Abstract
There is a lack of consensus about the measurement of the muscle viscoelastic features in stroke patients. Additionally, the psychometric properties of the most-commonly used clinical tools remain controversial. Our objective is to investigate the validity and reliability of myotonometry to assess viscoelastic muscle features in stroke survivors. Pubmed, PEDro, Scopus and Cinahl were systematically searched to include studies reporting the psychometric properties of myotonometric devices used in people after stroke. The QUADAS-2 and the COSMIN checklists were used to assess the methodological quality of the studies and the psychometric properties of myotonometry. Nine studies were included in the qualitative synthesis and data from five of these were pooled in a meta-analysis. Overall, low to moderate risk of bias and applicability concerns were observed. Pooled data from intra-rater reliability for muscle tone showed a mean coefficient of correlation of 0.915 (95% CI: 0.880–0.940, I 2 = 69.2%) for upper limbs, and a mean coefficient of 0.785 (95%CI: 0.708–0.844, I 2 = 4.02%) for lower limbs. Myotonometry seems to be a valid and reliable complementary tool to assess muscle viscoelastic properties in stroke survivors, although definite conclusions about concurrent validity need further research.
Similar content being viewed by others
Introduction
Stroke is among the leading causes of death and disability-adjusted life years worldwide, and shows an increasing prevalence, with over 70 million stroke survivors expected by 20301. Only in the European Union, the annual costs related to stroke treatment are estimated to be approximately 38 billion euros2.
In stroke patients, paresis is caused by a disruption of central motor command, which leads to stretch-sensitive (spastic) muscle overactivity, and adaptative changes in the viscoelastic properties of the soft tissues3. Spasticity is described as a velocity-dependent increase in muscle tone (hypertonia). It affects 4 to 27% of survivors in the acute or sub-acute phase and 17 to 42.6% in the chronic stage4, and is strongly associated with a reduced quality of life, loss of independence, and depression5.
The spastic hypertonia syndrome is currently understood as a multifactorial disorder, which accounts for biomechanical, histological and structural muscle changes6, such as shorter optimal fascicle length7,8, reduced muscle thickness7, and increased non-reflex stiffness or decreased muscle compliance, as a result of the adaptative muscle shortening9.
Muscle tone can be evaluated with non-instrumented, e.g., clinical scales, or instrumented tools, e.g., electrophysiological or mechanical measures. The original or modified Ashworth scale (MAS) and Tardieu scale (TS) are the most common non-instrumented measures in the clinical setting10. The Ashworth Scale (AS) and the MAS quantify the muscle response to an external movement, and they are used as a ‘gold standard’ to assess muscle tone in many studies10. Yet, these are subjective scales11, which tend to cluster12, and reveal little evidence of their psychometric properties10. The TS and the Modified Tardieu Scale (MTS) grade the muscle response to different stretching velocities13, and have demonstrated moderate to high reliability10, although these psychometric data are mainly related to patients with cerebral palsy14. As regards instrumented measures, a combination of neurophysiological and mechanical devices is the most recommended approach10. However, their clinical use is also controversial15 due to the lack of standardization of the measurement protocols and the need for costly equipment and specific training16. Therefore, cheap, objective, and easy-to-use devices are required in a clinical context.
Myotonometry represents a novel and non-invasive method to characterize the biomechanical and viscoelastic muscle properties17 such as compliance, stiffness, tone, elasticity, relaxation time and creep. Currently, there are two types of hand-held myotonometric devices. The Myotonometera measures the muscle deformation capacity in response to a number of repeated perpendicular forces (from 0.25 kg to 2 kg, at intervals of 0.25 kg) applied to the skin, during rest or isometric contraction, through a metal probe instrumented with a linear range of transducers18. Muscle compliance is derived from the slope of the muscle displacement-force function, as an inverse of stiffness18. The MyotonPROb, an improved version of the Myoton 3b, applies a brief mechanical impulse to the skin to record different oscillation parameters of the muscle response19. Dynamic stiffness refers to the soft tissue resistance to an external force and is calculated using the damped natural oscillation response, as registered by an in-built accelerometer20. Muscle tone is quantified by the natural frequency of the acceleration signal, while muscle elasticity, which is inversely proportional to the decrement, is determined by the sequential oscillations when the muscle restores its shape from deformation21. Stress relaxation time reflects the duration of the muscle recovery process21, and muscle creep is defined as the gradual elongation of the muscle under a constant tensile stress22. In healthy participants, the Myotonometera demonstrates moderate to good intra- and inter-rater reliability, and appears not to be valid for the measurement of stiffness23. The MyotonPROb has been validated for measurements of elasticity24 and stiffness20 in healthy participants, and reports good to excellent intra- and inter-rater reliability in healthy skeletal muscles25. To date, this is the first study aiming to summarize the available evidence regarding the psychometric properties of myotonometry in patients after stroke. This systematic review investigates the validity and reliability of myotonometry to evaluate upper and lower limb muscle viscoelastic properties in post-stroke patients, and aims to describe the assessment protocol with the highest quality evidence.
