MMP-9) is a member of proteinases that plays an essential role in the remodeling of the extracellular matrix19. The overexpression of MMP-9 in ectopic endometrial lesions primary seems to exacerbate the angiogenesis and invasion of ectopic implants20,21.
It was claimed that resveratrol could ameliorate endometriosis progression22. Its effect is due to suppression the expression of growth factors23, decrease cell proliferation24, reduction of the size of the ectopic implant25, induction of apoptosis26, reduction of the inflammation25 and oxidative stress27, and inhibition the invasion, adhesion, and angiogenesis of endometriotic ectopic lesions22. Also, Resveratrol has shown to suppress the expression of VEGF and MMP-9 in rat-induced endometriosis25 and decrease the expression of VEGF, TGF-β, and MMP-9 in other diseases28,29,30.
Considering the importance of VEGF, TGF-β and MMP-9 in the development of EM and the inhibitory effect of resveratrol on the expression of these factors in other cell types in different diseases, and given that to date no study has been performed on the effect of resveratrol on gene and protein expression of these factors in ESCs, the aim of the present study was to investigate the effect of resveratrol treatment on the gene and protein expression of VEGF, TGF-β and MMP-9 in ectopic (EESCs) and eutopic (EuESCs) endometrial stromal cells in women with endometriosis in comparison with non-endometriotic controls (CESCs).
Materials and methods
Study population
The present study was performed on 40 patients with peritoneal endometriosis and 15 non-endometriotic controls. The inclusion criteria were: being at reproductive age (19–45 years old), at the proliferative phase of the menstrual cycle, the III-IV stages of peritoneal endometriosis according to the revised American Fertility Society system (rAFS)31 for patients and non-endometriotic lesions for control group based on the laparoscopy. The control group was undergone laparoscopy for diagnostic reasons and because of benign gynecological problems.
The exclusion criteria were: any history of malignancy, autoimmune or metabolic disorders, taking any hormonal medications or dietary supplements within the last three months before the surgery, pregnancy, lactation, and cigarette smoking.
All individuals signed written informed consent before participating in the study, and all participants' privacy was respected. The study protocols was approved by the Ethics Committee for Medical Research of Iran University of Medical Sciences (Code: IR.IUMS.rec.1395.9221324203). All methods were performed in accordance with the relevant guidelines and regulations.
Sample collection
All sample collection and tissue extraction details were explained earlier23. Ectopic and eutopic endometrial samples were collected using laparoscopic sampling and biopsy curette, respectively. All endometriotic cysts (endometrioma) size were ≥ 5 cm in diameter. Tissue samples were put in sterile tubes containing Dulbecco's modified Eagle's medium (DMEM)-F12 (Sigma-Aldrich, St. Louis, MO, USA) culture medium with 1% Penicillin–Streptomycin antibiotics (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and quickly transferred to the laboratory on ice. A part of all samples was taken to the pathology laboratory to confirm endometriosis. The phase of the menstrual cycle was confirmed by the histological dating of ectopic endometrial implants. In the case of virgins and other patients with only the ectopic tissue, the confirmation of the cycle phase done by the last menstrual period (LMP).
Isolation, culture and purification of endometrial stromal cells (ESCs)
The digestion of endometrial tissue samples and the purification and culture of stromal cells performed as we described earlier23. Briefly, in the sterile condition, ectopic and eutopic endometrial tissues from endometriotic women and normal endometrial tissues from the control women were minced into smaller pieces and digested in the presence of 2 mg/ml Collagenase A (Roche, Pleasanton, CA, USA) and 300 mg/ml DNase (Roche, Pleasanton, CA, USA). Then the obtained cells were cultured in T25 culture flasks (SPL Life Sciences, Korea), ans stored in an atmosphere of 5% CO2 at 37 °C in DMEM-F12 (Sigma-Aldrich, St. Louis, MO, USA) containing 1% Penicillin–Streptomycin antibiotics (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and 10% fetal bovine serum (FBS) (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and adherent stromal cells were allowed to multiply. The cultured cells were passaged three times and when they reached to about 80% confluency they were used for the treatment. Some tissue samples especially ectopic tissues were excluded due to the cultural contamination, inproper pathology results, or not obtained the desired cells. At the end, from 40 endometriotic and 15 non-endometriotic control tissues, 8 ectopic, 13 eutopic, and 11 control tissues were treated. The purification of the ESCs was approved by immunofluorescent staining and flow cytometry. These cells were characterized as a panel of vimentin+, nestin+, cytokeratin−, CD10+, CD44+, CD73+, CD105+, CD34−, and CD45− cells. as we described earlier23.
