Abstract
Primary prevention of premature death is a public health concern worldwide. Circulating microRNAs (miRNAs) have been described as potential diagnostic biomarkers for diseases as cancer and cardiovascular disease (CVD). This case-cohort study aimed to investigate the potential relationship between circulating miRNAs and the risk of premature death. A total of 39,242 subjects provided baseline serum samples in 1988–1990. Of these, 345 subjects who died of intrinsic disease (< 65 years old) and for which measurable samples were available were included in this study. We randomly selected a sub-cohort of 879 subjects. Circulatring miR-21, miR-29a, and miR-126 were determined using qRT-PCR. Conditional logistic regression models were used to analyse the data with respect to stratified miRNA levels. Multivariable logistic regression revealed that subjects with high circulating miR-21 and miR-29a individual levels had a significantly higher risk of total death, cancer death, and CVD death than those with medium miR-21 and miR-29a individual levels. Conversely, subjects with low circulating miR-126 levels had a significantly higher risk of total death than those with medium levels. This suggests that circulating miRNAs are associated with the risk of premature death from cancer and CVD, identifying them as potential biomarkers for early detection of high-risk individuals.
Similar content being viewed by others
Introduction
Premature death is broadly defined as the occurrence of mortality before the average age of death in a certain population. Although premature death (i.e. death before 65 years of age) accounts for only a small proportion of total mortality, its high impact on public health raises significant clinical and economic concerns. Despite the increasing average life expectancy in developed countries (e.g. currently over 80 years in Japan), a substantial proportion of adults still die prematurely1,2,3. Therefore, primary prevention of premature death is a considerable public health concern worldwide.
Over the past few decades, a considerable number of studies have been conducted on factors associated with premature death. Although the causes of these deaths vary, there appear to be some common causative factors4,5. For example, smoking cigarettes and/or being exposed to secondhand tobacco smoke are leading causes of premature death, and heavy drinking has also been implicated as a causative factor6,7. These lifestyles can increase the risk of cancer, heart disease, stroke, lung disease, and many other health problems that are associated with premature death. However, the identification of reliable biomarkers associated with premature death could complement the assessment of traditional risk factors and allow the identification of high-risk individuals with greater accuracy.
MicroRNAs (miRNAs), a class of small non-coding RNAs 18–25 nucleotides in length, are involved in a wide array of biological processes, including cell apoptosis, differentiation, development, proliferation, and metabolism. As miRNAs can be detected in bodily fluids (e.g., plasma, serum, urine, and saliva), there is much interest in studying the function and effects of these so-called “circulating miRNAs”. Several studies have demonstrated that circulating miRNAs can be used as biomarkers for various diseases such as cancers and cardiovascular disease (CVD)8,9,10,11,12,13,14. The circulating miRNAs miR-21, miR-29a, and miR-126 are particularly well studied due to their potential as novel biomarkers. For example, it has been reported that circulating level of miR-29a is correlated with clinical stage of colorectal cancer15. More recently, Yamada et al. reported that circulating miR-29a is upregulated in early stage of colorectal neoplasia, suggesting that circulating miR-29a may be increased in any clinical stages including early colorectal cancer16. miR-29a may be also useful for the prediction of type 2 diabetes17. Furthermore, Jiang et al. showed that miR-21 peripheral blood levels are increased in atherosclerosis, suggesting its potential as a biomarker for CVD18. In addition, miR-21 may be a potential biomarker for breast cancer and chronic kidney disease10,19. Also, Jianhong et al. showed that circulating miR-21 is up-regulated at all clinical stages in gastric cancer patients, suggesting that circulating miR-21 level is up-regulated throughout cancer progression20. Finally, circulating miR-126 has been reported as a potential biomarker for both type 2 diabetes21, non-alcoholic fatty liver disease22and lung cancer23 and may also reflect the progression of CVD24 As these diseases cause premature death, it is logical to hypothesise that circulating miR-21, miR-29a, and miR-126 levels may be associated with premature death.
We designed this study to investigate whether circulating miRNAs could represent new risk factors for premature death. The Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC Study) is a large-scale population-based cohort study that has formed the basis of multiple reports on the relationships between disease risk and serum components, lifestyle, and living conditions of the Japanese population25,26,27,28. The JACC Study makes it possible to examine the significance of a predictive marker by analysing its levels in the serum of individuals who died prematurely due to various diseases such as cancer and CVD.
The present large prospective study examined the relationships between circulating miRNAs (miR-21, miR-29a, and miR-126) and premature death using a case-cohort study design as part of the JACC Study. Our results identify potential new risk factors for premature death that could complement the assessment of traditional risk factors and help to identify high-risk individuals with greater precision.
Results
During the follow-up period, premature death was observed in 775 subjects, of which 345 had available baseline serum samples for analysis in this study. Of these premature deaths, 210 were due to cancer and 43 to coronary artery disease (CAD). Detailed data of the underlying causes of death as classified by the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) codes are described in Supplementary Tables S1-S3 online.
