Abstract
Soil salinity affects soil quality and reduces plant performance. Arbuscular mycorrhizal fungi (AMF) can enhance the tolerance of plants under salinity stress. Cultivation of eucalyptus (Eucalyptus camaldulensis), which exhibits high water use efficiency, is possible in saline areas to produce raw materials for the pulp industry. We determined the effects of arbuscular mycorrhizal fungi (AMF) on the growth and survival of eucalyptus seedlings under saline conditions. Three different clones of eucalyptus seedlings were pre-inoculated with three salt-tolerant AMF species, namely Glomus sp.2, Gigaspora albida and G. decipiens, and without pre-inoculation. The seedlings were grown in a greenhouse for 45 days. They were then transferred to individual pots, filled with field soil and subsequently treated with NaCl solution until electro-conductivity (EC) reached 10, 15 and 20 dS m−1. They were watered for 90 days under nursery conditions. The results show that increased salinity levels reduced plant performance, fractional AMF root colonization, spore number, and eucalypt K/Na ratio. AMF significantly increased chlorophyll and decreased leaf proline concentrations by more than 50% and 20% respectively and increased the K/Na ratio three- to six-fold compared with non-inoculated plants. Pre-inoculation with AMF before outplanting also improved plant performance by more than 30% under salinity stress compared to non-inoculated plants. We conclude that AMF can alleviate the negative impacts of salinity on plant physiological and biochemical parameters.
Similar content being viewed by others
Introduction
Saline soils in the twenty-first century are increasing1. Increased salinization of arable land may have a large negative global effect, predicted to result in a 30% loss of land within the next 25 years, and up to 50% by the middle of the twenty-first century2. Soil salinity is a serious problem for agriculture, particularly in arid and semi-arid regions3. Salinity limits plant growth and crop productivity. Under salinity conditions, the three main problems for plant growth include osmotic stress (physiological drought), the toxic effect of ions, notably sodium (Na) ions, and nutrient imbalance4. Arbuscular Mycorrhizal Fungi (AMF) form symbiotic associations with the roots of many plant species. AMF species occur naturally in saline environments5. Recent studies have highlighted the benefits of AMF for host plants by improving soil quality, enhancing growth, regulating substances, and resistance to plant pathogens and environmental stress6,7,8,9. Through selective ion uptake they can improve the ionic balance, as expressed in the ratio between K+ and Na+ (K/Na ratio)10. Furthermore, AMF can increase enzyme activities, protect enzymes from damage, and enhance antioxidant production. Under salinity stress, mycorrhizal plants grow better than non-AMF plants due to increased nutrient uptake, photosynthesis, water use efficiency, the production of osmoprotectants, higher K/Na ratios, and compartmentalization of Na within certain plant tissues11. These beneficial effects of AMF depend on the behavior of individual fungal species and strains12. Eucalyptus or river red gum (Eucalyptus camaldulensis Dehnh.) is a fast-growing plant native to Australia. The species can grow in a wide range of soils, from very poor to rich soils. It is one of the economically most important trees in Thailand, being used as raw material in the production of pulp, oil, furniture and housing13. Three eucalyptus species; E. alba Blume (white gum), E.microtheca F. Muell. (coolibah), and E. camaldulensis have been investigated earlier for their salt tolerance14. Eucalyptus camaldulensis is the first choice for many growers in Thailand as it can adapt to the saline soils in the northeastern region of Thailand, such as in Khon Kaen and Kalasin province, where soils range from slightly to strongly saline (EC 4–16 dS m−1)15. The species is also tolerant to various climate conditions. Cultivation of this species is therefore one option for producing wood in areas with saline soils. An effective way of expanding saline-land usage in Thailand could be the use of saline-tolerant strains of AMF, isolated from the eucalyptus rhizosphere in saline soils, to improve plant tolerance. This study therefore aimed to investigate the contribution of AMF to survival and growth of eucalyptus seedlings under salinity stress. We executed a three-factorial experiment with saline-tolerant strains of AMF, different eucalyptus clones (C), and soil salinity levels (S).