Methods
This systematic review was performed following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations and was registered in the PROSPERO database (CRD42018105751).
Research question and study selection
The research question for this systematic review was: Are the myotonometric devices valid and reliable tools for the assessment of the muscle viscoelastic properties in post-stroke patients? Thus, inclusion criteria for this review were as follows:
Participants
To be included, participants had to: (1) be adults aged over 18 years, (2) have been clinically diagnosed with a first-event stroke according to the current World Health Organization definition26.
Studies
This review was restricted to studies aiming to evaluate the psychometric properties of different myotonometric devices. The reference standard for validity studies had to be commonly used muscle property assessment tools. Self-reported scales were excluded. Participants had to: (1) be adults aged over 18 years, (2) have been clinically diagnosed with a first-event stroke according to the current World Health Organization definition. Finally, to be included, studies had to evaluate at least one muscle viscoelastic property of interest in adults with a stroke (e.g., tone, stiffness, compliance, or elasticity). Only published papers written in English were admitted at this stage. No publication date restriction was established. As for exclusion criteria, studies not using myotonometric devices as an assessment tool were excluded.
Data sources and search strategy
The following electronic databases were searched: Pubmed, PEDro, Scopus and Cinahl, starting in December 2018 and finishing on June 2019.
The search strategy included all available records with the following Medical Subject Heading Terms: Musc* tone or Spastic* or Musc* Stiffness or Musc* Propert* AND Valid* or Reliab* or Reproducib* or Accura* or Psychometr* AND Stroke or Brain Injur* or Cerebrovascular Accident* or Hemipleg* or Hemipares* or Aploplex* (Table S1). Myoton* or tonomet* were not included as descriptors in the search strategy to avoid exclusion of papers using devices of similar mechanism but different names.
Data extraction and quality assessment
First, articles were selected by screening of the title and abstract by two independent reviewers (PGG and AMHR) and duplicates were removed. The full text of eligible records were thoroughly read to determine whether the inclusion/exclusion criteria were met. Any doubt was discussed and solved with the help of other researcher (MJCH).
When articles suitable for inclusion in this research were identified two reviewers (AMHR and MJCH) independently extracted the following information from each study using a standardized form: measured psychometric properties of myotonometric devices; characteristics of participants (e.g., sample size and study groups, mean age, sex distribution, and mean time post-stroke); assessed muscles; myotonometric tool and outcome measures; reference standard for validity studies; raters and timing of measurements for reliability studies; evaluation protocol; and main results.
The quality of studies regarding risk of bias and applicability concerns was measured using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool27, which it is recommended by the Cochrane Collaboration for critical appraisal of research on diagnostic test accuracy28. The QUADAS-2 tool lists four main domains: patient selection, index test, reference standard, and flow and timing27. The reference standards differed among the included studies, hence the use of myotonometry was compared to several stated measures of mechanical, viscoelastic and functional properties of the muscle. Following previous guidelines29, no article was excluded based on the selected reference standard. We also used the COnsensus-based Standards for the selection of health status Measurement Instruments (COSMIN) Risk of Bias checklist30,31. Boxes 6 to 10 from the checklist were chosen, whenever applicable, to assess risk of bias regarding reliability, measurement error, criterion validity, hypothesis testing for construct validity and responsiveness. This newly updated checklist has been developed from the original COSMIN32, to be used exclusively in systematic reviews of measurement properties of patient reported outcome measures. Every item is ranked on a 4-point scale from very good to inadequate, and the lowest rating of any item within a box is used to determine the risk of bias (“worse score counts”)30. The two reviewers independently rated the quality of studies and, if necessary, a final consensus was reached in the case of disagreement.
Data synthesis and meta-analysis
The results for criterion and construct validity and reliability were described and the overall clinimetric evidence of the myotonometric devices was summarized. Where possible, reliability studies providing similar data (e.g., intra or inter-rater reliability for lower or upper limbs muscles) were pooled and the meta-analysis was conducted assuming a random-effects model. Data from overlapping samples were screened to avoid bias in the quantitative analysis.
For criterion validity, the strength of correlations was interpreted as low (< 0.25), fair (0.25–0.50), moderate to good (0.50–0.75) and good to excellent (> 0.75)33. For relative reliability interpretation, Intraclass Correlation Coefficients (ICCs) scores of less than 0.4 were considered poor, 0.4 to 0.59 as fair, 0.6 to 0.74 as good and > 0.75 as excellent34. For absolute reliability interpretation, a standard error of measurement (SEM) < 10% was considered small; standard real difference (SRD) was considered acceptable when < 30% and excellent when < 10%, and the narrower limits of agreement (LOA) indicates a higher stability. These benchmarks were established in accordance with previous research35 because quantification of absolute reliability statistics is controversial36. Comprehensive Meta-Analysis version 3.0 software was used to construct forest plots, and Review Manager version 5.0 was used for risk of bias and applicability concerns summary graph.