Table 1 shows the results of the statistical analysis comparing various characteristics of the premature death cases and controls. Perhaps unsurprisingly, the categories of age, sex, smoking status, and alcohol consumption displayed significant differences between the control and premature death groups (p < 0.001). Interestingly, serum levels of circulating miR-21 and miR-29a were significantly higher in the premature death group than in the control group (p = 0.001 and 0.01, respectively).
Table 2 shows age- and sex-adjusted odds ratios (ORs) and 95% confidence intervals (CIs) for premature death with respect to the three categories of serum miRNA levels. Cases with high circulating miR-21 levels (> 75%) had a significantly higher risk of all death, cancer death, and CVD death than those with medium levels of this miRNA (OR [95% CI]: 1.93 [1.42–2.64], 1.74 [1.19–2.54], and 3.06 [1.42–6.74], respectively). Cases with high circulating miR-29a levels also had a significantly higher risk of all death, cancer death, and CVD death than those with medium levels of this miRNA (OR [95% CI]: 2.03 [1.48–2.78], 1.83 [1.25–2.69], and 3.13 [1.49–6.74], respectively). Interestingly, cases with low circulating miR-29a levels also had a significantly higher risk of all death and cancer death than those with medium levels of this miRNA (OR [95% CI]: 1.40 [1.01–1.94] and 1.56 [1.05–2.30], respectively). There were no significant associations with regard to circulating miR-126 levels. To control for the potential impact of subclinical symptoms of cancer at the time of baseline sampling, we repeated the analysis after excluding cases occurring within two years of the baseline survey. The results after this exclusion still showed a significant increase in the risk of premature death associated with serum levels of circulating miRNAs (data not shown).Table 3 shows the ORs and 95% CIs for premature death following analysis by the multivariable logistic regression model. Cases with high circulating miR-21 levels (> 75%) had a significantly higher risk of all death, cancer death, and CVD death than those with medium levels of this miRNA (OR [95% CI]: 1.98 [1.39–2.83], 1.84 [1.20–2.81], and 3.36 [1.39–8.33], respectively). Interestingly, cases with low circulating miR-21 levels also had a significantly higher risk of all death than those with medium levels of this miRNA (OR [95% CI]: 2.44 [1.02–5.94]). Cases with high circulating miR-29a levels had a significantly higher risk of all death, cancer death, and CVD death than those with medium levels of this miRNA (OR [95% CI]: 1.99 [1.39–2.84], 1.96 [1.27–3.00], and 3.23 [1.42–7.50], respectively). On the other hand, cases with low circulating miR-29a levels had a significantly higher risk of cancer death than those with medium levels of this miRNA (OR [95% CI]: 1.55 [1.03–2.34]). Cases with low levels of circulating miR-126 had a significantly higher risk of all death than those with medium levels of this miRNA (OR [95% CI]: 1.49 [1.06–2.10]). To control for the potential impact of subclinical symptoms of cancer at the time of baseline sampling, we repeated the analysis after excluding cases occurring within two years of the baseline survey. The results after this exclusion still showed a significant increase in the risk of premature death associated with serum levels of circulating miRNAs (data not shown).
Discussion
The present large prospective study examined associations between circulating miRNAs and premature death using a case-cohort study design. To our knowledge, this is the first study to show the association of circulating miRNA levels with premature death due to cancer and CVD. We observed that high serum levels of circulating miR-21 and miR-29a were most significantly associated with increased risk of premature death. On the other hand, low serum levels of circulating miR-126 were associated with increased risk of premature death. These results indicate that changes in serum levels of circulating miRNAs might be a novel risk factor preceding premature death events and could therefore be used as biomarkers to assess premature death risk many years before disease onset.
Over the past decades, many studies have examined environmental factors associated with the risk of premature death such as smoking and alcohol consumption4,29,30,31. We designed this prospective cohort study to identify new predictive factors independent of these lifestyle risk factors with the aim of improving prognostic information. A unique aspect of this study was the use of serum markers to identify factors associated with premature death. The focus on miRNA was particularly novel as these have not been previously assessed in this kind of research study. Although we adjusted our analysis for lifestyle risk factors such as smoking, exercise, and alcohol consumption, circulating miRNAs were significantly associated with premature death (Tables 2 and 3). These results suggest that circulating miRNA levels may represent novel factors for predicting premature death independently of traditional lifestyle-related risk factors.
The biological mechanisms of miR-21, miR-29a, and miR-126 have been described in multiple studies to date. MiR-21, one of the most widely studied abnormal miRNAs, is known to be upregulated in numerous tumours such as breast cancer, lung cancer, gastric cancer, colorectal cancer, hepatocellular carcinoma, pancreatic cancer, and ovarian carcinoma32. It has been proposed that circulating miRNAs such as miR-21 could be used as non-invasive biomarkers for cancer. Multiple studies have shown that the level of circulating miR-21 can distinguish cancer patients from healthy individuals and predict disease outcomes33,34. However, no previous studies have related miR-21 levels to the risk of developing cancer. Our findings showed that miR-21 serum levels were significantly associated with the risk of cancer death, suggesting that altered miR-21 levels may precede the onset of the disease. Indeed, many previous studies indicated that miR-21 plays an important role in the oncogenic process such as its association with inflammation, proliferation, apoptosis, invasion, and metastatic potential32,35. Also, these processes are well known to induce the development of metabolic dysfunction, an underlying cause of CVD36. The easily detectable imbalance of circulating miR-21 may therefore predict the pro-thrombotic, pro-inflammatory, pro-vasoconstrictive, and pro-proliferative phenotype that precedes the development of multiple clinical diseases, including cancer and CVD.
