Abstract
We previously reported that synovial mast cells (MCs) from patients with rheumatoid arthritis (RA) produced TNF-α in response to immune complexes via FcγRI and FcγRIIA. However, the specific functions of synovial MCs in RA remain unclear. This study aimed to elucidate those functions. Synovial tissues and fluid were obtained from RA and osteoarthritis (OA) patients undergoing joint replacement surgery. Synovium-derived, cultured MCs were generated by culturing dispersed synovial cells with stem cell factor. We performed microarray-based screening of mRNA and microRNA (miRNA), followed by quantitative RT-PCR-based verification. Synovial MCs from RA patients showed significantly higher prostaglandin systhetase (PTGS)1 and PTGS2 expression compared with OA patients’ MCs, and they produced significantly more prostaglandin D2 (PGD2) following aggregation of FcγRI. PGD2 induced IL-8 production by human group 2 innate lymphoid cells, suggesting that PGD2-producing MCs induce neutrophil recruitment into the synovium of RA patients. PTGS2 mRNA expression in RA patients’ MCs correlated inversely with miRNA-199a-3p expression, which down-regulated PTGS2. RA patients’ synovial fluid contained significantly more PGD2 compared with OA patients’ fluid. Synovial MCs might regulate inflammation in RA through hyper-production of PGD2 following FcRγ aggregation. Our findings indicate functional heterogeneity of human MCs among diseases.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease characterized by immune cell-mediated destruction of the architecture of joints. Proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and IL-17, are thought to play pivotal roles in that joint architecture destruction in RA patients. In RA patients, the number of degranulated mast cells (MCs) is increased in synovial tissues and correlates with the disease activity1,2,3,4. Such MC mediators as histamine and tryptase in synovial fluid (SF) are also increased in these patients2,4,5,6. We previously reported that aggregated IgG induces TNF-α production by human synovium-derived, cultured MCs via FcγRI and FcγRII7 and that IL-33 synergistically enhances immune complex-induced TNF-α and IL-8 production in those same cells8. These results suggest that activation of MCs may exaggerate inflammation in RA. On the other hand, IL-33- and immune complex-triggered activation of human peripheral blood-derived, cultured MCs reportedly down-regulated monocyte-mediated immune responses via IL-10 and histamine release9, suggesting that MCs might have anti-inflammatory functions. The mRNA levels of MC-specific genes were reportedly inversely associated with disease severity in RA patients9. C-reactive protein and the tryptase serum level were negatively correlated in RA patients10, Thus, MCs might have both beneficial and harmful roles in inflammation, depending on their site and time of action and the etiology of the inflammatory response.
We previously compared the expression levels of Fc receptors and protease phenotypes of synovial MCs from RA and osteoarthritis (OA) patients and found no significant differences between the two groups7. Thus, the specific function or functions of synovial MCs of RA patients have not yet been elucidated. We hypothesized that RA patients’ synovial MCs have special characteristics and play specific roles in the inflammatory response in RA.
Upregulation of the cyclooxygenase and lipoxygenase pathways of arachidonic acid is thought to be involved in the development of rheumatic diseases, and targeting this pathway might lead to improved treatment strategies11. Indeed, prostaglandins (PGs) such as PGE2 and PGI2, and leukotrienes (LTs) such as LTB4, have been considered to play important roles in the onset and development of arthritic diseases in animals and humans11,12,13,14. However, some PGs, such as the nonenzymatic PGD2 metabolite 15-deoxy-PGJ2, have anti-inflammatory effects depending on the disease context11. PGD2 may exert pro-inflammatory or anti-inflammatory effects in different biologic systems15. PGD2 is a ligand for two receptors, D prostanoid (DP) and chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2)15. PGH2 is converted to PGD2 by two enzymes, hematopoietic PGD synthase (HPGDS) and lipocalin-type PGDS (LPGDS). HPGDS is present in hematopoietic cells, including MCs16, and also in antigen-presenting cells17 and T helper 2 (Th2) lymphocytes18. On the other hand, LPGDS is present mainly in neuronal cells, but also in macrophages in pathologic situations15. PGD2 is produced in articular tissues of mice during the development of collagen-induced arthritis and plays an anti-inflammatory role, acting through the DP receptor19. CRTH2 deficiency was reported to exacerbate complete Freund’s adjuvant (CFA)-induced joint inflammation by attenuating infiltration of macrophages20. HPGDS-derived PGD2 was reported to ameliorate joint inflammation by attenuating vascular permeability and subsequent angiogenesis, and daily administration of a DP agonist attenuated CFA-induced hyperpermeability and angiogenesis21. A clinical study found high concentrations of PGD2 in the SF of patients with RA, and PGD2 and HPGDS expressions were inversely associated with serum C-reactive protein (P < 0.01)22. These findings suggest that PGD2 is an active agent in the resolution of inflammation in experimentally induced murine arthritis and in human RA.
In this study, we show that human synovial MCs from RA patients produced greater amounts of PGD2 via the PTGS2-miR-199a-3p axis compared with MCs from OA patients. Elevated PGD2 production in RA patients’ MCs might play an important role in the pathogenesis of RA.
Results
Comparison of gene expression profiles between synovial MCs from OA and RA patients
We first compared the gene expression profiles between synovium-derived, cultured MCs from OA patients (n = 3 donors) and those from RA patients (n = 3 donors) using DNA chips. A total of 419 genes among approximately 42,545 full-length genes and expressed sequence tags were significantly more than two fold higher in synovium-derived, cultured MCs from RA patients than in OA patients. Figure 1A shows a part of hierarchical clustering on the basis of those gene expression data. Upregulation of the cyclooxygenase and lipoxygenase pathways of arachidonic acid is thought to be involved in the development of rheumatic diseases, and targeting these pathways might lead to improved treatment strategies11. For that reason, from among those upregulated genes, we focused on prostanoid synthetases, including prostaglandin synthetase (PTGS)1, PTGS2, thromboxane synthetase 1 (TBXAS1) and leukotriene C4 synthetase (LTC4S) (Fig. 1A). DNA chip analysis showed that the normalized expression level of HPGDS was high compared with the other prostanoid synthetases and did not differ significantly between MCs from OA and RA patients (Fig. 1B). The expression levels of PTGES, PTGES2 and PTGIS were quite low. The expression levels of prostanoid receptors (Fig. 1C) and MC-related genes (Fig. 1D) did not appear to differ significantly between MCs from OA and RA patients. We confirmed the above DNA chip results using quantitative RT-PCR (Fig. 1E,F). The expression levels of mRNA for PTGS1, PTGS2 and TBXAS1 were significantly higher in RA patients’ MCs than in OA patients’ MCs (Fig. 1E). The expression levels of mRNA for LTC4S showed the tendency of being high in RA patients’ MCs compared with OA patients’ MCs. Furthermore, we confirmed those findings in synovium-derived, cultured MCs by using freshly isolated synovial MCs from OA and RA patients (Fig. 1F).