Materials and methods
AMF inoculum and plant preparation
Three AMF species, which are most frequent in saline soil areas in Khon Kaen province, viz., Glomus sp.2 (KKU-BH-001), Gigaspora albida (KKU-BP-001) and G. decipiens (KKU-BP-002), were isolated from the rhizosphere of eucalyptus from planting sites on saline soil in Ban Phai (EC 6.92 dS m-1) and Ban Haeat district (EC 5.35 dS m−1). These AMF isolates were selected after screening their salt tolerance by growing them in soils supplemented with a strong NaCl solution (EC 20 dS m−1) using a minor modification of the sandwich technique16. Briefly, AMF spores (20–30 spores) were surface-sterilized by 2% chloramine-T and washed with sterilized distilled water 4–5 times. Thereafter, surface-sterilized AMF spores were placed between two sheets of gridline sterile filter membranes with pore size diameter of 45 μm and covered by plastic frame slides that are called Hepper units. Hepper units were embedded in Petri dishes containing sterilized saline soil. Soils in each dish were watered with 25 mL sterilized distilled water and incubated in the dark at room temperature for 15–20 days. After incubation, spore germination was checked by removing the Hepper unit from the plate, cleaning by tap water, and staining with acetic glycerin solution with trypan blue. After staining, the filter membrane was gradually separated, and spore germination was observed under a stereomicroscope (Nikon SMZ445). The AMF species were subsequently propagated in maize (Zea mays L.) by the pot culture technique in a sterilized sandy loam. Pots were then placed in a greenhouse under natural lighting conditions for three months. Colonized root fragments (fractional root colonization 70–90%) and spores (24 spores g−1 dry soil) were used as inoculum. Forty-five days old eucalyptus cuttings from three clones that differ in salt tolerance were used: commercial clone H4, which can grow in sand, clone P6, which can grow in loam, and non-commercial clone H8, which can grow in sandy loam. Cuttings were obtained by using the patent of SCG packaging public company limited, Phoenix Pulp & Paper Public Co. Ltd. and Siam Forestry Co., Ltd, Thailand. Cuttings were grown in sterilized coconut dust and subsequently inoculated with 40 g inoculum in the mycorrhizal treatments, and 40 g sterilized inoculum in the non-mycorrhizal treatment.
Experimental design
The eucalyptus cuttings were transplanted into individual pots that were filled with 20 kg field soil, with the following properties: pH 4.87, EC 5.72 dS m−1, soil organic matter 3.5 g kg−1, total N 195 mg kg−1, total P 50 mg kg−1, total K 5,950 mg kg−1, exchangeable Ca 100 mg kg−1 and Na 464 mg kg−1. The experiment was a 3 × 3 × 4 complete factorial experiment in a randomized complete block design (RCBD) with three salinity levels (10, 15 and 20 dS m−1), three eucalyptus clones (H4, H8 and P6) and four AMF treatments (Glomus sp.2 KKU-BH-001, G. albida KKU-BP-001, G. decipiens KKU-BP-002, and a treatment without AMF pre-inoculation). Each treatment had three replicates. After fourteen days, to avoid plant shock from salinity, 5% of NaCl solution was gradually added to the soil every seven days to increase the initial EC from 5.72 (0% NaCl) to 10, 15 and 20 dS m−1, respectively. All eucalyptus cuttings were watered with 1,000 mL distilled water every three days, and excess water in saucer was reused in order to maintain salinity. Every six days before watering the pots we took soil samples to check the EC. Assessment of plant and fungal performance parameters was conducted at 90 days.
Assessment of plant and fungal parameters
Plant fresh and dry weight (g), and plant height (cm) were measured. Eucalyptus roots were scanned by an Epson scanner V700 PHOTO and analyzed with WINRHIZO Pro2004a (REGENT Instruments Inc., Qc, Canada). We assessed root length and root diameter and calculated on that basis specific root length, root surface area, and root tissue density.
Mycorrhizal root colonization was determined after staining with acetic glycerin solution with trypan blue and scoring root fragments with the method proposed by Trouvelot et al.17,18. Spore density (number of spores g−1 dry soil) was observed after sucrose centrifugation19.
Intensity of AMF colonization (I) was calculated using the following equation:
where “n5” means AMF root colonization level 5 (90–100%), “n4” is level 4 (50–90%), “n3” is level 3 (10–50%), “n2” is level 2 (1–10%), “n1” is level 1 (0–1%) and N is the total number of root segments.