Treatment of endometrial stromal cells with resveratrol
Based on the results of MTT test and the pilot study23, all ESCs from the three study groups were seeded 30 × 104 in 24-well plates (SPL Life Sciences, Korea) to have confluency about 80% for resveratrol treatment. After 3 h treated with the pre-determined optimized concentration of 100 µM resveratrol (Sigma-Aldrich, St. Louis, MO, USA) and stimulated with 100 ng/ml Lipopolysaccharide (LPS) (Sigma-Aldrich, St. Louis, MO, USA)32, and incubated for three-time points 6, 24 and 48 h.
Extraction of RNA and quantitative real-time PCR
For RNA isolation all ESCs were stored in Trizol (Qiagen, Hilden, Germany) at −80 °C. Total RNA was isolated according to the manufacturer’s instructions. Extracted RNA was reverse transcribed to complementary DNA (cDNA) using reverse transcription-polymerase chain reaction (RT-PCR) kit (Fermentas, Thermo Fisher Scientific, Waltham, MA, USA). The gene expressions of VEGF, TGF-β, and MMP-9 were quantified by real-time PCR with Syber premix Extaq (Biofact, Daejeon, Korea) according to the protocol by Rotor-Gene Q (Qiagen, Hilden, Germany). The gene expressions were normalized using Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA as an internal control. The primer pairs and the size of the amplicons are shown in Table 1. The PCR conditions were mentioned in details earlier23. It included a holding step on 95° for 15 min (for enzyme activation), which followed by 40 cycles of 95 °C for the 20 s, extension at 60 °C for 40 s (GAPDH at 58 °C for 40 s) and the melting step at 60° to 99°. All reactions were run in duplicate.
Measurement of VEGF, TGF-β, and MMP-9 protein
The concentration of VEGF, TGF-β, and MMP-9 protein in the cell supernatant was examined by a standard enzyme-linked immunoassay (ELISA) kit (Duoset; R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocol.
Statistical analysis
Statistical analyses were carried out using GraphPad Prism software 6.01 (GraphPad Software. Inc). Based on the results of the Kolmogorov–Smirnov test, all data was analyzed using the non-parametric tests, including the Wilcoxon sign-ranked test, Mann–Whitney, and Kruskal–Wallis tests. For the gene expression analysis, the fold change and relative expression were compared by calculating the 2−∆Δct and 2−∆ct, respectively. P-value < 0.05 was considered as the statistically significant level.
Results
The Basal expression of VEGF gene and protein in ESCs
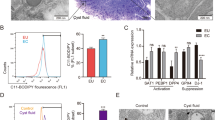
Based on the results of real-time PCR, the VEGF gene was expressed significantly more in EESCs compared to EuESCs and CESCs (Both P < 0.01) in the basic state (Fig. 1a). Moreover, according to the results of ELISA, the VEGF protein had significantly higher expression in EESCs compared to EuESCs and CESCs (P < 0.01 and P < 0.001 respectively) (Fig. 1b).
The basal expression levels of VEGF, TGF-β and MMP-9 genes and proteins in ESCs. The basal expression of VEGF, TGF-β and MMP-9 genes and proteins were measured in EESCs (n = 8) and EuESCs (n = 13) from endometriotic women and CESCs from non-endometriotic controls (n = 11) by real-time PCR and ELISA. (a) The basal expression of VEGF gene, (b) The basal expression of VEGF protein, (c) The basal expression of TGF-β gene, (d) The basal expression of TGF-β protein, (e) The basal expression of MMP-9 gene, (f) The basal expression of MMP-9 protein. *P-value < 0.05, ** P-value < 0.01 and *** P-value < 0.001 by non-parametric tests. VEGF: Vascular endothelial growth factor, TGF-β: Transforming growth factor-β, MMP-9: Matrix metalloproteinase-9, ESCs: Endometrial stromal cells, EuESCs: Eutopic endometrial stromal cells, EESCs: Ectopic endometrial stromal cells, CESCs: Control endometrial stromal cells.
The Basal expression of TGF-β gene and protein in ESCs
According to the results of real-time PCR and ELISA the basal gene and protein expression of TGF-β had no statistically significant differences among EESCs, EuESCs and CESCs (Fig. 1c,d).
The Basal expression of MMP-9 gene and protein in ESCs
Analysis of real-time PCR and ELISA methods revealed that the basal expression of MMP-9 gene and protein was significantly more in EESCs in comparison with EuESCs (Both gene and protein P < 0.05) and CESCs (Both gene and protein P < 0.01) (Fig. 1e,f).
Resveratrol decreased the expression of VEGF gene in all ESCs
The real-time PCR method demonstrated that treatment with resveratrol (100 µM) reduced the expression of VEGF gene significantly in EESCs at 24 (P < 0.05) and 48 h (P < 0.01) and in EuESCs and CESCs only at 48 h (Both P < 0.05) (Table 2). Also, the effect of 100 µM resveratrol treatment was more noticeable in EESCs in comparison with EuESCs and CESCs at 48 h (Both P < 0.05) (Supplementary file).