Comparison of gene expression profiles between synovial MCs from OA and RA patients. (A) A part of hierarchical clustering on the basis of the gene expression data in synovium-derived, cultured MCs obtained from three RA patients compared with MCs from three OA patients. Total RNA was isolated from the cultured MCs without any stimulus. Each row of colored bars represents one gene, and each column represents one donor. Colored bars show the magnitude of the response of each gene, according to the scale shown. Arrows indicate genes that are associated with enzymes involved in eicosanoid biosynthesis. (B–D) Comparison of gene expression levels of enzymes involved in eicosanoid biosynthesis (B), receptors for eicosanoid (C) and MC-related molecules (D) in synovium-derived, cultured MCs from three OA patients (open bars) and three RA patients (closed bars). Data are shown as normalized expression levels of DNA chip (mean ± SEM). (E) Comparison of gene expression levels of enzymes involved in eicosanoid biosynthesis in synovium-derived, cultured MCs obtained from OA (open bars, n = 5) and RA (closed bars, n = 8) patients. The data are shown as the ratio of the enzyme mRNA level to the GAPDH mRNA level (mean ± SEM). Significance was determined using the Mann–Whitney U test (*P < .05). (F) Comparison of gene expression levels of enzymes involved in eicosanoid biosynthesis in fleshly isolated, synovial MCs from OA (open bars, n = 3) and RA (closed bars, n = 3) patients.
Comparison of histamine release and eicosanoid synthesis by synovium-derived, cultured MCs from OA and RA patients following FcR γ-chain cross-linking
Histamine release from synovium-derived, cultured MCs following FcγRI and FcεRI aggregation did not differ significantly between OA and RA patients (Fig. 2A,E), while PGD2 production by the RA patients’ MCs was significantly higher than by the OA patients’ MCs (Fig. 2B,F). Following FcγRI aggregation, MCs from both OA and RA patients produced very small amounts of LTC4 and LTB4 (Fig. 2C,D). Following FcεRI aggregation, LTC4 synthesis in RA patients’ MCs was significantly higher than in OA patients’ MCs (Fig. 2G), while LTB4 synthesis was significantly lower in RA patients’ MCs than in OA patients’ MCs (Fig. 2H).
Comparison of histamine release (A,E) and PGD2 (B,F), LTC4 (C,G) and LTB4 synthesis (D,H) by synovium-derived, cultured MCs obtained from OA and RA patients following FcγRI aggregation (A–D) and FcεRI aggregation (E–H). Data are shown as % HR (histamine release) or pg/mL (/105 cells, mean ± SEM). Significance was determined by Sidak’s-Bonferroni test (*P < .05).
PGD2 induced IL-8 production by human group 2 innate lymphoid cells (ILC2s)
We investigated the pathogenic roles of PGD2-producing MCs in RA. We first investigated whether PGD2 induced IL-6, IL-8 and TNF-α production by human synovium-derived, cultured MCs, but found that it did not (data not shown). Group 2 innate lymphoid cells (ILC2s) were reported to be involved in the pathogenesis of RA23,24. MCs and ILC2s are located in close proximity in such tissues as the skin25 and lung26. ILC2s were found in the synovium of RA patients23,24. PGD2 induced production of Th2 cytokines such as IL-5 and IL-13 by ILC2s27. Thus, we investigated the effects of PGD2 on production of IL-6, IL-8, IL-9 and TNF-α by ILC2s. We found that PGD2 induced production of IL-8, but not IL-6 and IL-9, by ILC2s (Fig. 3A,B,D). TNF-α was spontaneously released by ILC2s and PGD2 did not enhance the production (Fig. 3C). This suggests that higher amounts of IL-8 produced by ILC2s in RA patients’ synovial tissues in response to IgG-mediated MC-induced PGD2 might cause neutrophil recruitment into the synovium of those patients.
PGD2 induced IL-8 production by human group 2 innate lymphoid cells (ILC2s). IL-8 (A), IL-6 (B), TNF-α (C) and IL-9 production (D) by ILC2s in response to PGD2. The results are the mean ± SEM for three different donors. Significance was determined by Sidak’s-Bonferroni test (*P < .05, **P < .01, and ****P < .0001). N.D. means not detected (below the level of detection).
Effects of co-culture of OA patients’ synovial MCs with RA patients’ synovial fibroblasts on PTGS1, PTGS2, TBXAS1 and LTC4S expression in OA patients’ MCs
The SCF–Kit system alone is insufficient to fully drive the maturation of MCs, since culture of immature MCs with fibroblasts, but not with SCF alone, can induce differentiation into mature MCs28. We reported that group III phospholipase A2 secreted from MCs couples with fibroblastic LPGDS to form PGD2, which facilitates MC maturation via PGD2 receptor DP29. Thus, we hypothesized that RA patients’ fibroblasts might influence the phenotypes of MCs. We first compared the expression levels of mRNAs for PTGS1, PTGS2, TBXAS1 and LTC4S between the RA patients’ and OA patients’ synovial fibroblasts. No significant differences were found (Fig. 4A). Next, synovium-derived, cultured MCs from OA patients were cultured with and without RA patients’ fibroblasts for 96 h. The MCs were then collected, and the expression levels of mRNAs for PTGS1, PTGS2, TBXAS1 and LTC4S were compared between the MCs cultured with and without the fibroblasts. No differences in the expression levels were found (Fig. 4B).
Effects of co-culture of OA patients’ synovial MCs with RA patients’ synovial fibroblasts on expression levels of mRNA for PTGS1, PTGS2, TBXAS1 and LTC4S in the OA patients’ MCs. (A) Comparison of expression levels of mRNA for PTGS1, PTGS2, TBXAS1 and LTC4S in fibroblasts obtained from OA (open bars, n = 4) and RA (closed bars, n = 4) patients. The data are shown as the ratio of the enzyme mRNA level to the β-actin mRNA level (× 10–4, mean ± SEM). (B) Effects of co-culture of OA patients’ synovium-derived, cultured MCs (n = 5) with RA patients’ fibroblasts (n = 2) on expression levels of mRNA for PTGS1, PTGS2, TBXAS1 and LTC4S in the OA patients’ MCs. The data are shown as the ratio of the enzyme mRNA level in the MCs alone to that in the MCs after co-culture with the RA patients’ fibroblasts (mean ± SEM).