Plant nutrient analysis
Plant N concentration was determined after digestion by the Kjeldahl method and analyzed by the FLA method20, while plant P and K concentrations were determined by the wet oxidation method21 and Na concentration determined by flame photometer22.
Leaf relative water content (LRWC)
Leaf disc samples (10 mm diameter) were punched from each plant after 90 days to determine the tolerance of mycorrhizal and non-mycorrhizal plants at each salinity level. We calculated LRWC using the following equation23:
where FW is leaf fresh weight, DW is leaf dry weight after 24 h of drying at 70 °C, and TW is leaf turgid weight after being soaked in distilled water for 24 h.
Leaf chlorophyll concentration
Leaf chlorophyll concentration (chlorophyll a, chlorophyll b, and total chlorophyll) was determined by the method described by Arnon24. Fresh leaves (0.5 g) were ground with 20 mL of 80% acetone. The homogenate was then centrifuged at 4,000 rpm for 15 min. The supernatant was read using a spectrophotometer (Thermo Scientific GENESYS 10S UV/Vis Spectrophotometer, model EW-02654–22) at absorbance readings at 645 (A645) and 663 (A663) nm. The chlorophyll content was calculated using the following formulae:
Proline concentration
Proline concentrations were determined using the method described by Bates et al.25. Fresh leaves (0.5 g) were homogenized in 10 mL of 3% sulfosalicylic acid and then sieved through Whatman’s No. 1 filter paper. Then 2 mL filtrate solution were mixed with 2 mL of acid-ninhydrin and glacial acetic acid in a test tube, respectively. The reaction mixture test tubes were placed in a water bath at 100 °C for 1 h and then placed in ice to stop the reaction. The mixture was extracted by 4 mL toluene and the chromophore containing the toluene was separated to measure absorbance of 520 nm using a Thermo Scientific GENESYS 10S UV/Vis Spectrophotometer (model EW-02654–22). The calculated proline concentration was then compared with the proline standard.
Statistical analysis
The treatment effects and the interactions were tested by three-way analysis of variance (ANOVA) using the Statistix program version 8.0. All data complied with the ANOVA assumptions of homoscedasticity and normality. Means were compared between treatments using Tukey's Honestly Significant Difference (HSD) at a 0.05 probability level.
Results and discussion
Results of the analysis of variance are provided in Table 1. In almost all cases, salinity and AMF were significant sources of variation. Interactions between AMF and clone were significant sources of variation (except for leaf P concentration), indicating species-specific AMF responses on different eucalyptus clones. Eucalyptus clone and the other interactions were significant sources of variation for a number of parameters as well.
AMF colonization and spore density
Control plants (plants that were not pre-inoculated) were also colonized by AMF, which was caused by the experiment, which, after pre-inoculation or not in sterilized soil, was executed in non-sterile field soil, however, colonization levels were much lower than in the pre-inoculated seedlings. Spore density and fractional root colonization significantly (P ≤ 0.05) declined with increasing salinity levels (Table 2). Mycorrhizal colonization and spore density were very significantly correlated (r = 0.64; n = 36; P < 0.001). The significant interaction between AMF and salinity level for both parameters (Table 1) indicated that the protective effect of pre-inoculation diminished at higher salinity levels. The interaction between AMF and eucalyptus clone was also significant for fractional root colonization, suggesting species-specific responses to different eucalyptus clones. Root colonization and spore densities with clone H4 and H8 were highest with G. albida, while eucalyptus clone P6 showed highest spore densities and root colonization with Glomus sp.2. AMF are generally characterized as showing little or no host specificity, however plant species or plant variety-specific responses to individual species of AMF have been observed before26,27. Our results are consistent with earlier studies that showed that salinity inhibited spore germination, suppressed the growth of hyphae after initial infection, and reduced the number of arbuscules28,29,30,31.
Plant performance
Both AMF and salinity were significant sources of variation for root and shoot biomass, whereas clone was only a significant source of variation for root parameters. The interaction between AMF and clone was also significant, again demonstrating AMF species-specific responses in combination with different clones (Table 1). Salinity decreased plant performance parameters, with a larger effect at higher salinity levels, whereas pre-inoculated plants produced more biomass than control plants. At all salinity levels, plants pre-inoculated with G. albida usually showed higher biomass than plants pre-inoculated with the other AMF species (Table 3). However, at the salinity level of 15 dS m−1, eucalyptus clone P6 pre-inoculated with Glomus sp.2, was significantly heavier than when pre-inoculated with the other AMF species. These data fit with the selectivity of the different AMF for different clones as assessed by fractional root colonization and spore density. Negative effects of salinity have been reported for many glycophytes, such as Allium cepa L., Medicago sativa L., Triticum aestivum L. and Hordeum vulgare L.28,29,32 and the alleviation of these negative effects of salinity by AMF, and plant and fungal species specificity with respect to this protective effect has also regularly been reported31,33,34,35.