miR-29a has also been shown to regulate several biological functions underlying important physiological and pathological processes, including the cell cycle, proliferation, differentiation, apoptosis, and senescence37. A large body of literature has shown a significant role for miR-29a in various diseases, including cancer38. Although the majority of these studies have reported that miR-29a functions as a potent tumour suppressor, a few reports have demonstrated an oncogenic function of this miRNA. Circulating miR-29a has been reported as a promising biomarker for the early detection of cancer. However, higher levels of miR-29a in plasma conferred lower survival rates in lung cancer39. In addition, the pre-treatment level of circulating miR-29a is an independent prognostic marker for poor disease-free survival in hepatitis B virus-related HCC patients40. The evidence that miR-29a may have a dual role in regulating normal physiology and disease is consistent with the results of our study, which showed that both high and low levels of miR-29a are associated with premature death (Tables 2 and 3).
Our finding showed that low miR-126 levels correlated with lower premature death risk. This result may be explained by the fact that miR-126 prevents endothelial inflammation and dysfunction. Harris et al. indicated that miR-126 may control vascular inflammation by influencing leukocyte adhesion to endothelium via its effects on vascular cell adhesion molecule 1 expression41. Also, Jin et al. showed that circulating miR-126 levels were negatively correlated with levels of inflammatory cytokines such as tumour necrosis factor-α, interleukin (IL)-1β, and IL-642. Another report showed that low plasma miR-126 levels might have a negative impact on vascular endothelial growth factor resistance and endothelial dysfunction in patients with type 2 diabetes43. Furthermore, increased levels of circulating miR-126 were reported to correlate with a lower predisposition to major adverse events in patients with stable CAD, a form of CVD44. Our finding showing that low miR-126 levels correlated with lower premature death risk is consistent with those of the previous reports because decreased levels of miR-126 may reflect endothelial dysfunction and subsequent impairment of the peripheral angiogenic system in the general population.
Although circulating miRNAs have been described as non-invasive biomarkers for multiple pathologies, including cancer and CVD, it is not clear whether the changes in miRNA levels precede the disease, appear at the early stages, or are a consequence of the disease state. Only a few studies have been performed based on large prospective cohorts in the general population, and little is known about the value of miRNAs as biomarkers for risk stratification of future events45. To our knowledge, the prognostic value of miRNAs for the general population has been evaluated in only two large-scale studies. Zampetaki et al. evaluated circulating miRNAs regarding their applicability in primary prevention of CVD. In 820 apparently healthy subjects, the three circulating miRNAs miR-126, miR-223, and miR-197 were associated with CAD in the general population, suggesting these miRNAs as biomarkers in primary prevention46. Also, Willeit et al. utilised the prospective population-based Bruneck Study (n = 810; survey year 1995) to show that circulating miR-122 is associated with the risk of developing metabolic syndrome and type 2 diabetes in the general population47.
In this study, we showed that circulating miRNAs (miR-21, miR-29a, and miR-126) are significantly associated with the risk of premature death due to cancer and CVD in the general population. To control for the potential impact of subclinical symptoms of cancer at the time of baseline sampling, we repeated the analysis after excluding cases occurring within two years of the baseline survey. Results after exclusion still showed a significant increase in the risk of premature death associated with serum levels of circulating miRNAs, suggesting that alterations in miRNA levels may precede the early stages of the disease. The findings of the current study combined with those of previous studies therefore suggest that changes in circulating miRNA patterns are not only the result of disease but may also precede the early stages of disease onset. Thus, circulating miRNAs may be useful tools as biomarkers for the risk of disease.
The cause and significance of changes in circulating miRNAs are important matters to be addressed in future studies. The risk of developing diseases such as cancer and CVD is known to result from the interaction between genetic and environmental factors. Given what is known about the action of miRNAs, it is easy to envisage how they could regulate gene expression related to environmental or lifestyle factors before, during, or after disease onset. For example, tobacco and alcohol consumption are leading risk factors in the development of diseases such as cancer, CVD, and liver injury. These lifestyles likely promote disease development by affecting gene expression and signalling pathways controlling important processes such as apoptosis, angiogenesis, and inflammation. These complex gene expression changes resulting from the interaction between genetic and environmental factors can be regulated by miRNAs. Thus, studying changes in circulating miRNAs may be crucial for our understanding of the interaction between genetic and environmental factors. In the future, it will be important to better elucidate the cause and significance of changes in circulating miRNAs, including the effect of genetic variations.