Resveratrol decreased the expression of TGF-β gene in EuESCs and EESCs
The TGF-β gene expression had significant reduction by treatment with resveratrol (100 µM) in EuESCs and EESCs at 48 h (P < 0.05 and P = 0.01 respectively). The TGF-β gene expression had no significant changes in EuESCs and EESCs at 6 and 24 h, and in CESCs at all three time intervals (Table 2). There was no significant difference in the effect of resveratrol treatment between EESCs and EuESCs at 48 h (Supplementary file).
Resveratrol decreased the expression of MMP-9 gene in all ESCs
The MMP-9 gene expression was significantly reduced by resveratrol (100 µM) in EuESCs at 24 (P < 0.01) and 48 h (P < 0.05) and in EESCs and CESCs at 48 h (Both P < 0.05). The gene expression of MMP-9 did not show significant changes in EuESCs at 6 h, and in EESCs and CESCs at 6 and 24 h (Table 2). In addition, resveratrol had a greater effect on EESCs compared with EuESCs and CESCs at 48 h, but this was not statistically significant (Supplementary file).
Resveratrol decreased the expression of VEGF protein in EuESCs and EESCs
The use of ELISA method revealed that the protein expression of VEGF was significantly reduced in EuESCs and EESCs at 48 h by 100 µM resveratrol (P < 0.05 and P < 0.01 respectively). Resveratrol treatment had no significant effect at 6 and 24 h in these cells. The VEGF protein expression did not change significantly in CESCs at any of the treatment times (Fig. 2). Although, the effect of resveratrol treatment on reducing VEGF protein expression in EESCs was greater than that of EuESCs, this difference was not statistically significant. (Supplementary file).
Resveratrol Decreased the Expression of VEGF Protein in EuESCs and EESCs. ESCs from endometriotic women (8 EESCs and 13 EuESCs) and non-endometriotic controls (11 CESCs) were cultured with or without of 100 µM resveratrol. After 6, 24, and 48 h, the protein expression of VEGF was examined using ELISA. (a) 6 hr (Res + vs Res-), (b) 24 hr (Res + vs Res-), (c) 48 hr (Res + vs Res-). *P-value < 0.05, ** P-value < 0.01 by non-parametric tests. VEGF: Vascular endothelial growth factor, EuESCs: Eutopic endometrial stromal cells, EESCs: Ectopic endometrial stromal cells, CESCs: Control endometrial stromal cells.
Resveratrol decreased the expression of TGF-β protein in EuESCs and EESCs
The effect of treatment with 100 µM resveratrol on the expression of TGF-β protein was the same as its gene expression. Resveratrol could reduce the expression of this factor in EuESCs and EESCs at 48 (Both P < 0.05). The expression of TGF-β protein showed no significant changes at 6 and 24 h in EuESCs and EESCs, and at any treatment times in CESCs (Fig. 3). The effect of resveratrol treatment between EESCs and EuESCs had no significant difference at 48 h (Supplementary file).
Resveratrol Decreased the Expression of TGF-β Protein in EuESCs and EESCs. ESCs from endometriotic women (8 EESCs and 13 EuESCs) and non-endometriotic controls (11 CESCs) were cultured with or without of 100 µM resveratrol. After 6, 24, and 48 h, the protein expression of TGF-β was examined using ELISA. (a) 6 hr (Res + vs Res-), (b) 24 hr (Res + vs Res-), (c) 48 hr (Res + vs Res-). *P-value < 0.05 by non-parametric tests. TGF-β: Transforming growth factor-β, EuESCs: Eutopic endometrial stromal cells, EESCs: Ectopic endometrial stromal cells, CESCs: Control endometrial stromal cells.
Resveratrol decreased the expression of MMP-9 in all ESCs
Resveratrol (100 µM) decreased significantly the MMP-9 protein in EuESCs, at 24 and 48 h and in EESCs and CESCs at 48 h (All P < 0.05). The MMP-9 protein production had no significant changes at 6 in EuESCs at 6 and 24 h in EESCs and CESCs (Fig. 4). In addition, the effect of treatment with 100 µM resveratrol at 48 h on the reduction of MMP-9 protein production was not statistically significant among three groups (Supplementary file).
Resveratrol Decreased the Expression of MMP-9 Protein in all ESCs. ESCs from endometriotic women (8 EESCs and 13 EuESCs) and non-endometriotic controls (11 CESCs) were cultured with or without of 100 µM resveratrol. After 6, 24, and 48 h, the protein expression of MMP-9 was examined using ELISA. (a) 6 hr (Res + vs Res-), (b) 24 hr (Res + vs Res-), (c) 48 hr (Res + vs Res-). *P-value < 0.05 by non-parametric tests. MMP-9: Matrix metalloproteinase-9, EuESCs: Eutopic endometrial stromal cells, EESCs: Ectopic endometrial stromal cells, CESCs: Control endometrial stromal cells.