Leaf relative water content (LRWC) was also significantly affected by salinity (S), AMF, eucalyptus clone (C), and the interaction of AMF × C and S × C (Table 1). Salinity reduced, but mycorrhizal plant increased LRWC. Again, eucalyptus clone H8 that was pre-inoculated with G. albida and clone P6 pre-inoculated with Glomus sp.2, showed the highest positive mycorrhizal effect (Table 3). There are several reasons why the AMF plants have a higher LRWC, (1) AMF roots have higher hydraulic conductivity at low water potential36; (2) AMF induce alterations to the root system37; (3) mycorrhizal plants have higher stomatal conductance38; (4) AMF accumulate solutes and improve plant osmotic adjustment39, and (5) improved water relation by AMF hyphae40.
Root length and root surface area were both significantly affected by salinity level, AMF and interaction of AMF × C. In the case of root surface area, the interaction of S × C was also significant (Table 1). Root length was significantly positively correlated with LRWC. Root diameter showed a significant negative correlation with root length, specific root length, and root tissue density (Table 4). Salinity reduced, and pre-inoculation with mycorrhiza increased, root length and root surface area (Table 3). Seedlings pre-inoculated with Glomus sp.2 had larger root diameter than control seedlings and seedlings pre-inoculated by both Gigaspora species, an effect described before41 and likely due to hormonal effects.
Plant nutrient concentration
AMF, salinity, and eucalyptus clone were all significant sources of variation, and many interactions were significant as well (Table 1). Especially the interaction of AMF × S was significant for N, Na and the K/Na ratio, but not for P and K. Concentrations of N, P and K in plant shoots decreased with high salinity, while those of Na increased. The mycorrhizal effect on lowering Na concentrations was stronger than the mycorrhizal effect in increasing K concentrations; in combination, pre-inoculation with AMF increased the K/Na ratio three- to sixfold. Pre-inoculation with AMF increased leaf nutrient concentrations compared to the non-inoculated control across all salinity levels. Eucalyptus clones H4 and H8 benefitted most when pre-inoculated with G. albida, showing higher N, P, K, and lower Na concentrations than the control whereas P6 was positive when pre-inoculated with Glomus sp.2 (Table 5). Many studies have reported that increasing salinity levels lowered N and K concentrations, for example in pepper (Piper nigrum L.), olive (Olea europaea L.), peanut (Arachis hypogaea L.) and faba bean (Vicia faba L.)42,43,44,45. High concentrations of K can maintain K/Na ratio and photosynthetic rate. Higher phosphorus (P) uptake in all pre-inoculated plants is consistent with the major role of AMF in extending the depletion zone of P in the rhizosphere and increasing P uptake. Both a higher-affinity uptake system and a lower threshold concentration for absorption by AMF than by plant roots are major mechanisms of higher P uptake46,47.
Leaf chlorophyll concentration
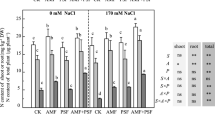
Leaf chlorophyll concentration, an important physiological indicator for plant photosynthetic capacity, was significantly affected by all three main factors (salinity, AMF, eucalyptus clone) and by all two-way and three-way interactions (Table 1). Salinity significantly reduced leaf chlorophyll concentration (Fig. 1) likely caused by repression of specific enzymes of the photosynthesis system and reduction of nutrient uptake such as Magnesium (Mg) and Nitrogen (N) for chlorophyll biosynthesis48,49. Mycorrhiza significantly increased leaf chlorophyll concentration. This result is likely due to enhanced nutrient uptake and reduced Na concentrations in the plants, resulting in overall higher photosynthetic capability50. In some combinations of eucalyptus clone and AMF species, there was a major effect when increasing salinity levels from 10–15 dS m−1, whereas in other combinations a major decline was observed only when salinity increased from 15 to 20 dS m−1. Due to the fact that two-way and three-way interactions were significant, other patterns were difficult to explain. Eucalyptus clones H4 and H8 pre-inoculated with G. albida had higher chlorophyll concentration compared to other AMF treatments, while eucalyptus clone P6 pre-inoculated with Glomus sp.2 had higher leaf chlorophyll concentration than the other AMF treatments.
