Abstract
Insecticide resistant Aedes populations have recently been reported in Pakistan, imposing a threat to their control. We aimed to evaluate the susceptibility of Aedes aegypti and Aedes albopictus populations from Lahore to WHO-recommended insecticides and to investigate metabolic and target-site resistance mechanisms. For this purpose, we first carried out bioassays with the larvicides temephos and pyriproxyfen, and the adulticides malathion, permethrin, deltamethrin, alpha-cypermethrin, and etofenprox. We looked for Knockdown resistance mutations (kdr) by qPCR, High-Resolution Melt (HRM), and sequencing. In order to explore the role of detoxifying enzymes in resistance, we carried out synergist bioassay with both species and then checked the expression of CYP9M6, CYP9J10, CYP9J28, CYP6BB2, CCAe3a, and SAP2 genes in Ae. aegypti. Both species were susceptible to organophosphates and the insect growth regulator, however resistant to all pyrethroids. We are reporting the kdr haplotypes 1520Ile + 1534Cys and T1520 + 1534Cys in high frequencies in Ae. aegypti while Ae. albopictus only exhibited the alteration L882M. PBO increased the sensitivity to permethrin in Ae. aegypti, suggesting the participation of P450 genes in conferring resistance, and indeed, CYP928 was highly expressed. We presume that dengue vectors in Lahore city are resistant to pyrethroids, probably due to multiple mechanisms, such as kdr mutations and P450 overexpression.
Similar content being viewed by others
Introduction
Lahore, the second-largest Pakistani city, also known as Pakistan’s cultural capital, has faced several outbreaks of dengue in the last two decades. The biggest of which was in 2011 when more than 20,000 people were hospitalized and 350 died1. Dengue virus has four serotypes, and all four have been reported in Lahore2. However, serotype 2 (genotype IV) was more prevalent and was responsible for high mortality and morbidity in Lahore3. The most recent outbreaks in the country took place in 2019 when more than 47,000 patients were admitted to hospitals (up to November) due to mild dengue fever, severe hemorrhagic fever, or shock. Lahore was once again the hotspot of the infection4.
Dengue virus is transmitted to humans by the bite of a female infected Aedes aegypti Linnaeus, 1762 and Aedes albopictus Skuse, 1895 (Diptera: Culicidae). The recent spread of the vector population and global environmental conditions, such as the average temperature increase, has promoted the disease's transmission beyond the tropics5,6. In the absence of a preventive vaccine and potent treatment, effective vector management remains the best option to keep dengue under control7. Both Aedes species are abundant in Pakistan, with Ae. albopictus found at high altitude and in peri-urban areas and Ae. aegypti most abundant in urban settlements. In Lahore, both mosquito species are found, but Ae. aegypti is relatively more abundant8. Apart from dengue, chikungunya was detected in patients from Karachi and Lahore9,10. So far, there is no evidence of Zika and yellow fever transmission in Pakistan.
Since the beginning of the dengue outbreaks in Lahore, neurotoxic insecticides like pyrethroids (PY), mainly permethrin and deltamethrin, have been used as adulticides, while the organophosphate (OP) temephos as a larvicide. When a case of dengue is reported, comprehensive vector surveillance and health education activities are conducted in all houses at a radius of approximately 200 m from the index house. Potential breeding sites are searched and mechanically eliminated, temephos is applied in containers that cannot be managed physically. Alpha-cypermethrin (10% SC) is applied as a residual spray on the expected vector's resting sites. All houses or industrial areas present in a 50 m radius are also sprayed with this pyrethroid. Deltamethrin was used for vector control before the introduction of alpha-cypermethrin. In addition to case response, larvicide temephos is used during routine vector surveillance in addition to the mechanical elimination of potential vector breeding sites in the Punjab province. The option of space spray is used during outbreaks only under the advice of a technical committee. All vector control practices, including space spraying and larvicides applications, are applied by the public sector. Community is well educated to eliminate possible breeding places from their houses and workplaces mechanically. Legal action is taken against the occupants of the premises in non-compliance with the instructions regarding eliminating mosquito breeding sites in their premises11. Resistance against pyrethroids in Aedes has been reported from various parts of Punjab12 and Khyber Pakhtunkhwa provinces13. Similarly, in the Indian capital city of New Delhi, which is only 425 km from Lahore, pyrethroid-resistant Ae. aegypti are prevalent14. Resistance to temephos in Aedes has also been shown in several districts of Punjab province, including Lahore and its neighbor districts15. On the other side, Insect Growth Regulators (IGRs) were effective against Aedes in the area, at least until 200816.
Neurotoxic insecticides interfere with the central nervous system of insects. Pyrethroids (PYs) and the organochlorines (OCs) target the voltage-sensitive sodium channel (NaV, also commonly referred to as Vssc or Vgsc). This protein comprises four homologous domains (I-IV), each containing six transmembrane subunits of α-helix (S1–S6) and a P-loop connecting S5 and S6 segments. The first four segments make the voltage-sensing domain (S1–S4), while the last two segments (S5 and S6) along with their respective P-loops assemble in such a way to form the pore, which is responsible for the action potential of ions, particularly Na+. Upon binding, PY and OC keep this pore or channel open for more extended periods resulting in fast contractions, paralysis, and eventual death, known as the knockdown effect17,18,19. Pyrethroids are either type I or type II based on their chemical structure. Type I (for example, permethrin) lack an alpha-cyano group, while type II (for example, deltamethrin and cypermethrin) have an alpha-cyano group close to phenyl benzyl alcohol moiety. On the other hand, organophosphates (OPs) and carbamates (CAs) target the acetylcholinesterase (AChE, EC 3.1.1.7) coded by the ace-1 gene18. Insect Growth Regulators (IGRs) mimic the growth hormones, not directly killing the insect but affecting their growth, development, physiology, and behavior, ultimately provoking the insect death20.