Discussion
The results revealed that the basal expression of VEGF and MMP-9, but not TGF-β in EESCs were significantly higher compared to EuESCs and CESCs. To date, some studies assessed the concentration of these factors in PF or endometrial implants of the endometriotic patients21,33,34,35 and according to our knowledge, the present study is the first to compare the expression of VEGF, TGF-β and MMP-9 in EESCs, EuESCs, and CESCs.
The findings of the previous studies, consistent with the present study, have shown that VEGF expression in endometrial tissue and PF of patients with endometriosis is increased compared to controls, although, it does not differ significantly between the different stages of the disease33,36. In the only discordant study, the VEGF concentration in PF of patients with genital endometriosis and healthy control women was not significantly different34. VEGF receptors gene expression was also higher in ectopic endometrial lesions than in eutopic tissue21,37.
VEGF is one of the most important angiogenic factors in endometriosis. It can increase cell proliferation, cell migration, and vascular permeability13,38. The most important cells secreting this factor in endometriosis are eutopic and ectopic stromal cells, peritoneal macrophages, and neutrophils that increase the expression of this factor in response to elevated inflammatory conditions13. Increased levels of reactive oxygen species (ROS) due to oxidative stress in endometriosis can also increase VEGF expression and its angiogenesis in in-vivo and in-vitro39.
The few studies that have examined the expression of TGF-β in endometriosis have shown contradictory findings. For example, in a study of Sokolov et al. the concentration of TGF-β in PF did not differ significantly between the women with genital endometriosis and healthy controls34, but two other studies, showed that levels of TGF-β in serum and PF were higher in patients than in controls, and this level, especially in PF, increased with increasing severity of the disease35,40.
Several studies have shown the role of TGF-β in regulating the immune system and inflammation41. TGF-β enhances the growth and angiogenesis of ESCs, especially ectopic cells, and plays an essential role in the development of endometriotic lesions13. Increased expression of this factor in endometriosis seems to occur in response to increased inflammatory conditions and oxidative stress in the peritoneal cavity42. As is evident in our study, there was no significant difference in TGF-β expression in the stromal cells of the study groups. Previous studies reported that peritoneal mesothelial cells are the most important source of this factor in peritoneum-related diseases such as peritoneal endometriosis, followed by peritoneal macrophages, ectopic endometrial tissue including ESCs17, it appears that the increased expression of this factor in the serum and PF of patients with endometriosis than in controls has been reported in some previous studies35,40, may be due to the increased production of this cytokine by peritoneal mesothelial cells and then other sources and in the meantime, the ESCs evaluated in the present study, have less role in the production of this factor. Previous studies have also shown that the concentration of TGF-β in the peritoneum of individuals with endometriosis changes during the menstrual cycle and its highest concentration is seen in the secretory phase and in the premenstrual phase18,41. However, in our study, we measured the expression of TGF-β in the proliferative phase.
In the case of MMP-9, the only study comparing the expression of this factor in ectopic endometrial lesions with eutopic endometrium is Machado's study on an induced model of endometriosis in rats and reported findings consistent with the present study21.
Studies have shown that chronic inflammation increases MMP-9 expression. Expression of MMP-9 by EESCs and EuESCs increases in endometriosis in response to inflammatory conditions in the peritoneal cavity, which is higher in ectopic than eutopic lesions and activation of the NF-κB and MAP-kinase signaling and other inflammatory pathways, as well as to increased oxidative stress43,44; This assists the replacement, growth, and invasion of endometriotic implants20,21. Increased production and activity of MMP-9 increase the degradation and regeneration of extracellular matrix, angiogenesis, and VEGF secretion19,45.
The present study revealed that the gene and protein expression of VEGF, TGF-β, and MMP-9 in EESCs and EuESCs were reduced by resveratrol treatment. According to our knowledge, this is the first study to investigate the effect of resveratrol on VEGF expression in ESCs of patients with endometriosis. In the only animal study, resveratrol significantly reduced VEGF expression in endometriosis-induced rats25. Other previous in-vivo and in-vitro studies on the effect of resveratrol on VEGF expression in other diseases have also reported findings consistent with the present study29,46,47,48,49.