Effect of AMF pre-inoculation and salinity on leaf chlorophyll a (panels a,d,g); chlorophyll b (panels b,e,h) and total chlorophyll concentration (panels c,f,i) of eucalyptus three eucalyptus clones (H4; panels a–c), H 8: panels d–f) and P6 (panels g–i) after cultivation for 90 days. Mean values shown, in which the same letters above the bars represent no significant difference, according to HSD at P ≤ 0.05. Abbreviation: AMF1; Glomus sp.2, AMF2; G. albida, AMF3; G. decipiens, control; not pre-inoculated with AMF, S1; 10 dS m−1, S2; 15 dS m−1, S3; 20 dS m−1.
Leaf proline concentration
The accumulation of free amino acid, proline-reported modifications induced by water and salt stress, and an exogenous application of proline could play an important role in enhancing plant stress tolerance3,49. In saline conditions, plants can accumulate proline as a protective osmolyte, maintain an osmotic balance, stabilize proteins and membranes, protect plants against free radical-induced damage, and maintain appropriate NADP+/NADPH ratios51,52. Our study resulted that leaf proline concentrations were significantly affected by all main factors (AMF, S, C) and all two-way and three-way interactions (Table 1). Proline concentrations increased with increasing salinity and were lower in AMF pre-inoculated seedlings compared with control plants, At the lowest salinity level there were significant differences between varieties, with H8 showing lowest proline concentration and H4 showing highest concentrations. With increasing salinity levels, the differences between the eucalyptus clones attenuated. Clones H4 and H8 pre-inoculated with G. albida and P6 pre-inoculated with Glomus sp.2 had significantly lower proline concentrations across all salinity levels (Fig. 2). Proline concentrations were negatively correlated with the concentrations of chlorophyll a, chlorophyll b, and total chlorophyll (Table 6). Apparently, higher nutrient uptake, LRWC, and chlorophyll content due to the mycorrhizal symbiosis constitute an alternative way to alleviate salt stress without increasing proline production. Many authors have reported that proline concentrations increased in AMF plants compared to non-AMF plants53, while other authors have reported greater proline accumulation in non-AMF plants than AMF plants for example, in Ocimum basilicum L. and Arachis hypogaea L6,54. The underlying mechanisms deserve further study.
Effect of salinity level and AMF pre-inoculation on leaf proline concentration of eucalyptus clone H4 (A), H8 (B) and P6 (C) 90 days after planting. Mean values shown, in which the same letters above the bars represent no significant difference, according to HSD at P ≤ 0.05. Abbreviation: AMF1; Glomus sp.2, AMF2; G. albida, AMF3; G. decipiens, control, not pre-inoculated with AMF. S1; 10 dS m−1, S2; 15 dS m−1, S3; 20 dS m−1.
Conclusions
Salinity reduced the growth and performance of eucalyptus seedlings due to negative effects of Na on physiological and biochemical parameters. Salinity reduced the uptake of important mineral nutrients. AMF species mitigated these negative effects by increasing the uptake of major elements (N, P, K) and the reducing uptake of Na, resulting in a much more favorable K/Na balance of pre-inoculated plants than in non-inoculated ones. Pre-inoculation with AMF also reduced plant proline concentrations, the osmoprotectant that could help non-mycorrhizal plants to alleviate salt stress. However, enhanced proline production was a less successful strategy for plant salt tolerance compared to the mycorrhizal symbiosis. Different eucalyptus clones had specific relations with certain AMF species to reduce the negative impacts of salinity on the studied physiological and biochemical parameters. Eucalyptus clones H4 and H8 pre-inoculated with G. albida and clone P6 pre-inoculated with Glomus sp.2 resulted in AMF plants that had a better growth in saline soil. Pre-inoculation with AMF therefore seems an important practice to obtain healthy eucalyptus plants in saline soils.
References
Shrivastava, P. & Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 22, 123–131 (2015).