Relationship between expression levels of miR-199a-3p and PTGS2 mRNA in synovium-derived, cultured MCs from OA and RA patients
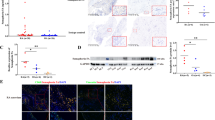
micro RNAs (miRNAs) are noncoding RNAs implicated in the regulation of gene expression underlying many relevant physiological processes, including cell activation. We investigated one mechanism causing different mRNA expression levels for prostanoid synthetases between RA and OA patients’ synovial MCs. That is, we compared miRNA expression profiles using microRNA array. Thirty miRNAs showed expression levels that were significantly more than three times higher in OA patients’ MCs compared with RA patients’ MCs (Table 1). Among those 30 miRNAs, miR-199a-3p was reported to down-regulate PTGS2 mRNA expression in OA chondrocytes30,31, cultured human fetal lung epithelial cells32, cultured human myometrial cells33 and human endometrial surface epithelial cells34. Thus, we performed quantitative RT–PCR to compare the miR-199a-3p expression levels between synovial MCs from RA and OA patients. The results showed that the miR-199a-3p expression level was significantly higher in the OA patients than in the RA patients (P < 0.05, Fig. 5A). Moreover, that expression level correlated inversely with the PTGS2 mRNA expression level in the RA patients’ MCs (r = − 0.698, P = 0.010; Fig. 5C), but not in the OA patients’ MCs (Fig. 5B). Tables 2 and 3 shows the characteristics of the patients with OA and RA, respectively.
Relationship between miR-199a-3p expression levels and PTGS2 mRNA expression levels in synovium-derived, cultured MCs from RA and OA patients. (A) Comparison of miR-199a-3p expression levels in synovium-derived, cultured MCs obtained from OA (n = 24) and RA (n = 12) patients. The data are shown as the ratio of the miR-199a-3p level to the RNU48 level (× 10–3, mean ± SEM). Significance was determined using the Mann–Whitney U test. (B,C) Plots of expression level of miR-199a-3p/RNU48 vs. expression level of PTGS2/GAPDH in synovium-derived, cultured MCs obtained from OA (n = 24, B) and RA (n = 12, C) patients. Each point represents one donor. (D) Illustration of procedure for transduction of miRNA mimic to synovium-derived, cultured MCs obtained from RA patients and activation of the cells (E). Expression levels of miR-199a-3p mRNA in control miRNA mimic-transduced (Ctrl) and miR-199a-3p mimic-transduced synovium-derived, cultured MCs obtained from RA patients (miR-199a-3p). The expression level of miR-199a-3p in control miRNA mimic-transduced cells was designated as 1. The results are the mean ± SEM for three different experiments. (F) Expression levels of PTGS2 mRNA in control miRNA mimic-transduced (Ctrl), and miR199a-3p mimic-transduced synovium-derived, cultured MCs obtained from RA patients (miR-199a-3p) in response to 10 ng/mL of TNF-α for 2 h. The expression level of PTGS2 mRNA in control miRNA mimic-transduced cells was designated as 1. The results are the mean ± SEM for three different experiments. *indicates P < .05.
To analyze the effect of miR-199a-3p on PTGS2 expression, we overexpressed miR-199a-3p in synovium-derived, cultured MCs obtained from RA patients by a transduction of miRNA mimics. Figure 5D depicts the method for transduction of miRNA mimic to synovium-derived, cultured MCs obtained from RA patients and activation of the cells. Briefly, miR199a-3p or control miRNA mimics were transduced to the cells, and then the cells were activated with TNF-α. In Fig. 5E, the miR199a-3p expression level was significantly increased in synovium-derived, cultured MCs obtained from RA patients transduced with an miR199a-3p mimic compared with control miRNA mimic (P < 0.05, Fig. 5E). We stimulated these MCs with TNF-α (10 ng/mL) for 2 h and analyzed PTGS2 expression in control miRNA mimic–transduced and miR199a-3p mimic–transduced cells. The PTGS2 expression in miR199a-3p mimic-transduced cells was significantly lower than in control miRNA mimic-transduced cells after 2 h of stimulation with TNF-α (P < 0.05, Fig. 5F).
Comparison of PGD2 and PGE2 concentrations in SF obtained from OA and RA patients
We performed EIA to measure the PGD2 and PGE2 concentrations in SF obtained from OA (n = 14) and RA (n = 10) patients. Tables 2 and 3 show the characteristics of the patients. The concentration of PGD2 (Fig. 6A), but not PGE2 (Fig. 6B), was significantly higher in the RA patients’ SF than in the OA patients’ SF.
Discussion
We present the first evidence that synovial MCs from RA and OA patients have different phenotypes and function. FcRγ aggregation induced significantly higher PGD2 production by the synovium-derived, cultured MCs from RA patients via miR-199a-3p/PTGS2 axis compared with the OA patients’ MCs.
Since the LTC4S expression level showed the tendency of being higher in the RA patients’ MCs than in the OA patients’ MCs (Fig. 1E), LTC4 production by the RA patients’ MCs following FcεRI aggregation was significantly higher (Fig. 2G). In contrast, LTB4 production by the RA patients’ MCs following FcεRI aggregation was significantly lower than that by the OA patients’ MCs (Fig. 2H). Since LTB4 and LTC4 are metabolites of LTA4, LTB4 synthesis in RA patients’ MCs may be relatively lower. LTB4 and LTC4 by MCs from both RA and OA patients after FcγRI aggregation was markedly lower than after FcεRI aggregation (Fig. 2C,D,G,H). FcεRI consists of heterotetramer αβγ2-chains. On the other hand, FcγRI consists of heterotrimer αγ2-chains. Since FcεRI β-chain reportedly amplified LT production by mouse MCs35, FcγRI aggregation might cause no or lesser amounts of LT production by human MCs as well. Thus, immune complexes might induce large amounts of only PGD2 among the arachidonic acid metabolites in RA patients’ synovial MCs.
We previously reported that co-culture of IL-3-dependent mouse bone marrow-derived MCs with mouse fibroblasts resulted in 1.4-fold and 387-fold up-regulation of PTGS1 and PTGS2 mRNA expression, respectively, in the MCs29. Thus, we hypothesized that fibroblasts from RA patients might influence PTGS1 and PTGS2 mRNA expression in OA patients’ MCs, but our co-culture experiment did not find that to be true. The reason is not clear, but it might be because IL-3-dependent mouse bone marrow-derived MCs are immature MCs, whereas human synovium-derived, cultured MCs are fully mature MCs7.
All of our OA patients and some RA patients had been treated with NSAIDs. NSAIDs inhibit PTGS enzymes36. However, NSAIDs do not affect PTGS1 and PTGS2 mRNA expression. Synovium-derived, cultured MCs from both OA and RA patients had been cultured under the same conditions for more than 12 weeks. Therefore, it would be difficult to conclude that NSAID treatment of patients might affect the gene expression profiles of the cultured MCs derived from synovia of OA and RA patients. We thus hypothesized that epigenetic modification might be the cause of the difference in the gene expression profiles of MCs derived from synovia of OA and RA patients.