Results
Study selection
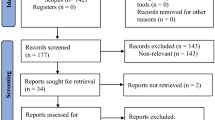
The PRISMA flow diagram (Fig. 1) shows the screening process followed to reach the final studies analyzed in this review. The initial electronic search identified 811 records, most of which were discarded at different stages. Two additional records were identified within the reference lists of other articles. Nine studies meeting the inclusion criteria were included in the qualitative synthesis37,38,39,40,41,42,43,44,45. For the quantitative analysis, three of them were excluded due to the heterogeneity in the reference standard test used43,44,45 whereas another paper was excluded because was the only one that investigated inter-observer reliability38. Therefore, only five studies were included in the meta-analysis37,39,40,41,42.
Methodological quality assessment
Figure 2 and Table S2 illustrate the assessment of methodological quality, based on the QUADAS-2 tool, in terms of risk of bias and applicability concerns. Criterion validity studies40,42,43,44,45 were assessed for all domains, whereas the rest of them were only evaluated for patient selection37,38,39,41. No single study showed a low risk of bias on all domains. Yet, all assessed studies used an acceptable protocol for the index test, except for Ryhdal et al.45, where the index test was conducted with knowledge of the results of the reference standard. The risk of bias was high in three studies for patient selection39,40,41, and in two studies for the reference standard, which was interpreted from the results of muscle strength and/or function40,42.
The COSMIN critical appraisal checklist (boxes 6 to 10) was applied to all nine studies (Tables S3–S8). Six reported the reliability and measurement error of the different tools, and were assessed as doubtful37,38,39,40,41 or inadequate40,42 methodological quality. Five studies included criterion validity, which inconsistently rated very good42,44, doubtful40,45 or inadequate methodological quality43. The three studies evaluating the construct validity of the Myotonometera or the MyotonPROb rated as having an adequate45, inadequate43 or doubtful42 methodological quality. Finally, a single study reported responsiveness and was considered with inadequate quality40.
Study design and population characteristics
For validity analysis, a sample of 188 first stroke survivors40,42,43,44,45 (30.31% women, mean age 56.53 years) in the chronic stage, mean time after stroke 32.02 months40,42,43,45 , were included together with 54 healthy controls (44.44% women, mean age 57.5 years)42,43,45. All the subjects were chronic stroke survivors (mean time post-stroke of 32.02 months). For reliability analysis, a sample of 121 individuals (35.53% women, mean age 54.29 years) was analyzed38,39,41,42. Within this sample, 80 subjects were in the chronic stage (mean time post-stroke of 22.11 months)41,42, 12 were in the sub-acute phase (3 to 9 months post-stroke)39 and the remaining 29 were in the acute stage (< 1 month post-stroke). The average sample size was 39.6 subjects (range 14 to 67) for validity studies and 34.5 (range 12 to 61) for reliability.
Three different myotonometric devices were included: Myotonometera43,44,45, Myoton-3 myometerb39,40,41 and MyotonPROb37,38,42. A detailed description of the study characteristics and main results for validity for upper and lower limbs are reported in Tables 1 and 2, respectively; and Tables 3 and 4 for reliability.
Validity
Criterion validity
Five of the studies40,42,43,44,45 measured criterion validity of myotonometric devices by comparison with other muscle property assessment tools. Different tests were used as the reference or gold standard. The MAS was the most commonly used reference test for the spastic condition assessment43,45. Dynamometry (muscle strength) was also used for comparison in two trials40,42, as well as muscle stretching tests with a torque motor44,45. In addition, Fröhlich et al.42 evaluated muscle and subcutaneous tissue thickness with an ultrasound, while Chuang et al.40 assessed arm functionality.
All studies collected data on the same patients at the same time with an appropriate interval between tests. Clear evaluation protocols were defined in all cases; myotonometric tests were performed at the resting muscle condition40,42,44, or during rest and voluntary muscle contraction43,45. Myotonometry was always conducted and interpreted prior to the reference standard except for by Rydahl and Brouwer45.
Data analysis was based on Pearson´s correlations (r)40,42,44,45, Spearman coefficients (ρ)4045 and Cramer´s V correlation43. Results from correlation analysis found moderate to high correlations (V = 0.64–0.81) between MAS scores and percentage differences of compliance at rest and muscle contraction43; low to fair correlations (ρ = 0.412–0.453) between MAS and differences of compliance at rest and 10% maximal voluntary contraction45, and no correlations between MAS and muscle compliance44. Similarly, low to fair correlations (r = 0.30–0.40) were reported between myotonometry scores and muscle strength40,42. When myotonometer values were correlated with muscle stretching tests, a negative association between muscle compliance and the area under the curve and the stretch stiffness at 100º/s were observed (r = − 0.556, − 0.607, respectively)44. In contrast, low to fair associations (r = 0.436–0.542) were obtained between differences in muscle compliance and muscle stiffness45.