The mechanisms of the effect of resveratrol on VEGF expression seems to be through activation of the sirtuin-1 molecule and inhibition of the NF-κB pathway50. Resveratrol can also inhibit VEGF through ACE-I-like activity. Thus, resveratrol inhibits positive feedback between angiotensin-II and VEGF. The in-vitro studies have shown that ACE-I-like factors can inhibit VEGF-induced endothelial cell migration and invasion and inhibit VEGF mRNA expression49,51. Resveratrol may also block the VEGF receptor response pathway by reducing MAP-kinase phosphorylation and inhibiting VEGF-induced angiogenesis by blocking tyrosine phosphorylation in the cadherin molecule46. Besides, resveratrol reduces VEGF expression and its invasion and angiogenesis by preventing the production and eliminating the ROS and reactive nitrogen species (RNS)39,52.
It seems that the difference in the effect of resveratrol on the reduction of VEGF gene expression in EESCs compared to EuESCs and CESCs is due to differences in inflammatory and micro-environmental conditions of these cells. Previous studies have shown that EESCs, EuESCs, and CESCs differ in cytokine expression, cell proliferation, invasion, metastasis, and response to nutritional interventions53,54.
The present study is the first to investigate the effect of resveratrol on TGF-β expression in ESCs of patients with endometriosis, and it is not possible to compare the results with similar studies. Therefore, the findings of this study were compared with those of animal studies on the effect of resveratrol on TGF-β levels in other diseases. Most of these studies consistent with the present study have shown that resveratrol can decrease TGF-β gene and protein expression28,55,56. In the only inconsistent study, a single-dose intraperitoneal injection of resveratrol had no significant effect on TGF-β levels in rats with acute liver injury, possibly due to the amount and timing of the intervention57.
Resveratrol has been reported to inhibit TGF-β transcription by blocking the NF-κB pathway55. Resveratrol can also reduce TGF-β expression by blocking the activator protein 1 (AP-1) molecule and removing ROS and reducing oxidative stress58. Resveratrol also down-regulates TGF-β expression and activity by down-regulating TGF-β signaling pathway molecules, including, Smad-2, 3,459. TGF-β is a pro-fibrotic factor that can increase the production of type IV collagen and fibrin28. Resveratrol treatment can prevent TGF-β-induced fibrotic tissue growth in ectopic lesions55.
The present study is the first to assess the effect of resveratrol treatment on MMP-9 expression in ESCs. The only animal study that investigated the effect of resveratrol on MMP-9 expression in endometriosis also reported the same results25. Other in-vivo and in-vitro studies also shown that resveratrol decreases MMP-9 mRNA and protein expression and suppresses the activity of this enzyme30,60,61. In the only inconsistent study, Gweon and Kim reported that resveratrol at different concentrations increased the activity and expression of MMP-9 in human fibrosarcoma cells. The cause of this contradictory finding may be the different inflammatory condition62.
MMP-9 is one of the proteins whose expression is enhanced by activation of the NF-κB pathway. It appears that resveratrol decreases the expression of this factor by suppressing the expression and activity of the NF-κB pathway60. Resveratrol inhibits NF-κB transcriptional activity by blocking phosphorylation and degradation of the IκB inhibitor molecule, thereby inhibiting NF-κB translocation and DNA binding and preventing expression of inflammatory cytokines and growth factors and angiogenesis including MMP-963. Resveratrol can also prevent MMP-9 expression by decreasing TGF-β expression, inhibiting MAP-kinase signaling pathway, reabsorption of ROS, and reducing oxidative stress64,65.
The present study had some advantages and limitations: As we mentioned earlier, it was the first study investigated the basal gene and protein expression and also the effect of resverstrol treatment on the gene and protein expression of VEGF, TGF-β and MMP-9 in ectopic (EESCs), and eutopic (EuESCs) endometrial stromal cells of women with endometriosis in comparison with non-endometriotic controls (CESCs). One of the limitations was that the present study was carried out only in the severe (III and IV) stages of the EM and at the proliferative phase. Also, it would have been better if we could assess the MMP-9 activity. It is also better to investigate the effect of resveratrol treatment on the expression of VEGF, TGF-β and MMP-9 in the peritoneal fluid mononuclear cells (PFMCs) and mesothelial cells as the important sources of these factors. Moreover, in order to better determine the effect of resveratrol on EM, further studies are needed on the effect of resveratrol treatment on cell proliferation, angiogenesis, invasion, adhesion, apoptosis, and other processes involved in the pathogenesis of EM.