This study was only possible because of the exceptional characteristics of the JACC Study, a large-scale cohort study conducted in Japan. The following unique characteristics of the JACC Study allow us to research factors associated with premature death: (1) follow-up of the general population for approximately 20 years, (2) inclusion of relatively young individuals (64 years old or less) at baseline (about 72%), (3) availability of serum at baseline, i.e. before development of the fatal disease. Our results are valuable not only for their implications for premature death prediction but also for future research on novel mechanisms of premature death. However, there are some limitations to our study. First, since not all of the cohort participants provided blood samples, there was the possibility of selection bias. Although serum from 29,410 individuals was sampled at the age of 40–64 in the JACC Study, only 25,418 samples were currently available. Previously, we have used serum samples in case–control studies48,49,50. However, this study was conducted using serum samples that were not used in previous case–control studies. Indeed, 775 premature deaths were observed during the follow-up period, but baseline serum samples were only available for 345 of these subjects. Second, only Japanese subjects were recruited in this study, potentially biasing the results towards a particular ethnic group. The recruitment of other ethnic groups may result in different circulating miRNA profiles. Third, although we were able to show the link between circulating miRNAs and premature death from cancer and CVD, the sample size of this study did not allow us to perform analyses regarding particular cancer types and other causes of death (see Supplementary Table S2 online). Therefore, further detailed investigations are required to more thoroughly understand the association of circulating miRNAs with premature death.
In conclusion, this study successfully utilised a case-cohort study design to demonstrate for the first time that circulating miRNAs (miR-21, miR-29a, and miR-126) are associated with the risk of premature death from cancer and CVD. Our results have implications for early prediction of premature death as well as the advancement of research on its underlying mechanisms.
Materials and methods
Study subjects and data collection
Details of the concept and design of the JACC Study have been described elsewhere27. Briefly, the JACC Study was started between 1988 and 1990 and enrolled a total of 110,585 participants (46,395 men and 64,190 women) aged 40–79 years living in 45 areas across Japan. The participants responded to self-administered questionnaires regarding their lifestyle (such as smoking and alcohol consumption status) and medical history of major diseases (including liver disease, cancer, and CVD).
Peripheral blood samples were collected from a total of 39,242 participants during municipal health screening examinations. This study focused on the 29,410 individuals that were sampled at the age of 40–64, of which 25,418 serum samples were currently available for use, because we have used serum samples in case–control studies48,49,50.
Serum samples (taken at the baseline survey) were selected from 775 patients who died of intrinsic disease before the age of 65. Of these, 345 measurable samples were available for the present study. A sub-cohort was randomly selected from those aged 40–64 at the baseline sample measurement for which serum samples were available. A total of 926 subjects were selected as the sub-cohort (an extraction rate of 3.6%) by gender, age class (every 5 years), area, and sample collection year. Of these, 17 cases of premature death and 63 cases of death over 66 years old were included. Subsequently, subjects were excluded based on the following criteria: missing dates for analysis, technical issues with sample vials, and unconfirmed history of cancer and CVD. The final study population included 1,224 subjects (345 premature death cases and 875 control subjects). All participants provided written informed consent. The JACC Study began before the ethical guidelines were first established by the Japanese government in 2002, and the Japanese ethical guidelines allows such established epidemiological studies to continue without obtaining additional personal informed consent from kin or legal guardian51,52,53,54. The study design and use of serum were approved by the Ethical Board at Hokkaido University School of Medicine, where the central office of the JACC Study was located. This study was complied with guidelines of the Declaration of Helsinki.
Follow-up
In the JACC Study, population registries in the municipalities were used for the follow-up of the subjects. In most areas, the follow-up was completed at the end of 2009; however, it was stopped at the end of 1999 in 4 areas, at the end of 2003 in another 4 areas, and at the end of 2008 in 2 areas. We discontinued the follow-up of those who had moved out of their area after the baseline survey. Individuals who re-located away from the study area were treated as dropouts, as deaths could not be confirmed in our follow-up system. We reviewed death certificates and classified the underlying causes of death as coded by the National Vital Statistics according to the ICD-10 (http://www.who.int/classifications/icd/en/). Cancer was defined using codes C00-C97, and CVD using codes I00-I99. All deaths that occurred in the cohort were ascertained by death certificates from public health centres.
Measurement of circulating miRNAs
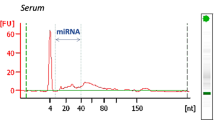
Serum was stored at − 80 °C, as this method has been proven to reliably maintain circulating miRNA levels55. Serum levels of the three circulating miRNAs were detected by quantitative real-time polymerase chain reaction (qRT-PCR) following a procedure that has been described in detail elsewhere56 In brief, serum miRNAs were isolated using TRIzol reagents according to the manufacturer’s protocol (Invitrogen, Foster City, USA). qRT-PCR was then performed with specific primers for miRNAs in an ABI PRISM 7900 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) using a miScript System (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. Relative expression levels of miRNAs were calculated using the comparative cycle threshold method. Caenorhabditis elegans miR-39 (cel-mir-39) was used to spike RNA samples as an external control to check the efficiency of either the RNA extraction or the cDNA synthesis. This is a widely used practical method in the measurement of circulating miRNAs57,58.