Finally, low to fair correlations (r = 0.29–0.46) were also observed between myotonometry scores and muscle thickness44, and arm function (r = 0.27–0.30)40.
Construct validity
Three studies assessed construct validity of myotonometry42,43,45. These trials aimed to determine if the devices could discriminate between healthy subjects and stroke patients, and between the involved and uninvolved extremities. Data analysis was based on analysis of variance (ANOVA).
Side effects were only observed for biceps brachii compliance (p: range, 0.03–0.05)43. The effect of group was significant for the stiffness of gastrocnemius medialis, which was higher in patients than in controls (p < 0.05), but not for all the other lower limb muscles and parameters42. For muscle compliance, scores for control and stroke groups were similar at rest43,45. When data was obtained during maximal voluntary contraction, significant differences (p: range, 0.01–0.00) between stroke and control participants were observed for the biceps brachii43, but not for the gastrocnemius medialis45. Yet, evaluation of the percentage difference in compliance (rest/contraction) revealed a significant difference between participants with and without stroke (p < 0.05) with smaller differences observed in those with stroke43,45 (Tables 3 and 4).
Reliability
For studies investigating reliability37,38,39,40,41,42, five of them exclusively assessed intra-rater reliability37,39,40,41,42, and only one assessed inter-rater reliability38. The length of time between testing sessions ranged from 15 min38 up to seven days42. All studies investigated the reliability of myotonometry for muscle tone assessment. In addition, five studies explored the reliability of stiffness and elasticity/decrement assessment38,39,40,41,42, whereas muscle creep was an outcome measure in only one trial38.
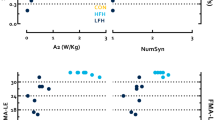
The ICC was commonly used as a statistical test for the evaluation of reliability. All studies, except for Fröhlich-Zwahlen et al.42, reported excellent intra-rater reliability, with results ranging from ICC = 0.72 to 0.96 for the upper limbs, and ICC = 0.62 to 0.92 for the lower limbs. When the data from intra-rater reliability for the upper limbs were pooled, it exhibited a mean coefficient of correlation of 0.915 (95% Confidence Interval (CI): 0.880–0.940, I2 = 69.26%) for muscle tone (Fig. 3A), 0.897 (95%CI: 0.874–0.915, I2 = 7.25%) for muscle elasticity (Fig. 3B) and 0.912 (95%CI: 0.89–0.93, I2 = 9.62%) for muscle stiffness (Fig. 3C). Pooled data for intra-rater reliability for lower limb muscle tone reported a mean coefficient of 0.785 (95%CI:0.708–0.844, I2 = 4.02%) (Fig. 4). A single study38 measured inter-rater reliability, with the results showing moderate to very-high reliability (ICC = 0.65 to 0.93 for the upper limbs, and ICC = 0.65 to 0.99 for the lower limbs). There were no differences for intra or inter-rater reliability of the evaluation of the affected or unaffected sides38,39,40. Five studies reported SEM percentages37,38,39,41,42, which ranged from 2 to 8.04% for muscle tone, 2.16 to 10.72% for muscle stiffness, 4.02 to 15.1% for muscle decrement/elasticity, and 3.05 to 5.71% for muscle creep. The SRD values were measured in three studies37,38,41 and varied from 6.65 to 18.7% for muscle tone, 9.82 to 16.5% for muscle stiffness, 13.5 to 24.4% for muscle decrement/elasticity, and 10.23% to 13.32% for muscle creep40. The percentages for the minimal detectable change ranged from 6.82 to 24.98% and were reported in one study39. Finally, the 95% LOA were evaluated in three studies37,38,41, and varied between + 4.37 to − 4.95 for muscle tone, + 88.40 to − 82.28 for muscle stiffness, + 0.76 to − 0.82 for muscle decrement/elasticity, and + 0.38 to − 0.39 for muscle creep. The high variability between studies for SEM, SRD and minimal detectable change percentages, and the 95% LOA, could be attributable to the different myotonometric devices used and the diversity of the assessment protocols (Tables 3 and 4).
Discussion
This systematic review and meta-analysis aimed to summarize the risk of bias and the findings of studies evaluating the psychometric properties of myotonometric devices used for the assessment of muscle viscoelastic properties related to the spastic hypertonia syndrome in stroke patients. A total of nine studies were included in the qualitative synthesis, and data from five of them were pooled in a meta-analysis. In general, low to moderate risk of bias and applicability concerns were observed. The following three reasons may account for lower scores: (1) no severe spasticity condition as inclusion criteria (MAS ≤ 2)39,40,41, which could be considered as a patient selection bias; (2) inappropriate time interval between measurements for reliability assessment38,42 and (3) the use of reference standards that are not likely to correctly classify the target condition40,42.