Conclusion
The present study showed that the basal gene and protein expression of VEGF and MMP-9 were higher in EESCs compared to EuESCs and CESCs. The treatment of EESCs and EuESCs with resveratrol could reduce the gene and protein expression of VEGF, TGF-β, and MMP-9. Further in-vitro and in-vivo studies are needed to determine the possible beneficial effects of resveratrol on EM progression.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Abbreviations
- VEGF:
-
Vascular endothelial growth factor
- TGF-β:
-
Transforming growth factor-β
- MMP-9:
-
Matrix metalloproteinase-9
- ESCs:
-
Endometrial stromal cells
- EuESCs:
-
Eutopic endometrial stromal cells
- EESCs:
-
Ectopic endometrial stromal cells
- CESCs:
-
Control endometrial stromal cells
- EM:
-
Endometriosis
- PF:
-
Peritoneal fluid
- GnRH:
-
Gonadotropin-releasing hormone
- LMP:
-
Last menstrual period
- DMEM:
-
Dulbecco's modified Eagle's medium
- LPS:
-
Lipopolysaccharide
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- ELISA:
-
Enzyme-linked immunoassay
- PCR:
-
Polymerase chain reaction
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- NF-κB:
-
Nuclear factor-κB
- MAP-kinase:
-
Mitogen-activated protein kinase
- ACE-I:
-
Angiotensin-converting-enzyme inhibitors
- ROS:
-
Reactive oxygen species
- RNS:
-
Reactive nitrogen species
- AP-1:
-
Activator protein 1
References
Tyagi, S. et al. Pytochemicals as candidate therapeutics: an overview. Int. J. Pharm. Sci. Rev. Res. 3(1), 53–55 (2010).
Melanie-Jayne, H. & Monique, S. The role of phytochemicals as micronutrients in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 17(6), 558–566 (2014).
Poulsen, M. et al. (2015) Resveratrol and inflammation: Challenges in translating pre-clinical findings to improved patient outcomes. Biochimica et Biophysica Acta Mol Basis Dis 6, 1124–1136 (1852).
Harikumar, K. B. & Aggarwal, B. B. Resveratrol: a multitargeted agent for ageassociated chronic diseases. Cell Cycle 7(8), 1020–1035 (2008).
Fukui, M. et al. Mechanism for the protective effect of resveratrol against oxidative stress-induced neuronal death. Free Radical Biol. Med. 49(5), 800–813 (2010).
Noh, K. et al. Resveratrol suppresses tumor progression via the regulation of indoleamine 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 431(2), 348–353 (2013).
Kasiotis, K. et al. Resveratrol and related stilbenes: Their anti-aging and anti-angiogenic properties. Food Chem. Toxicol. 61, 112–120 (2013).
Ashrafi, M. et al. Evaluation of Risk Factors Associated with Endometriosis in Infertile Women. Int J Fertil Steril 10(1), 11–21 (2016).
Laganà, A. et al. Unus pro omnibus, omnes pro uno: A novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Med Hypotheses. 103, 10–20 (2017).
Sourial, S., Tempest, N. & Hapangama, D. K. Theories on the Pathogenesis of Endometriosis. Int. J. Reprod. Med. 2014, 179515 (2014).
Gupta, S. et al. Role of oxidative stress in endometriosis. Reprod. Biomed. Online 13(1), 126–134 (2006).
Burney, R. O. & Giudice, L. C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 98(3), 511–519 (2012).
Gazvani, R. & Templeton, A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction 123, 217–226 (2002).
McLaren, J. et al. Vascular endothelial growth factor (VEGF) concentrations are elevated in peritoneal fluid of women with endometriosis. Hum Reprod. 11(1), 220–223 (1996).
Mahnke, J., Dawood, M. & Huang, J. Vascular endothelial growth factor and interleukin-6 in peritoneal fluid of women with endometriosis. Fertil Steril. 73(1), 166–170 (2000).
Hull, M. et al. Host-derived TGFB1 deficiency suppresses lesion development in a mouse model of endometriosis. Am J Pathol 180, 880–887 (2012).
Young, V. J. et al. The role of TGF-β in the pathophysiology of peritoneal endometriosis. Hum. Reprod. Update 23(5), 548–559 (2017).
Kupker, W., Schultze-Mosgau, A. & Diedrich, K. Paracrine changes in the peritoneal environment of women with endometriosis. Hum. Reprod. 4, 719–723 (1998).
London, C. A. et al. A novel antisense inhibitor of MMP-9 attenuates angiogenesis, human prostate cancer cell invasion and tumorigenicity. Cancer Gene Ther. 10, 823–832 (2003).
Weigel, M. T. et al. Differential expression of MMP-2, MMP-9 and PCNA in endometriosis and endometrial carcinoma. Eur. J. Obst. Gynecol. Reprod. Biol. 160, 74–78 (2012).
Machado, D. E. et al. Higher expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 (Flk-1) and metalloproteinase-9 (MMP-9) in a rat model of peritoneal endometriosis is similar to cancer diseases. J. Experim. Clin. Cancer Res. 29(4), 1 (2010).
Kolahdouz-Mohammadi, R. & Arablou, T. Resveratrol and endometriosis: In vitro and animal studies and underlying mechanisms (Review). Biomed. Pharmacother. 91, 220–228 (2017).