Continuous application of insecticides results in the selection of resistant insects. Resistance mechanisms in arthropod vectors are provoked by behavioral or physiological changes. Physiological responses in the form of target-site modifications alone can be sufficient to confer pyrethroids resistance in Aedes. One or more point mutations can achieve resistance to pyrethroids' knockdown effect (kdr) in the voltage-gated sodium channel gene (Nav). About 11 kdr mutations alone or in combinations are classically associated with PY and DDT resistance in Aedes. Of them, Val410Leu (IS6 segment), Ser989Pro (IIS6 segment), Val1016Ile/Gly (IIS6 segment), and Phe1534Cys (IIIS6 segment) are well studied21. A more recently described mutation, Thr1520Ile (IIIS6 segment), is also associated with PY resistance in Ae. aegypti populations from India22 and Thailand23. The 1534Cys is the only kdr mutation widespread on a global scale. On the other hand, mutation at the 1016 site varies geographically, as Val1016Gly is predominant in populations from Asia while Val1016Ile occurs in Latin American23. Surprisingly, Val1016Ile has also been detected in Vietnam as well24, and Val1016Gly in Panama25. Of other notable mutations, Val410Ile is restricted to Latin America and Ser989Pro to Asia, but they alone do not cause resistance21. The 1534C alone can confer resistance to PYs, and this resistance can reach higher levels when combined with other mutations like 989P, 1016Ile/Gly, or 1520I26. Contrary to pyrethroids, target site modification in OP resistance, Gly119Ser (G119S) in the ace-1 gene of other mosquito species is not likely to occur in the genus Aedes due to codon constraints7,27.
Metabolic resistance mechanisms are also very well investigated in Ae. aegypti, aiding in xenobiotic detoxification through enhanced expression or gene alterations28. Enzymes that detoxify neurotoxins are mainly from the cytochrome P450 dependent multi-function oxidases (CYP450 MFOs), esterases (ESTs), or glutathione-S-transferases (GSTs) families28. Around 160 MFOs29 have been reported to have altered expression in the resistant Aedes population, of which CYP9J22, CYP9J26, CYP9J28, CYP9M6, CYP9M9, CYP6BB2, and CYP9J10 have been strongly associated with PY resistance. The esterase gene CCEAe3a is generally overexpressed in OP resistant populations7,30. A recent observation was the overexpression of a sensory appendage protein (SAP2) in PY resistant Anopheles gambiae population from Burkina Faso31.
In this study, we have evaluated the susceptibility/resistance profile of Ae. aegypti and Ae. albopictus populations from Lahore to the insecticides in practice and the alternative compounds IGR pyriproxyfen. We investigated the main molecular mechanisms involved in insecticide resistance in Aedes populations from Lahore for the first time. These results will enrich the knowledge about insecticide resistance in Pakistani Aedes species and contribute to vector control monitoring actions that would ultimately reduce Pakistan's arboviruses burden.
Results
Bioassays with larvicides
Temephos
Dose–response assays determined the lethal concentrations (LCs) and resistant ratios (RRs) in Aedes aegypti (PAg) and Aedes albopictus (PAb) populations from Lahore, Pakistan, with serial dilutions from 0.001 to 0.018 mg/L (Fig. 1a). The LC50 for Aedes aegypti (PAg) and Aedes albopictus (PAb) populations were 0.006 mg/L and 0.008 mg/L, with respective RR50 as 1.50 and 2.04, employing Ae. aegypti Rock as a baseline reference. Accordingly, LC90 were 0.011 and 0.014, with RR90 of 2.0 and 2.3 for PAg and PAb, respectively (Table 1).
Larvicide bioassays with Aedes aegypti and Aedes albopictus from Lahore, Pakistan. (a) Dose–response bioassay to the organophosphate temephos, with mortality evaluated after 24 h of exposure. (b) Proportional mortality of each life stage to the diagnostic dose of the IGR pyriproxyfen. The adult emergence inhibition (AEI) was 100% in all experimental cases. (c) Susceptibility index (SI) average (with SEM error bars) for the pyrethroids permethrin and deltamethrin. The higher the SI, the less susceptible the population. Ae. aegypti Rockefeller strain (Rock) was used as a reference in all these larvicide bioassays.
Pyriproxyfen
We performed a qualitative bioassay (diagnostic dose assay) with the insect growth regulator (IGR) pyriproxyfen using a diagnostic dose of 0.3 µg/L. There was no mortality in the control condition, which generally lasted for 7 to 10 days until all pupae emerged into adults, indicating completion of tests. At this point, mortality ranged from 99.4% to 100% in the experimental condition, and the higher proportions of mortality occurred in the pupal stage. Rock exhibited the highest proportion of mortality in the larval stage (32.2%), compared to PAg (9.03%) and PAb (24.8%). Pyriproxyfen induced 100% of Adult Emergence Inhibition (AEI) in the reference lineage and in both PAg and PAb populations (Fig. 1b).