When criterion validity was analyzed, low to fair correlations were observed between muscle compliance, assessed with the Myotonometera, and the MAS scores43,44,45. These results are similar to those observed by Drenth et al.46, who analyzed the correlations between the MyotonPROb scores on the biceps brachii and the MAS for Paratonia. In this former study, no correlation was reported for elasticity, and poor associations were observed for muscle tone, stiffness and creep (ρ < 0.5). Similarly, low to fair correlations were reported between muscle strength and muscle tone, stiffness and elasticity when assessed with the Myoton-3b40 or the MyotonPROb42. In agreement with the present findings for criterion validity of myotonometry in the assessment of spasticity, Bar-On et al.47 observed poor correlations between the electrophysiological findings of the instrumented tests and the MAS scores. These findings were interpreted as a confirmation of the inadequacy of the clinical tests. Poor correlations were also obtained between the percentage differences in compliance between rest and muscle contraction and the total ankle stiffness assessed by a torque motor system45. Notwithstanding, Rydahl and Brouwer pointed out that stronger correlations could have been obtained if each muscle could be stretched in isolation45. These slight correlations are congruent with previous considerations for the measurement of spasticity. Previous research highlights that data obtained from the measurement of muscle resistance to passive movement cannot be directly associated with spasticity unless combined with neurophysiological measurement and vice versa8. Accordingly, ordinal measures have been proposed as valid clinical assessment tools for the evaluation of abnormal muscle resistance to passive motion, which has a partial reflexiogenic origin12.
On the contrary, the Myotonometera seems to discriminate between participants with and without stroke when changes in muscle compliance during muscle contraction are analysed43,45. This is consistent with previous research concluding the construct validity of using changes in muscle compliance as an indirect muscle strength measurement48. Hence, when differences in muscle compliance are evaluated in stroke survivors, smaller differences are considered to reflect a more spastic condition. The MyotonPROb scores also discriminate between stroke patients and control participants when muscle stiffness of the gastrocnemius medialis is evaluated42. Similarly, the MytonPRO also demonstrates to have a sufficient ability to discriminate between participants with and without paratonia46. Taking into account these results, the myotonometric devices seem to be a valid discriminative tool for the assessment of muscle viscoelastic property assessment in patients after stroke. However, this should be cautiously considered due to the small number of studies included. Well-known differences for validity results based on upper or lower limb muscles or type of myotonometric device were not observed. Additional research is required to establish a more solid discriminative pattern.
For reliability studies, results from meta-analysis reported excellent intra-rater reliability (ICC > 0.75) for muscle tone, elasticity and stiffness assessment in the upper limbs. Although it was not possible to pool the findings for the evaluation of muscle stiffness and elasticity of the lower limbs, a moderate to excellent intra-rater reliability was observed for all of these parameters. Similar relative reliability coefficients were obtained by Ko et al.49 when assessing lower limb muscle properties in spinal cord injury patients. The present findings are also consistent with those reported for non-neurological diseases50,51.
In general, intra-rater reliability was lower in the lower limbs than in the upper limbs. Similar results were reported for the overall intra and inter-rater reliability of the MAS52. It seems that the length and the weight of the lower limbs may hamper the assessment process. Two important issues need to be considered with regards to the reliability of the myotonometric devices. First, only the Myoton-3b and the MyotonPROb were used for reliability analysis. Second, inter-rater reliability of myotonometry has been scarcely investigated. In stroke patients, myotonometry shows moderate to excellent ICCs for all parameters in the evaluation of the upper limbs38. Very similar coefficients were reported in the muscles around the shoulder after breast cancer surgery51. Yet, inter-rater reliability in patients with paratonia is noticeably lower, which could be explained by the inherent variability of this type of muscle tone46. Finally, we observed a good absolute reliability (SEM 2–15.1%), which is congruent with previous research46,51. In summary, it can be concluded that myotonometric devices are highly reliable for assessing muscle viscoelastic properties in stroke patients, although new research focusing on inter-rater reliability is warranted.
There is compelling evidence suggesting that the assessment of the spastic hypertonia syndrome after stroke remains a challenge. Aloraini et al.10 conducted a systematic review aiming to appraise the most frequent clinical measures of spasticity after stroke reported by the literature. They summarized the psychometric properties of 15 tools (instrumented and non-instrumented) and concluded a need for objective clinical tools for the evaluation of this condition. Furthermore, the authors encouraged future research to focus on investigating the psychometric properties of clinical measures of spasticity that can be easily interpreted by clinicians. For more objective tools, particularly those that quantitatively assess spasticity by recording biomechanical and electrical signals during muscle stretching, it is concluded that no method has been sufficiently assessed on all psychometric properties. Nevertheless, these tools are more advisable than the isolated use of clinical scales47. Thus, it seems clear that no single measure should be used alone to assess the spastic condition16. With this in mind, myotonometric devices could be valid to identify those mechanical muscle properties associated with the spastic hypertonia syndrome.