Arablou, T. et al. Resveratrol reduces the expression of insulin-like growth factor-1 and hepatocyte growth factor in stromal cells of women with endometriosis compared with nonendometriotic women. Phytother. Res. 33(4), 1044–1054 (2019).
Rudzitis-Auth, J., Menger, M. D. & Laschke, M. W. Resveratrol is a potent inhibitor of vascularization and cell proliferation in experimental endometriosis. Hum. Reprod. 28(5), 1339–1347 (2013).
Tekin, Y. B. et al. Is resveratrol a potential substitute for leuprolide acetate in experimental endometriosis?. Eur. J. Obst. Gynecol. Reprod. Biol. 184, 1–6 (2015).
Taguchi, A. et al. Resveratrol Enhances Apoptosis in Endometriotic Stromal Cells. Am J Reprod Immunol. 75(4), 486–492 (2016).
Yavuz, S. et al. Resveratrol successfully treats experimental endometriosis through modulation of oxidative stress and lipid peroxidation. J. Cancer Res. Ther. 10(2), 324–329 (2014).
Rahal, K. et al. Resveratrol has antiinflammatory and antifibrotic effects in the peptidoglycan-polysaccharide rat model of Crohn’s disease. Inflamm. Bowel Dis. 18(4), 613–623 (2012).
Nagineni, C. N. et al. Resveratrol suppresses expression of VEGF by human retinal pigment epithelial cells: potential nutraceutical for age-related macular degeneration. Aging Dis. 5(2), 88–100 (2014).
Cheng, G. et al. Resveratrol inhibits MMP-9 expression by up-regulating PPAR α expression in an oxygen glucose deprivation-exposed neuron model. Neurosci. Lett. 451(2), 105–108 (2009).
AmericanFertilitySociety, Revised American. Fertility Society classification of endometriosis. Fertil Steril 43, 351–352 (1985).
Rashidi, N. et al. Lipopolysaccharide- and lipoteichoic acid-mediated pro-inflammatory cytokine production and modulation of TLR2, TLR4 and MyD88 expression in human endometrial cells. J. Reprod. Infertil. 16, 72–81 (2015).
Ying-ying, W. & Xiao-ling, F. The expression of hepatocyte growth factor (HGF) and vascular epithelial growth factor (VEGF) in peritoneal fluid of patients with endometriosis. J. Clin. Res. 1, 1 (2007).
Sokolov, D. I. et al. Study of cytokine profile and angiogenic potential of peritoneal fluid in patients with external genital endometriosis. Bull. Exp. Biol. Med. 140(5), 541–544 (2005).
Pizzo, A. et al. Behaviour of cytokine levels in serum and peritoneal fluid of women with endometriosis. Gynecol. Obstet. Invest. 54, 82–87 (2002).
Sel’kov, S. A. et al. Local production of interleukins and growth factors in external genital endometriosis. Bull. Experim. Biol. Med. 139(4), 444–447 (2005).
Yerlikaya, G. et al. Comprehensive study of angiogenic factors in women with endometriosis compared to women without endometriosis. Eur. J. Obst. Gynecol. Reprod. Biol. 204, 88–98 (2016).
Rocha, A. L. L., Reis, F. M. & Taylor, R. N. Angiogenesis and Endometriosis. . Obst. Gynecol. Int. 2013, 1 (2013).
Kuroki, M. et al. Reactive oxygen intermediates increase vascular endothelial growth factor expression in vitro and in vivo. J. Clin. Invest. 98(7), 1667–1675 (1996).
Young, V. J. et al. Transforming growth factor-b induced Warburg-likemetabolic reprogramming may underpin the development of peritoneal endometriosis. Clin. Endocrinol. Metab. 99(9), 3450–3459 (2014).
Young, V. J. et al. The peritoneum is both a source and target of TGF-b in women with endometriosis. PLoS ONE 9(9), e106773 (2014).
Zhao, W. et al. Oxidative stress mediates cardiac fibrosis by enhancing transforming growth factor-beta1 in hypertensive rats. Mol. Cell. Biochem. 317(1–2), 43–50 (2008).
Reddy, K. B. et al. Mitogen-activated protein kinase (MAPK) regulates the expression of progelatinase B (MMP-9) in breast epithelial cells. Int. J. Cancer 82(2), 268–273 (1999).
Yu, F. et al. Induction of MMP-9 expression and endothelial injury by oxidative stress after spinal cord injury. J. Neurotrauma 25(3), 1 (2008).
Zheng, H. et al. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 26, 3579–3584 (2006).
Garvin, S., Llinger, K. O. & Dabrosin, C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 231, 113–122 (2006).
Zhang, H. & Yang, R. Resveratrol inhibits VEGF gene expression and proliferation of hepatocarcinoma cells. Hepatogastroenterology 61(130), 410–412 (2014).