Pyrethroids (rapid knockdown assay)
For Rock, PAg and PAb, there was 100% knockdown in 0.1 ppm and 0.4 ppm for permethrin and deltamethrin. In total, we exposed 60 larvae of each strain to both pyrethroids. The susceptibility index (SI) average for deltamethrin was 2.7 in Rock, 3.0 in PAb and 3.7 in PAg, and for permethrin, it was 4.0 in Rock, 3.7 in PAb, and 5.3 in PAg (Fig. 1c). Although this data roughly suggested a higher SI to PAg for both pyrethroids, the difference among the strains was not significant, according to the ANOVA test: deltamethrin (P = 0.4640) and permethrin (P = 0.6892).
Bioassays with adulticides
Malathion
Both PAg and PAb exhibited 100% mortality showing susceptibility to the organophosphate malathion (20 μg/mL), similar to Rock.
Pyrethroids
Both species tested were resistant to the WHO diagnostic doses to the four pyrethroids, with the magnitude of resistance varying between species for different insecticides. As a whole, mortality rates were lower for permethrin and etofenprox compared to deltamethrin and alpha-cypermethrin (Fig. 2). Among pyrethroids, deltamethrin 0.03% caused the maximum mortality rates in both PAg (85.04%) and PAb (78.85%), while we observed minimum mortality rates with permethrin 0.25% for PAg (8.04%) and PAb (4.52%). In all experiments, control tubes showed no mortality, while Rock was 100% susceptible to all insecticides.
Adulticide bioassays with Aedes aegypti and Aedes albopictus from Lahore, Pakistan. PAg and PAb designate Ae. aegypti and Ae. albopictus populations, respectively. Dose-diagnostic tests with the pyrethroids permethrin (0.25%), etofenprox (0.5%), alpha-cypermethrin (0.03%) and deltamethrin (0.03%) in WHO test tubes, and the organophosphate malathion (20 µg) in bottle assays. Bars and errors indicate the mean mortality ± SEM. Populations below 90% of mortality (indicated by the red dotted line) are resistant. Aedes aegypti Rockefeller reached 100% mortality in all assays run in parallel.
The pyrethroid with the longest estimated time for 50% of the exposed mosquitoes (KdT50) to be knocked down was permethrin in PAg (145 min) and PAb (289 min), and the shortest KdT50 was induced by deltamethrin in PAg (40.4 min) and PAb (46.8 min). Resistance ratios based on knockdown timings (KdRR50) for the pyrethroids were higher for permethrin in PAb (8.4) and lower for deltamethrin in PAg (1.8) (Table 2).
Mechanisms of resistance
Synergistic assay
We tested if the synergist PBO would increase mortality to the pyrethroid permethrin to evaluate the possibility of metabolic resistance mechanisms involvement in permethrin resistance. Permethrin (0.25%) caused 22.3% mortality in PAg and 19.8% in PAb, in this assay, while when pre-exposed to 4% PBO, the mortality increased to 100% and 50% in PAg and PAb, respectively. There was no mortality in the control and PBO-only conditions.
Expression analyses
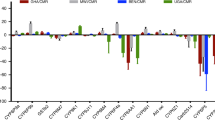
We assessed the levels of expression of five genes related to the metabolism of neurotoxic insecticides (CYP9M6, CYP9J10, CYP9J28, CYP6BB2, and CCEAe3a) and a gene of sensory appendage protein (SAP2) in the whole-body of PAg adult females, in comparison to the housekeeping gene Rps14. Comparing with the profile exhibited by Rock, the only gene with a significant relative fold change expression was the MFO P450 gene CYP9J28, 12.7 × more expressed in PAg (Supplementary Table S1). The SAP2 was nearly fourfold underexpressed in PAg than Rock. The fold change expression of these genes is found in Fig. 3, and more detailed data in Supplementary Table S1.
Kdr genotyping and sequencing
Aedes aegypti
We genotyped 45 PAg individuals for four kdr mutations: V410L (IS6 segment), S989P and V1016G (IIS6 segment), and F1534C (IIIS6 segment) with TaqMan SNP assay. All samples were similar to a wild type for the three SNPs in the IS6 and IIS6 NaV segments (V410, S989 and V1016). HRM analyses for the IIS6 segments were run for the exons 20 and 21 in independent reactions and indicated monomorphic sequences in exon 20 (N = 40 samples) and two variants in the exon 21 (N = 44 samples), though the distinct variant contained only one sample (#PAg_22). Sequencing of the exons 20 to 21 with the intron in between revealed two haplotypes: PAg-2s6_1 and PAg-2s6_2 (GenBank accession numbers MT707209 and MT707210, respectively). The polymorphisms were found only in the intron and justified the distinct variant observed in the HRM analysis for the #Pag_22 sample (Supplementary Figure S2). Sequences spanning exons 20–21 of the NaV gene in Ae. aegypti are phylogenetically divided into two groups, clades A or B23. In this context, both PAg haplotypes belonged to the clade B, as revealed when aligned with homologous sequences available on the GenBank (NCBI). PAg-2s6_1 was similar to a haplotype observed in Ae. aegypti populations from the Americas, Africa, Asia, and Australia (MN602762). PAg-2s6_2 was similar to haplotypes obtained in samples from Brazil, Australia and Kenya (MN602775) and Vietnam (MG257775, LC036556). This haplotype is also similar to the sequence of the reference lab strain LVP (MK977832) originally collected in Sierra Leone in the 1930s (Supplementary Figure S2).