Analysis
Statistical analyses were based on mortality during the follow-up period (1989–2009). To compare the baseline characteristics of premature death cases and control subjects, the paired t-test was used to test mean values, and the McNemar test was used for percentages of premature death risk factors. Group circulating miRNA levels were compared by Wilcoxon tests.
ORs for premature death were estimated using conditional logistic regression models. Subjects were categorised into 3 groups according to their serum circulating miRNA levels. The categories used in analyses of the distribution of sub-cohort subjects were defined as: < 25% (low), 25–75% (medium), and > 75% (high). miRNA levels in the premature death group were then categorised as low, medium, or high if they were < 25%, 25–75%, or > 75% of the average level of the sub-cohort (control) group, respectively. Multivariable logistic regression modelling was then performed to estimate the ORs and 95% CIs for premature death using the medium group (25–75%) as a reference group. Covariates for adjustment included sex, age, area of residence, body mass index (categorised as < 18.5 kg/m2, 18.5–24.9 kg/m2, and ≥ 25 kg/m2), systolic blood pressure (mm Hg), cigarette smoking status (never, former, and current), alcohol consumption status (never, ex-, and current), walking (≥ 30 min/day or not), exercise (≥ 1 h/week or not), and educational level (attended school up to 15–18 years old or > 18 years old). Data for the above factors were self-reported. For all covariates, missing values were included in the model as an additional category of variable. The data were analysed with R version 3.5.1 statistical software (R Core Team (2019). R: A language and environment for statistical computing. R Foundation for statistical Comuting, Vienna, Austria. https://www.R-project.org/)59. All statistical tests were 2-tailed, and a p-value of less than 0.05 was considered statistically significant.
References
Bauer, U. E., Briss, P. A., Goodman, R. A. & Bowman, B. A. Prevention of chronic disease in the 21st century: Elimination of the leading preventable causes of premature death and disability in the USA. Lancet 384, 45–52. https://doi.org/10.1016/S0140-6736(14)60648-6 (2014).
Mackenbach, J. P. et al. Trends in inequalities in premature mortality: A study of 3.2 million deaths in 13 European countries. J. Epidemiol. Community Health 69, 207–217. https://doi.org/10.1136/jech-2014-204319 (2015).
Shiels, M. S. et al. Trends in premature mortality in the USA by sex, race, and ethnicity from 1999 to 2014: An analysis of death certificate data. Lancet 389, 1043–1054. https://doi.org/10.1016/S0140-6736(17)30187-3 (2017).
Muller, D. C. et al. Modifiable causes of premature death in middle-age in Western Europe: Results from the EPIC cohort study. BMC Med. 14, 87. https://doi.org/10.1186/s12916-016-0630-6 (2016).
Stringhini, S. et al. Socioeconomic status and the 25 x 25 risk factors as determinants of premature mortality: A multicohort study and meta-analysis of 1.7 million men and women. Lancet 389, 1229–1237. https://doi.org/10.1016/S0140-6736(16)32380-7 (2017).
Holford, T. R. et al. Tobacco control and the reduction in smoking-related premature deaths in the United States, 1964–2012. JAMA 311, 164–171. https://doi.org/10.1001/jama.2013.285112 (2014).
Zaridze, D. et al. Alcohol and mortality in Russia: Prospective observational study of 151,000 adults. Lancet 383, 1465–1473. https://doi.org/10.1016/S0140-6736(13)62247-3 (2014).
Creemers, E. E., Tijsen, A. J. & Pinto, Y. M. Circulating microRNAs: Novel biomarkers and extracellular communicators in cardiovascular disease?. Circ. Res. 110, 483–495. https://doi.org/10.1161/CIRCRESAHA.111.247452 (2012).
Dufresne, S., Rebillard, A., Muti, P., Friedenreich, C. M. & Brenner, D. R. A review of physical activity and circulating miRNA expression: implications in cancer risk and progression. Cancer Epidemiol. Biomark. Prev. 27, 11–24. https://doi.org/10.1158/1055-9965.EPI-16-0969 (2018).
Fujii, R. et al. Associations of circulating microRNAs (miR-17, miR-21, and miR-150) and chronic kidney disease in a Japanese population. J. Epidemiol. https://doi.org/10.2188/jea.JE20180233 (2019).
Fujii, R. et al. Circulating microRNAs (miR-126, miR-197, and miR-223) are associated with chronic kidney disease among elderly survivors of the Great East Japan Earthquake. BMC Nephrol. 20, 474. https://doi.org/10.1186/s12882-019-1651-0 (2019).
He, Y. et al. Current state of circulating microRNAs as cancer biomarkers. Clin. Chem. 61, 1138–1155. https://doi.org/10.1373/clinchem.2015.241190 (2015).
Yamada, H., Itoh, M., Hiratsuka, I. & Hashimoto, S. Circulating microRNAs in autoimmune thyroid diseases. Clin. Endocrinol. 81, 276–281. https://doi.org/10.1111/cen.12432 (2014).