Wang, W. X., Vinocur, B. & Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 218, 1–14 (2003).
Ashraf, M. & Foolad, M. R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 59, 206–216 (2007).
White, P. J. Ion Uptake Mechanisms of Individual Cells and Roots: Short-distance Transport. In Marschner's Mineral Nutrition of Higher Plants (Third Edition 7–47. (2012).
Juniper, S. & Abbott, L. K. Soil salinity delays germination and limits growth of hyphae from propagules of arbuscular mycorrhizal fungi. Mycorrhiza 16, 371–379 (2006).
Al-Karaki, G. N. Nursery inoculation of tomato with arbuscular mycorrhizal fungi and subsequent performance under irrigation with saline water. Sci. Hortic. 109, 1–7 (2006).
Ben-Laouane, R., Baslam, M., Ait-El-Mokhtar, M., Anli, M., Boutasknit, A., Ait-Rahou, Y., Meddich, A. Potential of native arbuscular mycorrhizal fungi, rhizobia, and/or green compost as alfalfa (Medicago sativa) enhancers under salinity. Microorganisms, 8, article 1695. (2020).
Ait-El-Mokhtar, M., Baslam, M., Ben-Laouane, R., Anli, M., Boutasknit, A., Mitsui, T., Meddich, A. Alleviation of detrimental effects of salt stress on date palm (Phoenix dactylifera L.) by the application of arbuscular mycorrhizal fungi and/or compost. Front. Sustain. Food Syst., 4, article 131. (2020).
Ait-El-Mokhtar, M., Laouane, R. B., Anli, M., Boutasknit, A., Wahbi, S., & Meddich, A. Use of mycorrhizal fungi in improving tolerance of the date palm (Phoenix dactylifera L.) seedlings to salt stress. Scientia Horticulturae, 253, 429–438. (2019).
Giri, B., Kapoor, R. & Mukerji, K. G. Improved tolerance of Acacia nilotica to salt stress by arbuscular mycorrhiza, Glomus fasciculatum may be partly related to elevated K/Na ratios in root and shoot tissues. Microb. Ecol. 54, 753–760 (2007).
Augé, R. M., Toler, H. D., & Saxton, A. M. Arbuscular mycorrhizal symbiosis and osmotic adjustment in response to NaCl stress: a meta-analysis. Front. Plant Sci. 5, article 562. (2014).
Yang, S. J., Zhang, Z. L., Xue, Y. X., Zhang, Z. F., & Shi, S. Y. Arbuscular mycorrhizal fungi increase salt tolerance of apple seedlings. Bot. Stud. 55, article 70. (2014).
Reungchai, P. Eucalyptus Plantations in Thailand In Reports Submitted to the Regional Expert Consultation on Eucalyptus - Volume II. (1996).
Fathi, R. A. & Prat, D. Effects of saline stress on Eucalyptus seedlings. Annales des Sciences Forestières 46, 376–378 (1989).
Mohammad, Z., Shabbir, A. S. & Lee, H. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In: Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques. 43–53. (2018).
Hepper, C. M. Gemination and growth of Glomus caledonius spores: the effect of inhibitors and nutrients. Soil Biol. Biochem. 11, 269–277 (1979).
Koske, R. E. & Gemma, J. N. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 92, 486–488 (1989).
Trouvelot, A., Kough, J. L. & Gianinazzi-Pearson, V. Mesure du taux de mycorhization VA d'un système radiculaire. Recherche de methodes d'estimation ayant une signification fonctionnelle. In: Physiological and genetical aspects of mycorrhizae. 217–221. (1986).
Daniels, B. A., & Skipper, H. D. Methods for the recovery and quantitative estimation of propagules from soil. In: Methods & principles of mycorrhizal research. 29–35. (1982).
Jackson, M. L. Soil Chemical Analysis 498. (1967).
Hesse, P. R. A Textbook of Soil Chemical Analysis.: Chemical Pub. Co. (1972).
Martinez, B., Oliveira, A., Pedro, F., Oliveira, J. & Villa, R. Determination of the sodium concentration in Brazilian light and non-light powdered instant soups by flame photometry. Curr. Nutr. Food Sci. 11, 131–135 (2015).
Schonfeld, M. A. Water relations in winter wheat as drought resistance indicators. Crop Sci. 28, 526–531 (1988).