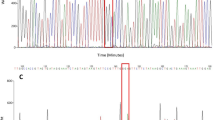
For the IIIS6 segment, however, 44 samples were kdr homozygous (1534 C/C), and one sample (that same #PAg_22) was heterozygote (1534 F/C), as obtained by TaqMan SNP assay for the F1534C SNP. In addition to this TaqMan assay, an HRM analysis in part of the exon 31 for this segment determined three variants (Fig. 4a), which when sequenced (see below), indicated variation in the 1520 (T1520I) in addition to the 1534 (F1534C) site. The genotypes were then determined as 1520 T/T + 1534 C/C (kdr homozygous at 1534) in 18.2% samples, 1520 I/I + 1534 C/C (kdr homozygous in both sites) in 27.3% samples, and 1520 T/I + 1534 C/C or 1520 T/T + 1534 F/C (heterozygous at 1520 or 1534 sites) in 54.5% samples (Fig. 4b). The exon 31 sequencing of the aforementioned #PAg_22 indicated its genotype as 1520 T/T + 1534 F/C. Summing up all of this information, the allelic frequencies in PAg were: 53.4% (IC), 45.5% (TC) and 1.1% (TF) (Fig. 4c). Three haplotypes were identified in the IIIS6 segment out of 13 sequenced samples (Supplementary Figure S2): the wild-type (PAg_3s6-1) without any nonsynonymous substitution, and two kdr haplotypes: one with the 1534C (PAg_3s6-2) and the other with both 1520I and 1534C kdr mutations (PAg_3s6-3). The PAg_3s6-1 haplotype was observed only under heterozygosis (overlapped peaks in the electropherogram) with PAg_3s6-2, confirming the #PAg_22 genotype (1520 T/T + 1534 F/C). We aligned these haplotypes with 69 other homologous sequences available on the NCBI GenBank and found that PAg_3s6-1 was similar to the wild-type haplotype described in Ae. aegypti populations from all continents. Likewise, PAg_3s6-2 was similar to the 1534C kdr sequences observed worldwide. PAg_3s6-3, with the double mutation 1520I + 1534C, was similar to sequences from Thai and Indian populations (Supplementary Figure S2).
Kdr genotyping in the NaV IIIS6 segment in Aedes aegypti from Lahore, Pakistan. (a) The high-resolution melting analyses (HRM) difference plot, where each line represents a sample, is grouped into three variants. Sequencing of some samples of each variant, summed with TaqMan genotyping of the F1534C SNP indicated their genotypes, which then rendered the genotypic (b) and allelic frequencies (c), considering the 1520 and 1534 sites.
Aedes albopictus
HRM analyses for each of the exons 20 and 21 showed two variants in PAb (N = 45 samples) in both reactions. When sequenced, four SNPs were found in the exon 20, indicating five haplotypes (Supplementary Figure S2). These observed SNPs were three nonsynonymous substitutions and the mutation L882M. A Blast search with the exon 20 of these five haplotypes matched with identical sequences of Ae. albopictus populations from Brazil and China (Supplementary Figure S2). The L882M substitution was also present in a sample from India (MF776970)32, although in a haplotype distinct to the Pakistani herein observed.
Concerning the exon 31, we obtained a 257 bp fragment from 13 samples, in which a total of six SNPs were identified, all synonymous substitutions. The sequences of eight haplotypes are available in (Supplementary Figure S2) (GenBank accession numbers: MT740758-MT740765). The haplotypes Pab_3s6-1 and Pab_3s6-2 were identical to sequences from Ae. albopictus from China and Malaysia, available on NCBI GenBank (Supplementary Figure S2).
Discussion
There are few insecticide resistance studies in Pakistani Aedes spp12,13,15,16,33,34,35,36,37,38,39,40, in a scenario where vector control on its own has been one of the neglected aspects of arthropod-borne infections. There were interesting projects about IR status and mechanisms selected in Anopheles mosquitoes during the late 1970s and early 1980s39,41,42,43. Nevertheless, the studies on this particular area were discontinued due to its geopolitical situation and other factors. Lahore, the second-most populous city in Pakistan, has faced various dengue outbreaks in the last decade. Before dengue vector control, several chemicals from pyrethroid, organophosphates, and organochlorine classes were employed in malaria vector control programs. They included deltamethrin, permethrin, malathion, DDT, among others. Here we evaluated the insecticide resistance status of Ae. aegypti and Ae. albopictus from Lahore to the larvicides temephos (organophosphate) and pyriproxyfen (IGR), as well to the adulticides permethrin, etofenprox, alpha-cypermethrin and deltamethrin (pyrethroids), and malathion (organophosphate). We evidenced that these species were still susceptible to both organophosphates and that the IGR is a suitable additional compound to be used in the region. On the other hand, Ae. aegypti and Ae. albopictus were resistant to all types of pyrethroids here evaluated. In addition to kdr mutations, an overexpressed P450 cyp gene might be playing a role in resistance to pyrethroids.
Temephos has been employed in Punjab, Pakistan, against Aedes spp. since 2011–2012, and resistance to this larvicide was detected in Ae. aegypti from several cities of that district, including Lahore, collected in 201615. Surprisingly, the LC50 of temephos in Ae. aegypti from this same city was around 13X lower in our study than that samples collected 2 years earlier (0.0815 against 0.006 µg/mL). A decrease in temephos resistance was indeed observed in laboratory lines maintained in the absence of selection pressure as well as in natural populations, some years later without the employment of temephos44 (Rahman et al. 45), likely due to a substantial fitness cost associated with physiological changes selected for resistance46. However, this decrease can be relatively slight and requires several generations or years in the field without selection pressure, and to our knowledge, temephos is still applied in Lahore. Further studies with new collections are necessary in order to understand this phenomenon better. In the case of Ae. albopictus from Lahore, there were records of resistance to the organophosphates larvicides chlorpyrifos, profenofos, and triazophos47, while in our results Ae. albopictus presented a temephos LC50 value similar to that of Ae. aegypti. Concerning the adulticide malathion, both Ae. aegypti and Ae. albopictus populations from Lahore were susceptible in collections performed in 201536, and maintained this status as we observed here.