Yamada, H. et al. Associations between circulating microRNAs (miR-21, miR-34a, miR-122 and miR-451) and non-alcoholic fatty liver. Clin. Chim. Acta 424, 99–103. https://doi.org/10.1016/j.cca.2013.05.021 (2013).
Faltejskova, P. et al. Circulating miR-17-3p, miR-29a, miR-92a and miR-135b in serum: Evidence against their usage as biomarkers in colorectal cancer. Cancer Biomark. 12, 199–204. https://doi.org/10.3233/CBM-130308 (2012).
Yamada, A. et al. Serum miR-21, miR-29a, and miR-125b are promising biomarkers for the early detection of colorectal neoplasia. Clin. Cancer Res. 21, 4234–4242. https://doi.org/10.1158/1078-0432.CCR-14-2793 (2015).
Jimenez-Lucena, R. et al. Circulating miRNAs as predictive biomarkers of type 2 diabetes mellitus development in coronary heart disease patients from the CORDIOPREV study. Mol. Ther. Nucleic Acids 12, 146–157. https://doi.org/10.1016/j.omtn.2018.05.002 (2018).
Jiang, Y. et al. Peripheral blood miRNAs as a biomarker for chronic cardiovascular diseases. Sci. Rep. 4, 5026. https://doi.org/10.1038/srep05026 (2014).
Khalighfard, S., Alizadeh, A. M., Irani, S. & Omranipour, R. Plasma miR-21, miR-155, miR-10b, and Let-7a as the potential biomarkers for the monitoring of breast cancer patients. Sci. Rep. 8, 17981. https://doi.org/10.1038/s41598-018-36321-3 (2018).
Wu, J. et al. Circulating MicroRNA-21 is a potential diagnostic biomarker in gastric cancer. Dis. Mark. 2015, 435656. https://doi.org/10.1155/2015/435656 (2015).
Zhang, T. et al. Circulating miR-126 is a potential biomarker to predict the onset of type 2 diabetes mellitus in susceptible individuals. Biochem. Biophys. Res. Commun. 463, 60–63. https://doi.org/10.1016/j.bbrc.2015.05.017 (2015).
Ando, Y. et al. Association of circulating miR-20a, miR-27a, and miR-126 with non-alcoholic fatty liver disease in general population. Sci. Rep. 9, 18856. https://doi.org/10.1038/s41598-019-55076-z (2019).
Grimolizzi, F. et al. Exosomal miR-126 as a circulating biomarker in non-small-cell lung cancer regulating cancer progression. Sci. Rep. 7, 15277. https://doi.org/10.1038/s41598-017-15475-6 (2017).
Khanaghaei, M. et al. Circulating miR-126 and miR-499 reflect progression of cardiovascular disease; correlations with uric acid and ejection fraction. Heart Int. 11, e1–e9. https://doi.org/10.5301/heartint.5000226 (2016).
Iso, H. et al. Smoking cessation and mortality from cardiovascular disease among Japanese men and women: The JACC Study. Am. J. Epidemiol. 161, 170–179. https://doi.org/10.1093/aje/kwi027 (2005).
Noda, H. et al. Walking and sports participation and mortality from coronary heart disease and stroke. J. Am. Coll. Cardiol. 46, 1761–1767. https://doi.org/10.1016/j.jacc.2005.07.038 (2005).
Tamakoshi, A. et al. Cohort profile of the Japan Collaborative Cohort Study at final follow-up. J. Epidemiol. 23, 227–232. https://doi.org/10.2188/jea.je20120161 (2013).
Yamada, H. et al. Coffee consumption and risk of colorectal cancer: The Japan Collaborative Cohort Study. J. Epidemiol. 24, 370–378. https://doi.org/10.2188/jea.je20130168 (2014).
Wang, H. et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 388, 1459–1544. https://doi.org/10.1016/S0140-6736(16)31012-1 (2016).
Rasky, E., Stolz, E., Burkert, N. T. & Grossschadl, F. Potentially preventable premature deaths in women and men from the two leading causes of death in Austria, mortality statistics of the nine federal states 2010–2012. BMC Public Health 15, 1177. https://doi.org/10.1186/s12889-015-2502-y (2015).
Roth, G. A. et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 132, 1667–1678. https://doi.org/10.1161/CIRCULATIONAHA.114.008720 (2015).
Pfeffer, S. R., Yang, C. H. & Pfeffer, L. M. The role of miR-21 in cancer. Drug Dev. Res. 76, 270–277. https://doi.org/10.1002/ddr.21257 (2015).
Ferraro, A. et al. Epigenetic regulation of miR-21 in colorectal cancer: ITGB4 as a novel miR-21 target and a three-gene network (miR-21-ITGBeta4-PDCD4) as predictor of metastatic tumor potential. Epigenetics 9, 129–141. https://doi.org/10.4161/epi.26842 (2014).
Shan, L. et al. Diagnostic value of circulating miR-21 for colorectal cancer: A meta-analysis. Cancer Biomark. 15, 47–56. https://doi.org/10.3233/CBM-140437 (2015).