Microarray-based screening found that the expression levels of thirty miRNAs were significantly more than three times higher in OA patients’ MCs than in RA patients’ MCs (Table 1). Among those 30 miRNAs, miR-199a-3p is a direct regulator of PTGS2 expression in OA chondrocytes30,31, cultured human fetal lung epithelial cells32, cultured human myometrial cells33 and human endometrial surface epithelial cells34. The miR-199a-3p/PTGS2 axis plays different roles in different cells and diseases. In human OA chondrocytes, miR-199a-3p directly suppressed the luciferase activity of a PGST2 3′UTR reporter construct and inhibited IL-β-induced PTGS2 protein, suggesting that miR-199a-3p may be an important regulator of human cartilage hemostasis and a new target for OA therapy30. In fact, epigallocatechin-3-O-gallate, the most abundant and active polyphenol in green tea, which has been reported to have anti-arthritic effects, inhibited PTGS2 mRNA/protein expression and PGE2 production by up-regulating miR-199a-3p expression in IL-1β-stimulated human OA chondrocytes31. The developmental decline in miR-199a/miR-214 expression in the fetal lung led to increased expression of critical targets, including PTGS2, NF-κB p50/p65, CREB1 and C/EBPβ that enhance surfactant protein-A expression and alveolar type II cell differentiation32. The levels of the clustered miRNAs, i.e., miR-199a-3p and miR-214, were significantly decreased in the myometrium of pregnant mice and humans, whereas the miR-199a-3p/miR-214 target, PTGS2, which induced synthesis of contracting PGs, was coordinately increased33,37. Overexpression of miR-199a-3p and miR-214 in cultured human myometrial cells inhibited PTGS2 protein and blocked TNF-α-induced myometrial cell contractility, suggesting their physiological relevance33. Epithelial sodium channel-dependent CREB activation led to suppression of miR-199a-3p and miR101, which in turn augmented PTGS2 up-regulation during embryo implantation34. We found that miR-199a-3p correlated inversely with PTGS2 expression in RA patients’ MCs (r = − 0.698, P = 0.010; Fig. 5C), suggesting that miR-199a-3p may be a regulator of PTGS2 mRNA expression in RA patients’ synovial MCs. Furthermore, we confirmed that PTGS2 mRNA expression by miR199a-3p mimic-transduced synovium-derived, cultured MCs obtained from RA patients was significantly lower than that by control miRNA mimic-transduced cells, when the cells were activated with TNF-α (P < 0.05, Fig. 5F). The 3′ UTR of the mTOR gene is reportedly targeted by miR-498, which was included in the list of thirty genes. Consequently, silencing of mTOR reduced PTGS2 expression in OA chondrocytes38. Thus, other miRNAs might also affect PTGS2 mRNA expression in RA patients’ synovial MCs.
PGD2 was reported to be detected in SF obtained from RA patients11,22,39. PGD2 is synthesized by various cells, including MCs16, antigen-presenting cells17, T helper 2 (Th2) lymphocytes18 and synovial fibroblasts40. The cell sources of PGD2 in RA patients’ SF have not been identified. In our study, OA and RA patients’ fibroblasts showed no significant differences in expression of PTGS1 and PTGS2 mRNAs. Thus, increased PGD2 synthesis would not be due to production by synovial fibroblasts. Our in vitro study found that synovial MCs (105 cells) from RA patients produced 4000 pg/mL PGD2 following FcγRI aggregation. Furthermore, PGD2 metabolites have been reported to be biomarkers of in vivo MC activation in RA patients41. Therefore, MCs might be one cell source of PGD2 in RA patients’ SF.
Upregulation of the PTGS1 and PTGS2 pathways of arachidonic acid (AA) is thought to be involved in the development of rheumatic diseases, and targeting these pathways might lead to improved treatment strategies11. Thus, to clarify the quantitative and qualitative changes in lipid mediators in the synovium of severe RA patients, we recently compared the profiles of lipid mediators in SF obtained from RA and OA patients using liquid chromatography-tandem mass spectrometry/mass spectrometry42. The concentrations (levels based on the area-under-the-curve/mL) of the majority of PTGS-1/2 products of AA appeared to be higher in SF from RA patients compared with OA patients. We determined the absolute concentrations (pmol/mL) of representative eicosanoids, including 6-keto PGF1α (a stable metabolite of PGI2), PGF2α, PGE2, PGD2 and 12-hydroxyheptadecatrienoic acid (HHT), in the SF from RA and OA patients. The PGF2α and PGE2 concentrations were significantly higher in the RA patients’ SF. Thus, although the PGD2 concentration in the SF did not differ significantly between the RA and OA patients in our previous study42, we confirmed our earlier findings regarding upregulation of the PTGS pathways in RA compared with in OA42.
This study has a number of limitations. First, all the samples used in this study were obtained from hospitalized patients who had undergone total knee replacement surgery. It was reported that RA patients in remission had significantly reduced synovial MC density compared with patients with clinically active RA43. Thus, the enrollees were limited to patients with severe clinical disease, hence limiting extrapolation of the findings to patients with milder clinical disease. Second, we cannot rule out the possibility that use of a wide range of antirheumatic drugs-including methotrexate, oral glucocorticoids, anti-TNF-α therapy and anti-IL-6 therapy that potently suppress specific inflammation, might be responsible for the differences in gene expression profiles in OA and RA patients’ MCs. However, the RA patients’ anti-TNF-α therapy and anti-IL-6 therapy had been discontinued 2–4 weeks before the total knee arthroplasty. Since expression of PTGS2 mRNA and protein is reportedly enhanced in various human cell types by such inflammatory cytokines as IL-1β and TNF-α44, anti-TNF-α therapy and anti-IL-6 therapy would not enhance PTGS2 mRNA expression levels.
PGD2 was reported to be active in the resolution of inflammation in experimentally induced arthritis in mice19,21 and in human RA22. Increased PGD2 may trigger an anti-inflammatory/pro-resolution cascade. That is because it spontaneously undergoes non-enzymatic dehydration and is converted into 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2). 15d-PGJ2 is a cyclopentenone PG that has been shown to be immuno-modulatory and anti-inflammatory due to its ability to inhibit NFκB signaling and cytokine release and to act as an agonist of PPARγ45. However, the role of MC-derived PGD2 in the pathogenesis of RA should be determined in MC-deficient experimental arthritis in mice reconstituted with bone marrow-derived MCs obtained from HPGDS-deficient mice. NSAIDs’ inhibition of cyclooxygenase-dependent PG synthesis ameliorates RA manifestation because they inhibit mainly production of PGE2, which exacerbates synovial inflammation in RA patients46.