Assessment protocols should be conducted under different muscle conditions (e.g., relaxation, contraction and stretching at different velocities) in order to be congruent with traditional5 and updated definitions of spasticity16. During the assessment, it would be necessary to take into account important variables, such as the patient and muscle position, the number of measurement repetitions, right-left side in case of bilateral evaluation, the presence of pain and/or fear or restiveness, contextual factors, e.g., temperature, noise, and the evaluation of different muscle spots located in the muscle belly, but also in the muscle tendon. After that, the myotonometric variables should be interpreted in combination with biomechanical measures, so that a distinction between neural and non-neural spasticity components can be achieved, and with the use of function scales12. The collection of different data would help clinicians to identify different aspects of the spastic condition, as a multifactorial phenomenon.
This study has some limitations. First, different types of myotonometric devices with different mechanisms were analyzed. Although scores from different devices are correlated, particularly during active muscle contraction, this was only studied in young healthy males53. Second, spasticity-related muscle adaptations, both in the upper54 and lower extremities55, may differ during different post stroke stages, although there is no clear evidence on this issue. In our review, only one research group investigated acute stroke patients37,38 and a single study included subacute stroke participants39, which made it difficult and no relevant to perform a subgroup analysis. Overall, findings about reliability were moderate to high for upper and lower limbs in all post-stroke stages, which is important to guide proper clinical decisions.
Third, the heterogeneity of the reference standard used in the validation studies made it impossible to carry out a concurrent validity meta-analysis. Finally, we noticed that some studies referred to the same sample population37,38,40,41. Some repeated information from these studies has only been considered once.
Conclusions
Myotometry seems to be a valid and reliable complementary tool when assessing muscle viscoelastic properties in stroke survivors. It is relatively easy and quick to administer, highly objective, and the devices are portable. The clinical interpretation of the different muscle parameters could help to quantify the spastic post-stroke condition.
Future research should focus on the validation of myotonometric devices using biomechanical and neurophysiological measurements as reference standards and analyse the inter-rater absolute and relative reliability for upper and lower limbs assessment. It would also be advisable to compare the psychometric properties of the different myotonometric devices between acute, subacute and chronic stroke patients. Finally, these studies need to provide reports on clear and reproducible protocols.
References
Feigin, V. L. et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 383, 245–254 (2014).
Rajsic, S. et al. Economic burden of stroke: a systematic review on post-stroke care. Eur. J. Heal. Econ. 20, 107–134 (2019).
Emergence of muscle overactivity. Gracies, J.-M. Pathophysiolgy of spastic paresis. II. Muscle Nerve 31, 552–571 (2005).
Wissel, J., Manack, A. & Brainin, M. Toward an epidemiology of poststroke spasticity. Neurology 80, 13–19 (2013).
Barnes, M., Kocer, S., Murie Fernandez, M., Balcaitiene, J. & Fheodoroff, K. An international survey of patients living with spasticity. Disabil. Rehabil. 39, 1428–1434 (2017).
Lieber, R. L., Steinman, S., Barash, I. A. & Chambers, H. Structural and functional changes in spastic skeletal muscle. Muscle Nerve 29, 615–627 (2004).
Dias, C. P. et al. Muscle architecture and torque production in stroke survivors: an observational study. Top. Stroke Rehabil. 24, 206–213 (2017).
De Gooijer-Van De Groep, K. L. et al. Estimation of tissue stiffness, reflex activity, optimal muscle length and slack length in stroke patients using an electromyography driven antagonistic wrist model. Clin. Biomech. 35, 93–101 (2016).
Gracies, J.-M. Pathophysiology of spastic paresis—I: paresis and soft tissue changes. Muscle Nerve 31, 535–551 (2005).
Aloraini, S. M., Gäverth, J., Yeung, E. & MacKay-Lyons, M. Assessment of spasticity after stroke using clinical measures: a systematic review. Disabil. Rehabil. 37, 2313–2323 (2015).
Ansari, N. N., Naghdi, S., Moammeri, H. & Jalaie, S. Ashworth Scales are unreliable for the assessment of muscle spasticity. Physiother. Theory Pract. 22, 119–125 (2006).
Platz, T., Eickhof, C., Nuyens, G. & Vuadens, P. Clinical scales for the assessment of spasticity, associated phenomena, and function: a systematic review of the literature. Disabil. Rehabil. 27, 7–18 (2005).