Sheedy, F. J. Turning 21: Induction of miR-21 as a Key Switch in the Inflammatory Response. Front. Immunol. 6, 19. https://doi.org/10.3389/fimmu.2015.00019 (2015).
Donato, A. J., Morgan, R. G., Walker, A. E. & Lesniewski, L. A. Cellular and molecular biology of aging endothelial cells. J. Mol. Cell. Cardiol. 89, 122–135. https://doi.org/10.1016/j.yjmcc.2015.01.021 (2015).
Lyu, G. et al. TGF-beta signaling alters H4K20me3 status via miR-29 and contributes to cellular senescence and cardiac aging. Nat. Commun. 9, 2560. https://doi.org/10.1038/s41467-018-04994-z (2018).
Alizadeh, M. et al. The potential role of miR-29 in health and cancer diagnosis, prognosis, and therapy. J. Cell. Physiol. 234, 19280–19297. https://doi.org/10.1002/jcp.28607 (2019).
Zhang, L. et al. MicroRNA-related genetic variants in iron regulatory genes, dietary iron intake, microRNAs and lung cancer risk. Ann. Oncol. 28, 1124–1129. https://doi.org/10.1093/annonc/mdx046 (2017).
Cho, H. J. et al. Low levels of circulating microRNA-26a/29a as poor prognostic markers in patients with hepatocellular carcinoma who underwent curative treatment. Clin. Res. Hepatol. Gastroenterol. 41, 181–189. https://doi.org/10.1016/j.clinre.2016.09.011 (2017).
Harris, T. A., Yamakuchi, M., Ferlito, M., Mendell, J. T. & Lowenstein, C. J. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc. Natl. Acad. Sci. U.S.A. 105, 1516–1521. https://doi.org/10.1073/pnas.0707493105 (2008).
Jin, F. & Xing, J. Circulating miR-126 and miR-130a levels correlate with lower disease risk, disease severity, and reduced inflammatory cytokine levels in acute ischemic stroke patients. Neurol. Sci. 39, 1757–1765. https://doi.org/10.1007/s10072-018-3499-7 (2018).
Zampetaki, A. et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ. Res. 107, 810–817. https://doi.org/10.1161/CIRCRESAHA.110.226357 (2010).
Jansen, F. et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J. Am. Heart Assoc. 3, e001249. https://doi.org/10.1161/JAHA.114.001249 (2014).
Lin, X. J. et al. A serum microRNA classifier for early detection of hepatocellular carcinoma: A multicentre, retrospective, longitudinal biomarker identification study with a nested case-control study. Lancet Oncol. 16, 804–815. https://doi.org/10.1016/S1470-2045(15)00048-0 (2015).
Zampetaki, A. et al. Prospective study on circulating MicroRNAs and risk of myocardial infarction. J. Am. Coll. Cardiol. 60, 290–299. https://doi.org/10.1016/j.jacc.2012.03.056 (2012).
Willeit, P. et al. Circulating MicroRNA-122 is associated with the risk of new-onset metabolic syndrome and type 2 diabetes. Diabetes 66, 347–357. https://doi.org/10.2337/db16-0731 (2017).
Pham, T. M. et al. A nested case-control study of stomach cancer and serum insulin-like growth factor (IGF)-1, IGF-2 and IGF-binding protein (IGFBP)-3. Eur. J. Cancer 43, 1611–1616. https://doi.org/10.1016/j.ejca.2007.04.014 (2007).
Pham, T. M. et al. Relationship between serum levels of insulin-like growth factors and subsequent risk of cancer mortality: Findings from a nested case-control study within the Japan Collaborative Cohort Study. Cancer Epidemiol. 34, 279–284. https://doi.org/10.1016/j.canep.2010.03.017 (2010).
Watanabe, Y. et al. Transforming growth factor-beta1 as a predictor for the development of hepatocellular carcinoma: A nested case-controlled study. EBioMedicine 12, 68–71. https://doi.org/10.1016/j.ebiom.2016.09.001 (2016).
Kikuchi, N. et al. Perceived stress and colorectal cancer incidence: the Japan collaborative cohort study. Sci. Rep. 7, 40363. https://doi.org/10.1038/srep40363 (2017).
Nakayama, T., Sakai, M. & Slingsby, B. T. Japan’s ethical guidelines for epidemiologic research: a history of their development. J. Epidemiol. 15, 107–112. https://doi.org/10.2188/jea.15.107 (2005).
Tamakoshi, A. Informed consent in epidemiologic research before the implementation of ethical guidelines. J. Epidemiol. 14, 177–181. https://doi.org/10.2188/jea.14.177 (2004).
Japanese Ministry of Education, Culture, Sports, Science and Technology & Ministry of Health, Labor and Welfare. Ethical Guidelines for Medical and Health Research Involving Human Subjects. (2016).
Balzano, F. et al. miRNA stability in frozen plasma samples. Molecules 20, 19030–19040. https://doi.org/10.3390/molecules201019030 (2015).