The IGRs emerged as a prominent alternative to neurotoxic larvicides48. The chitin synthesis inhibitor compounds like diflubenzuron and buprofezin have been tested in Ae. aegypti from Lahore, with diflubenzuron causing higher mortality at the pupal stage while buprofezin resulting in more larval deaths16. Both Ae. aegypti and Ae. albopictus we evaluated here showed 100% of adult emergence inhibition against the analog of juvenile hormone IGR, pyriproxyfen, with maximum mortality at the pupal stage, as previously observed in 201540. Also, as an alternative to chemicals, the biolarvicide Bti caused 100% mortality in the Aedes field populations from the same city16,34. Although resistance to pyriproxyfen and other IGRs is not common, there are reports of Brazilian and Malaysian populations resistant to pyriproxyfen and methoprene, respectively49,50. Therefore, although IGRs have emerged as a promising alternative to neurotoxicants, their effectiveness must be continuously monitored.
Although pyrethroids are not used as larvicides, we performed a rapid test with permethrin and deltamethrin, adopted as a preliminary method to indicate pyrethroid resistance in mosquito larvae51. Based on a similar assay, we did not evidence resistance to pyrethroid in Pakistani Ae. aegypti and Ae. albopictus larvae. However, both species were resistant to all tested pyrethroid adulticides. Levels of mortality were under 20% in adult bioassays with the pyrethroid type I permethrin, though around 80% to the type II pyrethroid deltamethrin. Resistance to permethrin and deltamethrin was previously recorded in Ae. aegypti from Lahore, collected in 201035. In 2015, both Ae. aegypti and Ae. albopictus from four towns in the Lahore district were found to be resistant to permethrin, lambda-cyhalothrin, and deltamethrin, in addition to DDT36.
Deltamethrin and permethrin resistant Ae. aegypti populations from Lahore showed a significant increase in mortality after being treated with synergist PBO15. Our study corroborated those findings by observing that a pre-exposure to 4% PBO increased mortality to permethrin (from 22 to 100% in Ae. aegypti and from 19.8% to 50% in Ae. albopictus), suggesting an influence of P450 detoxifying enzymes. Indicative of metabolic resistance in PY resistant Ae. aegypti from Lahore was previously shown by biochemical analysis, which indicated higher quantities of esterases, MFOs, GSTs and AchE33. Here we evaluated the expression of genes previously related to metabolic resistance, such as the P450 MFOs (CYP9J28, CYP9M6 and CYP6BB2) and the carboxylesterase CCEAe3a52,53,54. This trend has been identified in resistant Aedes population from Southeast Asia29,54,55,56, the Caribbean57, Central58, and South America59. Of them, we found CYP9J28 12-fold overexpressed in Ae. aegypti from Lahore. Its role has already been shown in vivo, when the transgenic expression of AaegCYP9J28 increased pyrethroid-resistance in D. melanogaster60. As far as the overexpression of carboxylesterase genes in Ae. aegypti is concerned, they are more associated with OP resistance61. Indeed, as we did not observe resistance to the OPs temephos and malathion, it makes sense that the CCEAe3a gene was not overexpressed in our samples from Lahore.
Knockdown resistance mutations in voltage-gated sodium channel (NaV) alone or in combinations have been reported from several Ae. aegypti populations from Southeast and South Asian countries including Malaysia62, Indonesia63, Thailand64, Singapore65, Myanmar66, India67, Sri Lanka68, Laos30, China69,70 and Saudi Arabia71. Here the Ae. aegypti Pakistani populations were genotyped to several kdr SNPs: V410L, described in populations from Latin America72, P989S and V1016G from Asia54,66,73 and F1534C, present in all continents23,74,75. Mutations on these sites alone or in combination are expected in populations resistant to pyrethroids and DDT, as recently reviewed21. We did not detect these classical SNPs at IS6 (V1014L) and IIS6 (S989P and V1016G) NaV segments, nor any additional nonsynonymous substitutions in the IIS6 segment. Interestingly, the sequences of this segment in worldwide Ae. aegypti populations are divided into two clades, A and B, distinguished mostly by indels in the intron between exons 20 and 21. Besides, all IIS6 kdr mutations described so far are in haplotypes from clade A23. Here all IIS6 sequences of Ae. aegypti belonged to clade B. On the other hand, the 1534C kdr mutation in the IIIS6 segment was present in all Ae. aegypti samples from Lahore here evaluated, as indicated by TaqMan genotyping assay specific for the F1534C SNP and confirmed by sequencing. Moreover, HRM analyses displayed a distinct profile that verified the presence of the SNP T1520I. Summing up TaqMan, HRM, and sequencing analyses, we evidenced two kdr haplotypes: T1520 + 1534C (45.5%) and the double kdr 1520I + 1534C (53.4%). The haplotype without kdr T1520 + F1534 (1.1%) was present in only one sample. This partially explains the resistance observed in the Pakistani Ae. aegypti population to the pyrethroids. The F1534C kdr has been described in Ae. aegypti populations from several Asian countries76. In PY-resistant Indian Ae. aegypti populations, a PCR–RFLP analysis followed by sequencing showed the presence of three haplotypes [T1520 + F1534 (21%), T1520 + 1534C (66%), and 1520I + 1534C (13%)] as we have reported in our findings. It was also observed that the F1534C mutation always occurred independently while T1520I was always found in association with F1534C14,23.