Since ILC2s were reportedly involved in the pathogenesis of RA23,24, we investigated the effect of PGD2 on RA-related cytokine production (IL-6, IL-8, IL-9 and TNF-α) by ILC2s. We found that PGD2 induced IL-8 production by ILC2s, suggesting that PGD2-producing MCs induce neutrophil recruitment into the synovium of RA patients.
In conclusion, we demonstrated that human synovial MCs might regulate inflammation through hyper-production of PGD2 in RA following FcRγ aggregation. These findings indicate that human MCs show functional heterogeneity among diseases.
Materials and methods
Patient enrollment and processing of SF
We enrolled RA and OA patients. The diagnosis of each patient was established by the treating doctor. SF and synovial tissue samples were obtained during total knee arthroplasty performed at the Department of Orthopeadic Surgery, Nihon University, after receiving informed consent. Two milliliters of SF were treated with hyaluronidase, followed by centrifugation at 860×g for 10 min. The supernatants were collected, and the tubes were filled with N2 gas. The samples were then frozen at − 80 °C.
Reagents
Human IgE was purchased from Calbiochem (San Diego, CA). Anti-FcεRIα monoclonal antibodies (mAbs) (clone AER-37) and anti-Kit mAb (clone YB5.B8) were purchased from Biolegend (San Diego, CA) and BD Biosciences (Franklin Lakes, NJ, USA), respectively.
Purification of dispersed synovial MCs and generation of synovium-derived, cultured MCs
Human synovial MCs were purified with anti-FcεRIα and anti-Kit mAbs using FACS Aria IIu (BD Biosciences). The purities of MCs were > 99%. Human synovium-derived cultured MCs were generated as described previously8. Briefly, fresh samples of synovial tissues were obtained after total knee arthroplasty at Nihon University, after obtaining informed consent. Treatment of RA patients with anti-TNF-α therapy and anti-IL-6 therapy had been discontinued 2–4 weeks before total knee arthroplasty, in accordance with the Japanese Guidelines for the use of Infliximab and Etanercept in RA47. Briefly, synovial cells were enzymatically dispersed and centrifuged using a density-gradient consisting of 22.5% HistoDenz solution (Sigma-Aldrich; St. Louis, MO, USA) and lymphocyte separation medium (LSM; MP Biomedicals; Santa Ana, CA, USA). Cells at the LSM interface and in the pellet fraction were collected and washed. The cells were then cultured in serum-free Iscove’s methylcellulose medium (Stem Cell Technologies Inc.; Vancouver, BC, Canada) and Iscove’s Modified Dulbecco’s Medium (IMDM; Thermo Fisher Scientific; Waltham, MA, USA) supplemented with 200 ng/mL recombinant human stem cell factor (rhSCF) (PeproTech; Rocky Hill, NJ, USA) and 50 ng/mL rhIL-6 (PeproTech). On day 42, methylcellulose was dissolved in PBS, and the cells were resuspended and cultured in IMDM containing 0.1% BSA, 100 ng/mL rhSCF and 50 ng/mL rhIL-6 (designated as MC medium).
Synovial fibroblasts
Fresh samples of synovial tissues were obtained after total knee arthroplasty at Nihon University, after obtaining informed consent. Synovial fibroblasts were obtained after culturing enzymatically-dispersed synovial cells7.
Isolation and expansion of human group 2 innate lymphoid cells (ILC2s)
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood of healthy volunteers by centrifugation using LSM. Lineage-negative cells were enriched from the isolated PBMCs using magnetic-activated cell sorting (MACS) and Microbeads (Miltenyi Biotec; GladBach, Germany) in accordance with the manufacturer’s protocol, with some modification. Briefly, 5 × 107 to 1 × 108 cells were suspended in 200 μL of cold MACS buffer (PBS containing 2% fetal bovine serum [FBS; Thermo Fisher Scientific] and 2 mM EDTA [Eastman Kodak Company; Rochester, NY, USA]) and incubated for 30 min at 4 °C with 50 μL of FcR blocking reagent (Miltenyi Biotec) and 25 μL of anti-human CD3, CD4, CD8, CD11b, CD14, CD16 and CD19 Microbeads (Miltenyi Biotec). The lineage-negative cells were washed with MACS buffer, centrifuged at 490×g for 5 min, resuspended in 500 μL of cold MACS buffer and applied to a MACS CS Column (Miltenyi Biotec) on a VarioMACS Separator (Miltenyi Biotec). The flow-through was collected as the lineage-negative fraction. The column was then washed 3 times with cold MACS buffer. The collected cells were treated for 10 min at room temperature with a Zombie NIR Fixable Viability Kit (Biolegend; San Diego, CA, USA) to label dead cells. The treated cells were then washed with PBS and incubated with Human TruStain FcX (Biolegend) for 15 min at room temperature to block non-specific binding. After washing with MACS buffer, the cells were stained for 30 min at 4 °C with FITC-Lineage Cocktail-1 (BD Biosciences; San Jose, CA, USA), PerCP-Cy5.5-anti-human CD45 mAb (clone HI30; BD Biosciences), PE-anti-human CD161 mAb (clone HP-3G10; Biolegend) and Brilliant Violet 421-conjugated anti-human CRTh2 mAb (clone BM16; BD Biosciences). Lin- CD45+ CD161+ CRTh2+ cells were isolated with FACSAria IIu (BD Biosciences). The sorted cells (designated as ILC2s; 2000 cells per well) were cultured in the presence of mitomycin C-treated PBMCs (2 × 105 cells per well) as feeder cells in 96-well U-bottom plates (CORNING; Corning, NY, USA) in Yssel’s medium (IMDM supplemented with 1% heat-inactivated human AB serum; Access Biologicals; Vista, CA, USA) and 100 IU/mL Teceleukin (rhIL-2; Kyowa Pharmaceutical Industry; Osaka, Japan). After 3 weeks of in vitro expansion the ILC2 number reached approximately 5 × 106 to 1 × 107 cells, which were used in subsequent experiments. ILC2s were stimulated with PGD2 (0–100 nM) for 24 h and the cytokine levels in the cell supernatants were measured by enzyme-linked immunosorbent assays (ELISAs).
RNA isolation and RT-PCR
Total RNA was isolated from the synovium-derived, cultured MCs by using a miRNeasy Micro kit (Qiagen, Hilden, Germany) and then quantified using NanoDrop ND-1000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. Real-time quantitative RT-PCR was performed as described previously8. Human gene-specific primers and probe sets for PTGS1, PTGS2, PTGES, LTC4S, LTC4S, TBXAS1, HPGDS, GAPDH, miR-199a-3p and RNU48 were designed using the Assay-on-Demand service (Thermo Fisher Scientific).