Haugh, A., Pandyan, A. & Johnson, G. A systematic review of the Tardieu Scale for the measurement of spasticity. Disabil. Rehabil. 28, 899–907 (2006).
Thibaut, A. et al. Spasticity after stroke: physiology, assessment and treatment. Brain Inj. 27, 1093–1105 (2013).
Wood, D. E. et al. Biomechanical approaches applied to the lower and upper limb for the measurement of spasticity: a systematic review of the literature. Disabil. Rehabil. 27, 19–32 (2005).
Burridge, J. H. et al. Theoretical and methodological considerations in the measurement of spasticity. Disabil. Rehabil. 27, 69–80 (2005).
Korhonen, R. K., Vain, A., Vanninen, E., Viir, R. & Jurvelin, J. S. Can mechanical myotonometry or electromyography be used for the prediction of intramuscular pressure?. Physiol. Meas. 26, 951–963 (2005).
Li, X., Shin, H., Zong, Y., Li, S. & Zhou, P. Assessing muscle compliance in stroke with the Myotonometer. Clin Biomech (Bristol, Avon) 50, 110–113 (2017).
Schneider, S., Peipsi, A., Stokes, M., Knicker, A. & Abeln, V. Feasibility of monitoring muscle health in microgravity environments using Myoton technology. Med. Biol. Eng. Comput. 53, 57–66 (2015).
Pruyn, E. C., Watsford, M. L. & Murphy, A. J. Validity and reliability of three methods of stiffness assessment. J. Sport Heal. Sci. 5, 476–483 (2016).
Ilahi, S. et al. Quantified biomechanical properties of lower lumbar myofascia in younger adults with chronic idiopatic low back pain and matched healthy controls. Clin. Biomech. 73, 78–85 (2020).
Kawczyński, A. et al. Trapezius viscoelastic properties are heterogeneously affected by eccentric exercise. J. Sci. Med. Sport 21, 864–869 (2018).
Pamukoff, D. N., Bell, S. E., Ryan, E. D. & Blackburn, J. T. The myotonometer: not a valid measurement tool for active hamstring musculotendinous stiffness. J. Sport Rehabil. 25, 111–116 (2016).
Feng, Y. N., Li, Y. P., Liu, C. L. & Zhang, Z. J. Assessing the elastic properties of skeletal muscle and tendon using shearwave ultrasound elastography and MyotonPRO. Sci. Rep. 8, 17064 (2018).
Chen, G. et al. Reliability of a portable device for quantifying tone and stiffness of quadriceps femoris and patellar tendon at different knee flexion angles. PLoS ONE 14, e0220521 (2019).
Coupland, A. P., Thapar, A., Qureshi, M. I., Jenkins, H. & Davies, A. H. The definition of stroke. J. R. Soc. Med. 110, 9–12 (2017).
Whiting, P. F. et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 155, 529–536 (2011).
Zeng, X. et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J. Evid. Based. Med. 8, 2–10 (2015).
Moore, M. & Barker, K. The validity and reliability of the four square step test in different adult populations: a systematic review. Syst. Rev. 6, 1–9 (2017).
Mokkink, L. B. et al. COSMIN risk of bias checklist for systematic reviews of patient-reported outcome measures. Qual. Life Res. 27, 1171–1179 (2018).
Terwee, C. B. et al. COSMIN methodology for evaluating the content validity of patient-reported outcome measures: a Delphi study. Qual. Life Res. 27, 1159–1170 (2018).
Terwee, C. B. et al. Rating the methodological quality in systematic reviews of studies on measurement properties: a scoring system for the COSMIN checklist. Qual. Life Res. 21, 651–657 (2012).
Portney, L. G. & Watkins, M. P. Fundations of Clinical Research: Applications to Practice (Vol. 892) (Upper Saddle River: Pearson/Prentice Halla, 2009).
Fleiss, J. L. Design and Analysis of Clinical Experiments (Wiley, New York, 2011).
Flansbjer, U. B., Holmbäck, A. M., Downham, D., Patten, C. & Lexell, J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J. Rehabil. Med. 37, 75–82 (2005).
Atkinson, G. & Nevill, A. M. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sport Med. 26, 217–238 (1998).
Lo, W. L. A. et al. Between-days intra-rater reliability with a hand held myotonometer to quantify muscle tone in the acute stroke population. Sci. Rep. 7, 14173 (2017).
Lo, W. L. A., Zhao, J. L., Li, L., Mao, Y. R. & Huang, D. F. Relative and absolute interrater reliabilities of a hand-held Myotonometer to quantify mechanical muscle properties in patients with acute stroke in an inpatient ward. Biomed. Res. Int. 2017, 4294028 (2017).
Chuang, L. L., Wu, C. Y., Lin, K. C. & Lur, S. Y. Quantitative mechanical properties of the relaxed biceps and triceps brachii muscles in patients with subacute stroke: a reliability study of the Myoton-3 myometer. Stroke Res. Treat. https://doi.org/10.1155/2012/617694 (2012).