In the case of Ae. albopictus, HRM analyses identified distinct variants in the analyses of both IIS6 and IIIS6 NaV segments. However, they all accounted for synonymous substitutions, as revealed by sequencing, except for one nonsynonymous in the IIS6 segment: L882M. Likewise, several mutations in PY-resistant Ae. albopictus populations from India were also reported32. Chinese Ae. albopictus presented a different mutation (F1534S) in pyrethroids resistant populations70. It is noteworthy that the diversity of haplotypes in Ae. albopictus was higher than Ae. aegypti, as expected since it is native to Asia while Ae. aegypti is an invasive species77. Consequently, lower diversity is expected in this species, compared to the autochthonous Asian tiger mosquito.
Conclusion
Ae. aegypti and Ae. albopictus from Lahore, Pakistan, were resistant to pyrethroids while susceptible to organophosphates and the IGR. Among compounds we tested herein, both larvicides temephos and pyriproxyfen, as well as the adulticide malathion, should be the most effective against both species. Synergist PBO increased mortality against permethrin, indicating the participation of metabolic resistance mechanisms. In fact, the P450 gene CYP9J28 was overexpressed in Ae. aegypti. Also, the kdr mutations T1520I and F1534C were present under high frequencies. Therefore, resistance to pyrethroids in Ae. aegypti from Lahore is likely related to multiple physiological mechanisms. These results' implications may be discussed with authorities responsible for Lahore's vector control actions, aiming to improve strategies against Aedes in Pakistan.
Methods
Sample collection and laboratory rearing
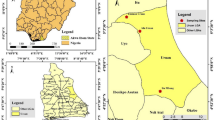
We collected larvae of Aedes aegypti and Aedes albopictus from different Union Councils (UCs)/neighborhoods of Mughulpura and airport areas of Lahore district with the help of sanitary agents and health workers recruited by the local health department for this purpose (Fig. 5).

source software QGIS version 2.18.24 (GNU General Public License), developed by the Open Source Geospatial Foundation Project (http://qgis.org).
Political map of Pakistan, divided into four provinces, with Lahore magnified in red. The map was generated with a free and open-
We visited different domestic and industrial areas. Larvae mainly infested uncovered or partially covered water storage tanks, buckets, and small pots made from various materials, including plastic, steel, copper, and cement, which contained water and were located in shaded places. Larvae were brought to the insectary of the Department of Zoology, GC University Lahore, identified into species level with the help of morphological characters78, and provided with fish food (Tetra- Marine Granules, Tetra). Emerged pupae were shifted to clean and disinfected cages, and adults were provided with a 10% sugar solution. Adults were starved overnight but allowed to feed on blood using an artificial feeder, Hemotek (PS-6 System, Discovery Workshops, Accrington, UK). Eggs of the F1 generation were shipped to Laficave, IOC/FIOCRUZ, Brazil, following the formalities of the Brazilian Ministry of Agriculture and the relevant authorities in Pakistan. Colonies were raised to F2 and F3 generations in our insectary at Fiocruz in the same fashion as mentioned before, while F1 males were preserved at − 20 °C for genotyping. The rabbit blood utilized to feed the mosquitoes was kindly provided by the Institute of Science and Technology in Biological models (ICTB), Fiocruz, under the license CEUA L-004/2018, approved by the Ethics Committee for the Use of Animals in Research of Oswaldo Cruz Institute (CEUA-IOC).
With the purpose of maximizing growth synchronization, we put the Aedes eggs to hatch in 1 L dechlorinated water, and after 3–4 h, 500 larvae were transferred to separate trays and grown to L3/L4 stage under standard lab conditions of temperature 26 °C ± 1 °C, light: dark 12 h: 12 h period and 70 ± 5% relative humidity46. The Ae. aegypti Rockefeller strain, the international reference for susceptibility to insecticides and vigor under laboratory conditions79, was reared in parallel and tested in all experiments as an internal control. We have referred to Ae. aegypti Rockefeller as Rock, and Ae. aegypti and Ae. albopictus Lahore populations as PAg and PAb, respectively.
Bioassays with larvae
Temephos
We followed the dose–response WHO protocol to calculate the resistance ratios (RR) to temephos in the evaluated populations80. Temephos technical grade (Pestanal Sigma-Aldrich) was dissolved in ethanol to give a series of concentrations ranging from 0.002 to 0.018 mg/L in order to obtain mortality from 5 to 99%, as recommended for probit analysis. Each concentration and a negative control condition (containing 300 µL of ethanol only) was replicated four times, each containing 100 mL solution with ~ 20 late L3 or early L4 larvae, in a disposable plastic cup of 150 mL capacity. Mortality was calculated 24 h after initial exposure. The tests were performed three times with both PAg and PAb populations simultaneously, always in parallel to Rock as an internal control.