Microarray experiments and data analysis
Synovium-derived, cultured MCs from 3 RA patients and 3 OA patients were screened for mRNAs and miRNAs using Agilent SurePrint G3 Human GE 8 × 60 k Microarray Kit and Agilent SurePrint G3 Human microRNA (miRNA) 8 × 60 k Microarray Kit (Agilent Technologies, Santa Clara, CA), respectively. One × 104 MCs were used in the microarray experiments. Data analysis was performed with GeneSpring12.5 (Agilent Technologies; Santa Clara, CA). To adjust for variation in the staining intensity between microarrays, the expression levels of all the genes on a given microarray chip were normalized by dividing the measurement by the 75th percentile of all measurements on that microarray. Quality control was achieved with the Filter Probe sets by Flags tool and, as result, 32,425 of 42,545 probes (mRNA) and 405 of 1368 probes (miRNA) were used for further analyses. mRNAs or miRNAs showing significant signal intensity (> 2.0-fold, adjusted P < 0.05; Mann–Whitney U-test with Benjamini–Hochberg false discovery rate correction) were determined to be differentially expressed (up- or down-regulated). The false discovery rate is a comparison of the number of times that the real data has a certain P value versus the number of times that randomized data has the same or better P value. The false discovery rate addresses the multiple comparisons problem that occurs when calculating P values for hundreds or thousands of categories, and protects against over-interpreting P values that do not have biological meaning48. Hierarchical clustering was performed on the basis of the gene expression data. Of the differentially expressed miRNAs, target miRNAs that interact with target genes were determined by a search of the literature. To further investigate the global molecular network, especially to identify upstream cytokines in pathological signaling cascades, the target genes were imported into IPA (version 27821452)49.
Ethical approval
This study was approved by the Ethics Committee of the Nihon University School of Medicine (RK-160112-2) and the Ethics Committee of National Research Center for Child Health and Development (approval number: 476). All the subjects provided written informed consent in accordance with the Helsinki Declaration of the World Medical Association.
Transduction of miRNA mimics
MISSION microRNA Mimics Negative Control 2 (HMC0003) and mimics for miR199a-3p (HMI0340) were purchased from Sigma-Aldrich. These small RNAs were transduced into synovium-derived, cultured MCs obtained from RA patients using MISSION siRNA Transfection Reagent (Sigma-Aldrich) and Nucleic Acid Transfection Enhancer (NATE, InvivoGen, San Diego, CA, USA) in accordance with the manufacturer’s instructions. Four hours later, the cells were washed with PBS to remove excess complexes, and the transduced cells were cultured with MC medium. Synovium-derived, cultured MCs obtained from RA patients transduced with control miRNA mimic or miR199a-3p mimic were activated with recombinant human TNF-α (R&D Systems, Minneapolis, MN, USA). Then the expression level of PTGS2 in these cells was analyzed by quantitative RT-PCR.
MC activation
For aggregation of FcεRI, MCs were sensitized with 0.5 μg/ml human IgE (Merck Millipore, Burlington, MA, USA) for 30 min at 37 °C. The cells were then washed once and resuspended in Hepes buffer or MC medium. The IgE-sensitized MCs were stimulated with polyclonal rabbit anti-human IgE (Agilent) for 30 min for the histamine and lipid mediator assays (2 × 103 MCs/100 μl). For aggregation of FcγRI, the MCs were incubated with 1 or 10 μg/ml of F(ab′)2 fragments of anti-human FcγRI (F(ab′)2αFcγRI clone 10.1), or mouse F(ab′)2 fragments of IgG1 (F(ab′)2mIgG1; Jackson ImmunoResearch Laboratories) for 30 min at 37 °C. The cells were washed once and resuspended in Hepes buffer or MC medium. FcγRI was cross-linked by incubating the MCs with the indicated concentrations of goat F(ab’)2 fragments of anti-mouse F(ab′)2 fragments of IgG (gF(ab′)2αmF(ab′)2; Jackson ImmunoResearch Laboratories) for 30 min for the histamine and lipid mediator assays (2 × 103 MCs/100 μL). Independent experiments were performed using MCs from different donors.
Mediator assays
Histamine was measured using an enzyme immunoassay (EIA) kit (Bertin Pharma; Montigny le Bretonneux, France). PGD2 was measured using a Prostaglandin D2-MOX EIA kit (Cayman Chemical Company; Ann Arbor, MI). LTC4, LTB4 and PGE2 were measured using EIA kits (R&D Systems; Minneapolis, MN). IL-6, IL-8 and TNF-α were also measured using ELISA kits (Biolegend). IL-9 was measured using a Human IL-9 Uncoated ELISA kit (Thermo Fisher Scientific). The percent histamine release was calculated as follows: (histamine released/total histamine content of unstimulated MCs) × 100%.
Co-culture of synovial MCs and fibroblasts
Synovial fibroblasts from RA patients were cultured in IMDM supplemented with 2% FBS for 48 h, and the fibroblasts were grown to confluence. Human synovium–derived, cultured MCs from OA patients were overlaid on the fibroblasts and cultured in IMDM containing 0.1% BSA, 100 ng/mL rhSCF and 50 ng/mL rhIL-6 for 96 h. The MCs were then purified with anti-FcεRIα and anti-Kit mAbs using FACS Aria IIu (BD Biosciences).
Statistical analysis
To evaluate the quantitative variables, the Mann–Whitney U test was used because of nonparametric distribution of the data. Figures 2 and 3 were analyzed by Sidak’s–Bonferroni method. Spearman rank correlation coefficients were calculated to determine the strength of correlations between continuous variables. P values were considered significant at P < 0.05.
References
Crisp, A. J., Chapman, C. M., Kirkham, S. E., Schiller, A. L. & Krane, S. M. Articular mastocytosis in rheumatoid arthritis. Arthritis Rheum. 27, 845–851 (1984).
Malone, D. G., Irani, A. M., Schwartz, L. B., Barrett, K. E. & Metcalfe, D. D. Mast cell numbers and histamine levels in synovial fluids from patients with diverse arthritides. Arthritis Rheum. 29, 956–963 (1986).
Tetlow, L. C. & Woolley, D. E. Mast cells, cytokines, and metalloproteinases at the rheumatoid lesion: Dual immunolocalisation studies. Ann. Rheum. Dis. 54, 896–903 (1995).
Tetlow, L. C. & Woolley, D. E. Distribution, activation and tryptase/chymase phenotype of mast cells in the rheumatoid lesion. Ann. Rheum. Dis. 54, 549–555 (1995).