Hiratsuka, I., Yamada, H., Munetsuna, E., Hashimoto, S. & Itoh, M. Circulating MicroRNAs in Graves’ disease in relation to clinical activity. Thyroid 26, 1431–1440. https://doi.org/10.1089/thy.2016.0062 (2016).
Munetsuna, E. et al. Association of subcutaneous and visceral fat with circulating microRNAs in a middle-aged Japanese population. Ann. Clin. Biochem. 55, 437–445. https://doi.org/10.1177/0004563217735124 (2018).
Yamada, H. et al. Longitudinal study of circulating miR-122 in a rat model of non-alcoholic fatty liver disease. Clin. Chim. Acta 446, 267–271. https://doi.org/10.1016/j.cca.2015.05.002 (2015).
R Development Core Team. A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, 2018).
Acknowledgements
The authors would like to thank Dr. Kunio Aoki and Dr. Yoshiyuki Ohno, Emeritus Professors of the Nagoya University School of Medicine and former Chairpersons of the JACC Study Group, for their encouragement and support during this study; Dr. Haruo Sugano, former Director of the Cancer Institute, Tokyo, for the substantial contributions to the initiation of the JACC Study; Dr. Tomoyuki Kitagawa, Director Emeritus of the Cancer Institute of the Japanese Foundation for Cancer Research and former Project Leader of the Grant-in-Aid for Scientific Research on Priority Areas of Cancer; Dr. Kazuo Tajima of the Aichi Cancer Center, former Project Leader of the Grant-in-Aid for Scientific Research on Priority Areas of Cancer Epidemiology; and Dr. Chigusa Date for contributing to the validation of FFQ and estimation of nutrient intake in the JACC Study. The present members of the JACC Study Group are as follws: The present members of the JACC Study Group are as follows: Dr. Akiko Tamakoshi (present chairperson of the study group), Hokkaido University Graduate School of Medicine; Drs. Mitsuru Mori and Fumio Sakauchi, Sapporo Medical University School of Medicine; Dr. Yutaka Motohashi, Akita University School of Medicine; Dr. Ichiro Tsuji, Tohoku University Graduate School of Medicine; Dr. Yosikazu Nakamura, Jichi Medical School; Dr. Hiroyasu Iso, Osaka University School of Medicine; Dr. Haruo Mikami, Chiba Cancer Center; Dr. Michiko Kurosawa, Juntendo University School of Medicine; Dr. Yoshiharu Hoshiyama, Yokohama Soei University; Dr. Naohito Tanabe, University of Niigata Prefecture; Dr. Koji Tamakoshi, Nagoya University Graduate School of Health Science; Dr. Kenji Wakai, Nagoya University Graduate School of Medicine; Dr. Shinkan Tokudome, National Institute of Health and Nutrition; Dr. Koji Suzuki, Fujita Health University School of Health Sciences; Dr. Shuji Hashimoto, Fujita Health University. School of Medicine; Dr. Shogo Kikuchi, Aichi Medical University School of Medicine; Dr. Yasuhiko Wada, Faculty of Nutrition, University of Kochi; Dr. Takashi Kawamura, Kyoto University Center for Student Health; Dr. Yoshiyuki Watanabe, Kyoto Prefectural University of Medicine Graduate School of Medical Science; Dr. Kotaro Ozasa, Radiation Effects Research Foundation; Dr. Tsuneharu Miki, Kyoto Prefectural University of Medicine Graduate School of Medical Science; Dr. Chigusa Date, School of Human Science and Environment, University of Hyogo; Dr. Kiyomi Sakata, Iwate Medical University; Dr. Yoichi Kurozawa, Tottori University Faculty of Medicine; Drs. Takesumi Yoshimura and Yoshihisa Fujino, University of Occupational and Environmental Health; Dr. Akira Shibata, Kurume University; Dr. Naoyuki Okamoto, Kanagawa Cancer Center; and Dr. Hideo Shio, Moriyama Municipal Hospital.
Funding
The JACC Study has been supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT); Grants-in-Aid for Scientific Research on Priority Areas of Cancer; Grants-in-Aid for Scientific Research on Priority Areas of Cancer Epidemiology from MEXT (Nos. 61010076, 62010074, 63010074, 1010068, 2151065, 3151064, 4151063, 5151069, 6279102, 11181101, 17015022, 18014011, 20014026, 20390156, 26293138 and 16H06277).
Author information
Authors and Affiliations
Contributions
H.Y. did the statistical analysis and wrote the first draft. K.S., R.F. and M.K. did statistical analysis. Y.W., H.I., Y.F., K.W. and A.T. did the data collection. S.H. and A.T. did the systematic literature search for the Research in Context section and the data interpretation, and critically revised the manuscript. All the authors were involved in the study design and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamada, H., Suzuki, K., Fujii, R. et al. Circulating miR-21, miR-29a, and miR-126 are associated with premature death risk due to cancer and cardiovascular disease: the JACC Study. Sci Rep 11, 5298 (2021). https://doi.org/10.1038/s41598-021-84707-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-84707-7
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.