Yu, H. et al. Resveratrol inhibits VEGF expression of human hepatocellular carcinoma cells through a NF-kappa B-mediated mechanism. Hepatogastroenterology 57(102–103), 1241–1246 (2010).
Zehai, T., Xin-yue, L. & Ping, Z. Resveratrol Inhibits the Secretion of Vascular Endothelial Growth Factor and Subsequent Proliferation in Human Leukemia U937 Cells. J. Huazhong Univ. Sci. Technol. 27(5), 508–512 (2007).
Kundu, O. K. & Surh, Y.-J. Cancer chemopreventive and therapeutic potential of resveratrol: mechanistic perspectives. Cancer Lett. 269, 243–261 (2008).
Saijonmaa, O. et al. Upregulation of angiotensin-converting enzyme by vascular endothelial growth factor. Am. J. Physiol. Heart Circ. Physiol. 280, H885–H891 (2001).
Lastra, C. A. N. D. L. & Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and clinical implications. Biochem. Soc. Trans. 35(5), 1156–1160 (2007).
Delbandi, A.-A. et al. Eutopic and ectopic stromal cells from patients with endometriosis exhibit differential invasive, adhesive, and proliferative behavior. Fertil. Steril. 100(3), 761–769 (2013).
Delbandi, A.-A. et al. 1,25-dihydroxy vitamin D3 modulates endometriosis-related features of human endometriotic stromal cells. Am. J. Reprod. Immunol. 75, 461–473 (2016).
Chávez, E. et al. Resveratrol prevents fibrosis, NF-κB activation and TGF-β increases induced by chronic CCl4 treatment in rats. J. Appl. Toxicol. 28, 35–43 (2008).
Chen, K.-H. et al. Resveratrol ameliorates early diabetic nephropathy associated with suppression of augmented TGF-β/smad and ERK1/2 signaling in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 190(1), 45–53 (2011).
Chan, C.-C. et al. Regulation by resveratrol of the cellular factors mediating liver damage and regeneration after acute toxic liver injury. J. Gastroenterol. Hepatol. 29, 603–613 (2014).
Kim, S. et al. Autoinduction of transforming growth factor 13 is mediated by the AP-1 complex. Mol. Cell. Biol. 10(4), 1492–1497 (1990).
Whyte, L. et al. Molecular mechanisms of resveratrol action in lung cancer cells using dual protein and microarray analyses. Cancer Res. 67(24), 12007–12017 (2007).
Banerjee, S., Bueso-Ramos, C. & Aggarwal, B. B. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Can. Res. 62, 4945–4954 (2002).
Sun, C.-Y. et al. Resveratrol as a novel agent for treatment of multiple myeloma with matrix metalloproteinase inhibitory activity. Acta Pharmacol. Sin. 27(11), 1447–1452 (2006).
Gweon, E. J. & Kim, S. J. Resveratrol induces MMP-9 and cell migration via the p38 kinase and PI-3K pathways in HT1080 human fibrosarcoma cells. Oncol. Rep. 29, 826–834 (2013).
Kundu, J. K. & Surh, Y.-J. Molecular basis of chemoprevention by resveratrol:NF-B and AP-1 as potential targets. Mutat. Res. 555, 65–80 (2004).
Yu, R. et al. Resveratrol inhibits phorbol ester and UV-induced activator protein 1 activation by interfering with mitogen-activated protein kinase pathways. Mol. Pharmacol. 60(1), 217–224 (2001).
Kim, E.-S., Kim, M.-S. & Moon, A. TGF-β-induced upregulation of MMP-2 and MMP-9 depends on p38 MAPK, but not ERK signaling in MCF10A human breast epithelial cells. Int. J. Oncol. 25(5), 1375–1382 (2004).
Acknowledgements
We thank all the participants in the present study. The study was funded by Iran University of Medical Sciences with the grant number 28107.
Author information
Authors and Affiliations
Contributions
T.A., R.K.M., N.A. and A.A.D. designed the project and planned the experiments. S.K.H. contributed to sample preparation. T.A., R.K.M., Z.M. and N.R. carried out the experiments. T.A., A.A.D., R.K.M., and Z.M. contributed to the data analysis and interpretation of the results. T.A. wrote the first draft of the manuscript. N.A. and A.A.D. critically reviewed the paper. A.A.D. supervised the project. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arablou, T., Aryaeian, N., Khodaverdi, S. et al. The effects of resveratrol on the expression of VEGF, TGF-β, and MMP-9 in endometrial stromal cells of women with endometriosis. Sci Rep 11, 6054 (2021). https://doi.org/10.1038/s41598-021-85512-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-85512-y
This article is cited by
-
Intervention of Phytochemicals During Endometriosis and Their Conceivable Mechanisms
Revista Brasileira de Farmacognosia (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.