Eklund, K. K. Mast cells in the pathogenesis of rheumatic diseases and as potential targets for anti-rheumatic therapy. Immunol. Rev. 217, 38–52. https://doi.org/10.1111/j.1600-065X.2007.00504.x (2007).
Nigrovic, P. A. et al. Mast cells contribute to initiation of autoantibody-mediated arthritis via IL-1. Proc. Natl. Acad. Sci. USA 104, 2325–2330 (2007).
Lee, H. et al. Activation of human synovial mast cells from rheumatoid arthritis or osteoarthritis patients in response to aggregated IgG through Fcgamma receptor I and Fcgamma receptor II. Arthrit. Rheum. 65, 109–119. https://doi.org/10.1002/art.37741 (2013).
Kashiwakura, J. et al. Interleukin-33 synergistically enhances immune complex-induced tumor necrosis factor alpha and interleukin-8 production in cultured human synovium-derived mast cells. Int. Arch. Allergy Immunol. 161(Suppl 2), 32–36. https://doi.org/10.1159/000350424 (2013).
Rivellese, F. et al. Ability of interleukin-33- and immune complex-triggered activation of human mast cells to down-regulate monocyte-mediated immune responses. Arthrit. Rheumatol. 67, 2343–2353. https://doi.org/10.1002/art.39192 (2015).
Rossini, M. et al. Serum levels of tryptase suggest that mast cells might have an antiinflammatory role in rheumatoid arthritis: Comment on the article by Rivellese et al.. Arthrit. Rheumatol. 68, 769. https://doi.org/10.1002/art.39506 (2016).
Korotkova, M. & Jakobsson, P. J. Persisting eicosanoid pathways in rheumatic diseases. Nat. Rev. Rheumatol. 10, 229–241. https://doi.org/10.1038/nrrheum.2014.1 (2014).
Brouwers, H., von Hegedus, J., Toes, R., Kloppenburg, M. & Ioan-Facsinay, A. Lipid mediators of inflammation in rheumatoid arthritis and osteoarthritis. Best Pract. Res. Clin. Rheumatol. 29, 741–755. https://doi.org/10.1016/j.berh.2016.02.003 (2015).
McInnes, I. B. & Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219. https://doi.org/10.1056/NEJMra1004965 (2011).
Chen, M. et al. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J. Exp. Med. 203, 837–842. https://doi.org/10.1084/jem.20052371 (2006).
Murata, T. & Maehara, T. Discovery of anti-inflammatory role of prostaglandin D2. J. Vet. Med. Sci. 78, 1643–1647. https://doi.org/10.1292/jvms.16-0347 (2016).
Urade, Y. et al. Mast cells contain spleen-type prostaglandin D synthetase. J. Biol. Chem. 265, 371–375 (1990).
Urade, Y., Ujihara, M., Horiguchi, Y., Ikai, K. & Hayaishi, O. The major source of endogenous prostaglandin D2 production is likely antigen-presenting cells. Localization of glutathione-requiring prostaglandin D synthetase in histiocytes, dendritic, and Kupffer cells in various rat tissues. J. Immunol. 143, 2982–2989 (1989).
Tanaka, K. et al. Cutting edge: Differential production of prostaglandin D2 by human helper T cell subsets. J. Immunol. 164, 2277–2280. https://doi.org/10.4049/jimmunol.164.5.2277 (2000).
Maicas, N., Ibanez, L., Alcaraz, M. J., Ubeda, A. & Ferrandiz, M. L. Prostaglandin D2 regulates joint inflammation and destruction in murine collagen-induced arthritis. Arthrit. Rheum. 64, 130–140. https://doi.org/10.1002/art.30656 (2012).
Tsubosaka, Y. et al. A deficiency in the prostaglandin D2 receptor CRTH2 exacerbates adjuvant-induced joint inflammation. J. Immunol. 193, 5835–5840. https://doi.org/10.4049/jimmunol.1303478 (2014).
Tsubosaka, Y. et al. Hematopoietic prostaglandin D synthase-derived prostaglandin D2 ameliorates adjuvant-induced joint inflammation in mice. FASEB J. 33, 6829–6837. https://doi.org/10.1096/fj.201802153R (2019).
Moghaddami, M., Ranieri, E., James, M., Fletcher, J. & Cleland, L. G. Prostaglandin D(2) in inflammatory arthritis and its relation with synovial fluid dendritic cells. Mediat. Inflamm. 2013, 329494. https://doi.org/10.1155/2013/329494 (2013).
Rauber, S. et al. Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nat. Med. 23, 938–944. https://doi.org/10.1038/nm.4373 (2017).
Omata, Y. et al. Group 2 innate lymphoid cells attenuate inflammatory arthritis and protect from bone destruction in mice. Cell Rep. 24, 169–180. https://doi.org/10.1016/j.celrep.2018.06.005 (2018).
Roediger, B. et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat. Immunol. 14, 564–573. https://doi.org/10.1038/ni.2584 (2013).
Barnig, C. et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci. Transl. Med. 5, 174ra126. https://doi.org/10.1126/scitranslmed.3004812 (2013).
Xue, L. et al. Prostaglandin D2 activates group 2 innate lymphoid cells through chemoattractant receptor-homologous molecule expressed on TH2 cells. J. Allergy Clin. Immunol. 133, 1184–1194. https://doi.org/10.1016/j.jaci.2013.10.056 (2014).
Gurish, M. F. & Austen, K. F. Developmental origin and functional specialization of mast cell subsets. Immunity 37, 25–33. https://doi.org/10.1016/j.immuni.2012.07.003 (2012).
Taketomi, Y. et al. Mast cell maturation is driven via a group III phospholipase A2-prostaglandin D2-DP1 receptor paracrine axis. Nat. Immunol. 14, 554–563. https://doi.org/10.1038/ni.2586 (2013).
Akhtar, N. & Haqqi, T. M. MicroRNA-199a* regulates the expression of cyclooxygenase-2 in human chondrocytes. Ann. Rheum. Dis. 71, 1073–1080. https://doi.org/10.1136/annrheumdis-2011-200519 (2012).
Rasheed, Z., Rasheed, N. & Al-Shobaili, H. A. Epigallocatechin-3-O-gallate up-regulates microRNA-199a-3p expression by down-regulating the expression of cyclooxygenase-2 in stimulated human osteoarthritis chondrocytes. J. Cell Mol. Med. 20, 2241–2248. https://doi.org/10.1111/jcmm.12897 (2016).
Mishra, R., Benlhabib, H., Guo, W., Lerma Cervantes, C. B. & Mendelson, C. R. Developmental decline in the microRNA 199a (miR-199a)/miR-214 cluster in human fetal lung promotes type II cell differentiation by upregulating key transcription factors. Mol. Cell. Biol. https://doi.org/10.1128/MCB.00037-18 (2018).