Pyriproxyfen
For the IGR pyriproxyfen, we performed dose-diagnostic assays. Each assay contained eight treatment replicas with pyriproxyfen (Sigma-Aldrich) at a diagnostic concentration of 0.3 μg/L. This dosage was previously obtained, as twice the CL99 for Rock49. Besides, we included six control replicas (with 1 mL of the solvent instead of the IGR solution) in a 250 mL solution. A 300 mL disposable, transparent plastic cup was used for each replica, into which we added 10 L4 larvae. To nourish the larvae, we introduced 15 mg of fish food (Tetramarine Granules, Tetra) at the start of the assay and 10 mg on the fourth day of the experiment. All the cups were covered with gauze to avoid eventual adult escaping. Mortality and life stage transformation were cumulatively recorded bi-weekly. Parallel assays were done with Rock. Data was recorded until all individuals in the control condition emerged into adults. Assays were performed three independent times, under standard environmental conditions of temperature (26 °C ± 1 °C), light: dark regiments (12 h: 12 h), and relative humidity (75 ± 5%). Unlike neurotoxic insecticides, for IGRs, mortality itself is not the critical parameter to evaluate, but the index of Adult Emergence Inhibition (AEI). Populations are considered to be resistant when AEI is lower than 90%. In addition to AEI, we recorded the mortality observed at each developmental stage: larvae, pupae, and adult (adults that remained attached to the exuviae and died).
Susceptibility Index of Aedes larvae against pyrethroids
We adopted a simplified knockdown assay51 to evaluate susceptibility to the knockdown effect to type I (permethrin) and type II (deltamethrin) pyrethroids in larvae. We prepared solutions with technical grades permethrin (Pestanal, Sigma-Aldrich) and deltamethrin (Pestanal, Sigma-Aldrich) by dissolving each in 1 mL acetone and then ethanol to make 250 ppm stock solutions. From this, we prepared a 20 mL solution for each pyrethroid, with 10 replicas for two concentrations (0.1 ppm and 0.4 ppm) in H2O. Two negative control cups with ethanol (32 µL) in 20 mL of H2O were run in parallel. We used one L4 larva per replica and registered the knockdown every 5 min, for half an hour. The KdT50, i.e., the time when 50% of the larvae were knocked down, was scored according to these categories: 1 (0–5 min), 2 (6–10 min), 3 (11–15 min), 4 (16–20 min), 5 (21–30 min) or 6 (> 30 min). The susceptibility index (SI) for each species against deltamethrin and permethrin was obtained by multiplicating the KdT50 categories of both concentrations (0.1 and 0.4 ppm). Population with the lower SI is considered more sensitive. The obtained SI values were the mean of three independent assays, with Rock in parallel as an internal control in all of them.
Adulticides
Pyrethroids
We followed the WHO-like tube tests procedure, with modifications81,82 for the tarsal contact tests with insecticides-impregnated filter papers. Papers (Whatman grade 1) were impregnated with diagnostic concentrations of four pyrethroids following WHO standard protocol and dosages: deltamethrin 0.03%, permethrin 0.25%, etofenprox 0.5% and alpha-cypermethrin 0.03%81. Solutions were prepared from technical grade insecticides (Pestanal Sigma-Aldrich) in acetone and silicone oil (used as a carrier) and evenly applied to a filter paper (Whatman grade 1). Papers were allowed to air dry for 72 h before use. Papers impregnated with just solvent were used as the control. In each assay, we put 15–20 female mosquitoes, 3–5 days old, non-blood-fed, in a resting tube (tube without insecticide) to acclimatize for 30 min. After that, they were gently blown into tubes having insecticides-impregnated papers, and knockdown was checked every 5 min for 2 h. This differed from the WHO protocol81, which recommends exposure time of 60 min. In the case of Rock, mortality was checked every two minutes. After they were exposed to insecticides, mosquitoes were shifted back to resting tubes, provided 10% sugar water, and mortality evaluated after 24 h.
Organophosphate
For the OP malathion bioassays, we used CDC bottle tests83, impregnating 250 mL glass bottles (Wheaton) with 20 µg/mL malathion (Cheminova Brasil Ltda, São Paulo) dissolved in acetone. For impregnating the bottles, 1 mL of the insecticide solution was distributed evenly to all parts of the bottle, including its cap, and left to air dry for at least 24 h before the test. In each bottle, 20–25 female mosquitoes, three to five days old, non-blood fed, were left for one hour, and mortality was recorded. Bottles impregnated with 1 mL acetone were used as control. This experiment was done in four replicates, three separate times. Standard conditions of temperature, humidity, and light–dark periods were maintained throughout the assays, as described earlier.
Synergist assay
In order to evaluate the occurrence of metabolic resistance mechanisms to the pyrethroid type I permethrin, we carried out WHO bioassays with the synergist PBO (Piperonyl Butoxide)84. This substance inhibits the action of Multi-Function Oxidases (MFOs) and can revert resistance85. For this purpose, papers were impregnated with technical grade PBO (Endura) and permethrin (Pestanal, Sigma-Aldrich), as described earlier. The test was composed of four conditions: (i) exposure to 4% PBO, (ii) a pre-exposure to 4% PBO and then to 0.25% permethrin, (iii) exposure to 0.25% permethrin only, and (iv) exposure only to solvent control. The susceptible strain, Rock, was carried out parallelly. Each assay consisted of four tubes, and each tube contained 20–25 female mosquitoes, 4–6 days old, non-blood-fed. All exposures lasted 1 h, after which mortality was checked in each assay. Mosquitoes were then transferred to resting tubes and provided 10% sugar solution. The number of dead mosquitoes was counted after 24 h, according to the standard criteria of WHO81.