Chuang, L. L., Wu, C. Y. & Lin, K. C. Reliability, validity, and responsiveness of myotonometric measurement of muscle tone, elasticity, and stiffness in patients with stroke. Arch. Phys. Med. Rehabil. 93, 532–540 (2012).
Chuang, L.-L. et al. Relative and absolute reliabilities of the myotonometric measurements of hemiparetic arms in patients with stroke. Arch. Phys. Med. Rehabil. 94, 459–466 (2013).
Fröhlich-Zwahlen, A. K., Casartelli, N. C., Item-Glatthorn, J. F. & Maffiuletti, N. A. Validity of resting myotonometric assessment of lower extremity muscles in chronic stroke patients with limited hypertonia: a preliminary study. J. Electromyogr. Kinesiol. 24, 762–769 (2014).
Leonard, C. T., Stephens, J. U. & Stroppel, S. L. Assessing the spastic condition of individuals with upper motoneuron involvement: validity of the Myotonometer. Arch. Phys. Med. Rehabil. 82, 1416–1420 (2001).
Li, X., Shin, H., Li, S. & Zhou, P. Assessing muscle spasticity with Myotonometric and passive stretch measurements: validity of the Myotonometer. Sci. Rep. 7, 44022 (2017).
Rydahl, S. J. & Brouwer, B. J. Ankle stiffness and tissue compliance in stroke survivors: a validation of Myotonometer measurements. Arch. Phys. Med. Rehabil. 85, 1631–1637 (2004).
Drenth, H. et al. Psychometric properties of the MyotonPRO in dementia patients with paratonia. Gerontology 64, 401–412 (2018).
Bar-On, L., Aertbeliën, E., Molenaers, G., Dan, B. & Desloovere, K. Manually controlled instrument spasticity assessments: a ystematic review of psycometric properties. Dev. Med. Child Neurol. 56, 932–950 (2014).
Gubler-Hanna, C., Laskin, J., Marx, B. J. & Leonard, C. T. Construct validity of myotonometric measurements of muscle compliance as a measure of strength. Physiol. Meas. 28, 913–924 (2007).
Ko, C. Y., Choi, H. J., Ryu, J. & Kim, G. Between-day reliability of MyotonPRO for the non-invasive measurement of muscle material properties in the lower extremities of patients with a chronic spinal cord injury. J. Biomech. 73, 60–65 (2018).
Hu, X. et al. Quantifying paraspinal muscle tone and stiffness in young adults with chronic low back pain: a reliability study. Sci. Rep. 8, 1–10 (2018).
Yeo, S. M. et al. Mechanical properties of muscles around the shoulder in breast cancer patients: intra-rater and inter-rater reliability of the MyotonPRO. Ann. Phys. Rehabil. Med. 61, e455 (2018).
Meseguer-Hanarejos, A.-B., Sánchez-Meca, J.-A. & Carles-Hernández, R. Inter- and intra-rater reliability of the modified Asworth scale: a sytematic rewiew and meta-analysis. Eur. J. Phys. Rehabil. Med. 54, 576–590 (2018).
Jarocka, E., Marusiak, J., Kumorek, M., Jaskólska, A. & Jaskólski, A. Muscle stiffness at different force levels measured with two myotonometric devices. Physiol. Meas. 33, 65–78 (2012).
Faturi, F. M., Lopes-Santos, G., Ocamoto, G. N. & Russo, T. L. Structural muscular adaptations in upper limb after stroke: a systematic review. Top. Stroke Rehabil. 26(1), 73–79 (2019).
Hunnicutt, J. L. & Gregory, C. M. Skeletal muscle changes following stroke: a systematic review and comparison to healthy individuals. Top. Stroke Rehabil. 24(6), 463–471 (2017).
Acknowledgements
This work was partially supported by the Ilustre Colegio Profesional de Fisioterapeutas de Andalucia (grant reference number 03729/19D/MA).
Author information
Authors and Affiliations
Contributions
Concept/idea/research design: M.I.G.B., A.M.H.R., P.G.G., M.J.C.H. Writing: M.I.G.B., A.M.H.R., P.G.G., M.J.C.H. Data collection: P.G.G., A.M.H.R., M.J.C.H. Data analysis: A.M.H.R., M.J.C.H. Consultation (including review of manuscript before submitting): M.I.G.B., A.M.H.R., P.G.G., M.D.C.V., M.J.C.H.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Garcia-Bernal, MI., Heredia-Rizo, A.M., Gonzalez-Garcia, P. et al. Validity and reliability of myotonometry for assessing muscle viscoelastic properties in patients with stroke: a systematic review and meta-analysis. Sci Rep 11, 5062 (2021). https://doi.org/10.1038/s41598-021-84656-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-84656-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.