Arnon, D. I. Copper enzymes in isolated chloroplasts Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24, 1–15 (1949).
Bates, L. S., Waldren, R. P. & Teare, I. D. Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207 (1973).
van der Heijden, M. G. A. & Horton, T. R. Socialism in soil? The importance of mycorrhizal fungal networks for facilitation in natural ecosystems. J. Ecol. 97, 1139–1150 (2009).
Zhang, Q., Sun, Q., Koide, R. T., Peng, Z., Zhou, J., Gu, X.., & Yu, M. Arbuscular mycorrhizal fungal mediation of plant-plant interactions in a marshland plant community. Sci. World J. 2014, article 923610 (2014).
Pfeffer, C. M. & Bloss, H. E. Growth and nutrition of guayule (Parthenium argentatum) in a saline soil as influenced by vesicular–arbuscular mycorrhiza and phosphorus fertilization. New Phytol. 108, 315–321 (1988).
McMillen, B. G., Juniper, S. & Abbott, L. K. Inhibition of hyphal growth of a vesicular-arbuscular mycorrhizal fungus in soil containing sodium chloride limits the spread of infection from spores. Soil Biol. Biochem. 30, 1639–1646 (1988).
Evelin, H., Kapoor, R. & Giri, B. Arbuscular mycorrhizal fungi in alleviation of salt stress: A review. Ann. Bot. 104, 1263–1280 (2009).
Latef, A. A. H. A. & He, C. X. Effect of arbuscular mycorrhizal fungi on growth, mineral nutrition, antioxidant enzymes activity and fruit yield of tomato grown under salinity stress. Sci. Hortic. 127, 228–233 (2011).
Aliasgharzadeh, N., Rastin, N. S., Towfighi, H. & Alizadeh, A. Occurrence of arbuscular mycorrhizal fungi in saline soils of the Tabriz Plain of Iran in relation to some physical and chemical properties of soil. Mycorrhiza 11, 119–122 (2001).
Wu, Q. S. & Zou, Y. N. Arbuscular mycorrhizal symbiosis improves growth and root nutrient status of citrus subjected to salt stress. Science Asia 35, 388–391 (2009).
Van der Heijden, M. et al. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396, 69–72 (1998).
Tawaraya, K. Arbuscular mycorrhizal dependency of different plant species and cultivars. Soil Sci. Plant Nutr. 49, 655–668 (2003).
Kapoor, R., Sharma, D. & Bhatnagar, A. K. Arbuscular mycorrhizae in micropropagation systems and their potential applications. Sci. Hortic. 116, 227–239 (2008).
Kothari, S. K., Marschner, H. & George, E. Effect of VA mycorrhizal fungi and rhizosphere microorganisms on root and shoot morphology, growth and water relations in maize. New Phytol. 116, 303–311 (1990).
Sheng, M. et al. Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza 18, 287–296 (2008).
Abdel Latef, A. A. H. & Chaoxing, H. Does inoculation with Glomus mosseae improve salt tolerance in pepper plants?. J. Plant Growth Regul. 33, 644–653 (2014).
Augé, R. M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11, 3–42 (2001).
Berta, G., Fusconi, A. & Trotta, A. VA mycorrhizal infection and the morphology and function of root systems. Environ. Exp. Bot. 33, 159–173 (1993).
Kaya, C. et al. The influence of arbuscular mycorrhizal colonisation on key growth parameters and fruit yield of pepper plants grown at high salinity. Sci. Hortic. 121, 1–6 (2009).
Porras-Soriano, A., Soriano-Martin, M. L., Porras-Piedra, A. & Azcón, R. Arbuscular mycorrhizal fungi increased growth, nutrient uptake and tolerance to salinity in olive trees under nursery conditions. J. Plant Physiol. 166, 1350–1359 (2009).
Al-Khaliel, A. S. Effect of salinity stress on mycorrhizal association and growth response of peanut infected by Glomus mosseae. Plant Soil Environ. 56, 318–324 (2010).
Rabie, G. & Almadini, A. M. Role of bioinoculants in development of salt-tolerance of Vicia faba plants under salinity stress. Afr. J. Biotech. 4, 210–222 (2005).
Balliu, A., Sallaku, G. & Rewald, B. AMF inoculation enhances growth and improves the nutrient uptake rates of transplanted, salt-stressed tomato seedlings. Sustainability 7, 15967–15981 (2015).