Williams, K. C., Renthal, N. E., Gerard, R. D. & Mendelson, C. R. The microRNA (miR)-199a/214 cluster mediates opposing effects of progesterone and estrogen on uterine contractility during pregnancy and labor. Mol. Endocrinol. 26, 1857–1867. https://doi.org/10.1210/me.2012-1199 (2012).
Sun, X. et al. Regulation of miR-101/miR-199a-3p by the epithelial sodium channel during embryo implantation: Involvement of CREB phosphorylation. Reproduction 148, 559–568. https://doi.org/10.1530/REP-14-0386 (2014).
Nunomura, S., Kawakami, Y., Kawakami, T. & Ra, C. The FcRbeta- and gamma-ITAMs play crucial but distinct roles in the full activation of mast cells induced by IgEkappa and Protein L. J. Immunol. 188, 4052–4064. https://doi.org/10.4049/jimmunol.1102796 (2012).
Ishiguro, H. & Kawahara, T. Nonsteroidal anti-inflammatory drugs and prostatic diseases. Biomed. Res. Int. 2014, 436123. https://doi.org/10.1155/2014/436123 (2014).
Renthal, N. E., Williams, K. C. & Mendelson, C. R. MicroRNAs-mediators of myometrial contractility during pregnancy and labour. Nat. Rev. Endocrinol. 9, 391–401. https://doi.org/10.1038/nrendo.2013.96 (2013).
Li, G. et al. circFADS2 protects LPS-treated chondrocytes from apoptosis acting as an interceptor of miR-498/mTOR cross-talking. Aging 11, 3348–3361. https://doi.org/10.18632/aging.101986 (2019).
Egg, D. Concentrations of prostaglandins D2, E2, F2 alpha, 6-keto-F1 alpha and thromboxane B2 in synovial fluid from patients with inflammatory joint disorders and osteoarthritis. Z. Rheumatol. 43, 89–96 (1984).
Frederick, E. D. et al. The low molecular weight fraction of human serum albumin upregulates COX2, prostaglandin E2, and prostaglandin D2 under inflammatory conditions in osteoarthritic knee synovial fibroblasts. Biochem. Biophys. Rep. 8, 68–74. https://doi.org/10.1016/j.bbrep.2016.08.015 (2016).
Cho, C., Nguyen, A., Bryant, K. J., O’Neill, S. G. & McNeil, H. P. Prostaglandin D2 metabolites as a biomarker of in vivo mast cell activation in systemic mastocytosis and rheumatoid arthritis. Immunity Inflamm. Dis. 4, 64–69. https://doi.org/10.1002/iid3.94 (2016).
Sano, Y. et al. Activation of inflammation and resolution pathways of lipid mediators in synovial fluid from patients with severe rheumatoid arthritis compared with severe osteoarthritis. Asia Pac. Allergy 10, e21. https://doi.org/10.5415/apallergy.2020.10.e21 (2020).
Ramirez, J. et al. Immunopathologic characterization of ultrasound-defined synovitis in rheumatoid arthritis patients in clinical remission. Arthrit. Res. Ther. 18, 74. https://doi.org/10.1186/s13075-016-0970-9 (2016).
Kuwano, T. et al. Cyclooxygenase 2 is a key enzyme for inflammatory cytokine-induced angiogenesis. FASEB J. 18, 300–310. https://doi.org/10.1096/fj.03-0473com (2004).
Buckley, C. D., Gilroy, D. W. & Serhan, C. N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 40, 315–327. https://doi.org/10.1016/j.immuni.2014.02.009 (2014).
Trebino, C. E. et al. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc. Natl. Acad. Sci. USA 100, 9044–9049. https://doi.org/10.1073/pnas.1332766100 (2003).
Koike, R., Takeuchi, T., Eguchi, K., Miyasaka, N. & Japan College of, R. Update on the Japanese guidelines for the use of infliximab and etanercept in rheumatoid arthritis. Mod. Rheumatol. 17, 451–458. https://doi.org/10.1007/s10165-007-0626-3 (2007).
Ng, C. S. et al. Lung ischaemia-reperfusion induced gene expression. Eur. J. Cardiothorac. Surg. 37, 1411–1420. https://doi.org/10.1016/j.ejcts.2010.01.001 (2010).
Kupershmidt, I. et al. Ontology-based meta-analysis of global collections of high-throughput public data. PLoS One https://doi.org/10.1371/journal.pone.0013066 (2010).
Acknowledgements
We thank Dr. Toshio Inoue, Division of Respiratory Medicine, Department of Internal Medicine, Nihon University School of Medicine, Tokyo, Japan (his present address is Technopro R&D, Tokyo, Japan) for his excellent technical help. We thank Dr. Shu Saito, Dr. Hyuho Lee and the staff members of Orthopedic Surgery of Nihon University Hospital for collecting synovial tissue samples.
Funding
This work was supported in part by a Grant-in-Aid for Early-Career Scientists (19K17687, awarded to S.T.), a Grant-in-Aid for Scientific Research from the MEXT of the Japanese Government (20K08811, awarded to Y.O), Nihon University Multidisciplinary Research Grants for 2018–2019 (Project No. So18-009, awarded to Y.O.) and for 2021–2022 (Project No. So21-00x, awarded to Y.O.), a Nihon University Research Grant for Social Implementation for 2020 (project No. Sya20-1201, awarded to Y.O.), and the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2015–2019 (Project No. S1511014, awarded to Y.O.).
Author information
Authors and Affiliations
Contributions
S.M., J.K., S.T., T.S.-S. and M.K. performed the experiments; J.K., S.T. and M.K. analyzed the data; S.M., J.K., S.T., K.N., M.K. and Y.O. interpreted the results of experiments; J.K. and S.T. prepared the figures; S.M., S.T. and Y.S. prepared the tables; Y.O. designed the research, conceived the manuscript and had primary responsibility for the writing; and all authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mishima, S., Kashiwakura, Ji., Toyoshima, S. et al. Higher PGD2 production by synovial mast cells from rheumatoid arthritis patients compared with osteoarthritis patients via miR-199a-3p/prostaglandin synthetase 2 axis. Sci Rep 11, 5738 (2021). https://doi.org/10.1038/s41598-021-84963-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-84963-7
This article is cited by
-
Network pharmacology and experimental validation methods to reveal the active compounds and hub targets of Curculigo orchioides Gaertn in rheumatoid arthritis
Journal of Orthopaedic Surgery and Research (2023)
-
Leveraging whole blood based functional flow cytometry assays to open new perspectives for rheumatoid arthritis translational research
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.