Bolduc, A., & Hijri, M. The use of mycorrhizae to enhance phosphorus uptake: A way out the phosphorus crisis. J. Biofertil. Biopest. 2, article 104 (2011).
Murkute, A. & Singh, S. K. Studies on salt stress tolerance of citrus rootstock genotypes with arbuscular mycorrhizal fungi. Hortic. Sci. 33, 70–76 (2006).
Selvakumar, G. & Thamizhiniyan, P. The effect of the arbuscular mycorrhizal (AM) fungus Glomus intraradices on the growth and yield of Chilli (Capsicum annuum L.) under salinity stress. World Appl. Sci. J. 14, 1209–1214 (2011).
Giri, B., Kapoor, R. & Mukerji, K. G. Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass, and mineral nutrition of Acacia auriculiformis. Biol. Fertil. Soils 38, 170–175 (2003).
Aggarwal, A., Kadian, N., Karishma, N., Tanwar, A. & Gupta, K. K. Arbuscular mycorrhizal symbiosis and alleviation of salinity stress. J. Appl. Natl. Sci. 4, 144–155 (2012).
Sannazzaro, A. I., Echeverria, M., Alberto, E. O., Ruiz, O. A. & Menendez, A. B. Modulation of polyamine balance in Lotus glaber by salinity and arbuscular mycorrhiza. Plant Physiol. Biochem. 45, 39–46 (2007).
Azooz, M., Shaddad, M. A. & Abdel Latef, A. The accumulation and compartmentation of proline in relation to salt tolerance of three sorghum cultivars. Indian J. Plant Physiol. 9, 18 (2004).
Elhindi, K. M., El-Din, A. S., & Elgorban, A. M. The impact of arbuscular mycorrhizal fungi in mitigating salt-induced adverse effects in sweet basil (Ocimum basilicum L.). Saudi J. Biol. Sci., 24, 170–179. (2017).
Acknowledgments
We are also indebted to Department of Microbiology, Faculty of Science, Khon Kaen University, The Center of Excellence on Biodiversity (BDC), Office of Higher Education Commission (BDC-PG2-159011). This work was partly supported from Chiang Mai University. We also thanks SCG packaging-The Siam Forestry Co., Ltd. for providing facilities and eucalyptus seedlings. Dr Samantha C. Karunarathana, Key Laboratory of Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Science, Kunming 65201, P.R, China is thanked for editorial help during preparation of the manuscript. We are grateful to three anonymous reviewers for their constructive comments on an earlier version of this manuscript.
Funding
This work was financially supported by Research and Researcher for Industry (RRI) of Thailand, Grant No. PHD58I0033.
Author information
Authors and Affiliations
Contributions
C.K. and S.B. designed, planned and executed the experiment and interpreted the results. S.L. and T.W.K. provided critical feedback and assisted in interpretation of the results. All authors (C.K., S.B., S.L., and T.W.K.) discussed the results and their interpretation, contributed to the final manuscript, and approved its submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Klinsukon, C., Lumyong, S., Kuyper, T.W. et al. Colonization by arbuscular mycorrhizal fungi improves salinity tolerance of eucalyptus (Eucalyptus camaldulensis) seedlings. Sci Rep 11, 4362 (2021). https://doi.org/10.1038/s41598-021-84002-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-84002-5
This article is cited by
-
Uncovering the impact of AM fungi on wheat nutrient uptake, ion homeostasis, oxidative stress, and antioxidant defense under salinity stress
Scientific Reports (2023)
-
Arbuscular mycorrhiza: advances and retreats in our understanding of the ecological functioning of the mother of all root symbioses
Plant and Soil (2023)
-
Deciphering the genetic and functional diversity of cultivable bacteria from chasmophytic pigweed (Chenopodium album) from Tsomoriri, Ladakh, India
3 Biotech (2022)
-
Relationships Between Mycorrhizal Attributes and Stable Carbon and Azote Isotopes in a Semi-arid Environment as Influenced by Durum Wheat Cultivars and Salinity Level
Journal of Soil Science and Plant Nutrition (2022)
-
Role of arbuscular mycorrhizal fungi as an underground saviuor for protecting plants from abiotic stresses
Physiology and Molecular Biology of Plants (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.