Calculations
In the case of both pyrethroids and organophosphates bioassays, we expected 100% mortality of the reference strain and no (zero) mortality in the control. If mortality in the control exceeded 20%, the test should be repeated. If less, Abbott's correction formula would be applied86. Temephos Lethal Concentrations (LC) were obtained by log x probit transformations followed by linear regression analyses87 with the sum of the values from the three assays. We calculated the resistance ratios (RR) by dividing the LC of both PAg and PAb by the LC of Rock, in the absence of a reference lab strain for Ae. albopictus at that time.
To estimate knockdown time to pyrethroids (KdT50), readings were likewise submitted to probit analysis. Accordingly, knockdown-time Resistant Ratios (KdT-RR50) were calculated by dividing KdT50 of PAg and PAb by the KdT50 of Rock.
Exploration of knockdown resistance (kdr) mutations
We evaluated the nucleotide diversity in the genomic region corresponding to IS6, IIS6, and IIIS6 NaV segments to investigate kdr mutations classically found in Ae. aegypti pyrethroid-resistant populations. Genomic DNA was isolated from male mosquitoes (n = 45) of both PAg and PAb with a column-based DNA extraction kit (NuleoSpin, Macherey–Nagel Laboratories), according to manufacturer's instructions. We did not use females to avoid eventual amplification of DNA inside their spermathecae, which would mislead interpretations about individual genotypes. Purified DNA was quantified with NanoDrop One (ThermoFisher Scientific) and diluted to 20 ng/µL in ultra-pure water.
SNPs genotyping
A TaqMan SNP genotyping qPCR approach was employed for kdr genotyping, essentially as described previously88, for the variations Val410Leu, Ser989Pro, Val1016Gly, and Phe1534Cys in PAg. We performed reactions independently for each SNP, consisting of 1X TaqMan Genotyping Master Mix (ThermoFisher), 1X of the respective Custom TaqMan SNP Genotyping Assay (Table 3), 20 ng of DNA and ultra-pure water q.s. 10 µL, run in a QuantStudio 6 Flex (Applied Biosystems), under standard conditions. The genotype callings were obtained by the online software Genotype Analysis Module V3.9 (Applied Biosystems, Thermo Fischer cloud platform). As a positive control, we used the DNA of Rockefeller strain (wild-type homozygote genotypes) in all SNP reactions, and the Rock-kdr strains89, which has the kdr homozygote genotypes: 410 Leu/Leu, 1016 Ile/Ile and 1534 Cys/Cys. Also, we employed a synthesized DNA fragment (gBlock, IDT) with the sequence of an Asian kdr haplotype (GenBank accession number MN602755)23 as the kdr homozygote genotypes in 989 Pro/Pro and 1016 Gly/Gly SNP reactions. An equimolecular quantity of Rock DNA was mixed with each kdr positive controls to obtain the respective heterozygote controls.
HRM reactions
To search for possible SNPs beyond the classical mutations tested with TaqMan qPCR, we developed a high-resolution melting analysis (HRM) for the genomic regions in the IIS6 and IIIS6 NaV segments of PAg and PAb. We performed three HRM reactions, each for part of the NaV gene exons 20, 21 (IIS6), and 31 (IIIS6) (Fig. 6). We focused on these exons because the known kdr sites 989, 1016, and 1534 are respectively placed in the exons 20, 21, and 31 of the Ae. aegypti NaV gene. Reactions were performed with 1X MeltDoctor HRM Master Mix kit (Thermo Fischer), 0.3 µM of each primer (Table 4), 20 ng DNA, and ultra-pure water q.s. 10 µL. The thermocycling program followed the standard qPCR conditions with an additional HRM step in a QuantStudio 6 (Applied Biosystems). The HRM curve analyses were performed with the QuantStudio Real-Time PCR Software v1.3 (ThermoFisher), which grouped the samples into distinct variants. To determine the variant genotypes, we sorted at least three samples of each variant to be sequenced.
Representation of the IIS6 (a) and IIIS6 (b) NaV segments with their respective amino acid (above) and nucleotide (bellow) sequences. The SNPs at 989, 1016, 1520, and 1534 sites are indicated inside brackets with the mutant alternative in red. The shaded colors indicate the amplified sequences in the HRM reactions, with their respective primer sequences underlined and orientation following the arrows.
DNA sequencing
The IIS6 and IIIS6 corresponding genomic regions were amplified with Phusion High-Fidelity DNA Polymerase PCR kit (New England BioLabs) in a 25 µL reaction, containing 1X Phusion HF buffer, 200 µM dNTP, 3% DMSO, 0.4 U Polymerase, 0.5 µM of each primer, 40 ng DNA and H2O q.s. 20 µL. The primers employed were 5para3: ACAATGTGGATCGCTTCCC x NaV_E21R: GCAATCTGGCTTGTTAACTTG for the IIS6 segment in both species and 31P: TCGCGGGAGGTAAGTTATTG x 31Q: GTTGATGTGCGATGGAAATG for the IIIS6 segment in Ae. aegypti, and 31F: GATCGCGGGAGGTAAGTT X 31R: CCGTCTGCTTGTAGTGATCG in Ae. albopictus (all primers described in 5′ to 3′orientation). Thermocycling conditions were 98 °C/30" for initial polymerase activation, followed by 35 cycles of 98 °C/30" for double-strand denaturation, 60 °C (in IIS6 reactions) or 61 °C (in IIIS6 reactions)/15" for primer annealing, and 72 °C/30" for enzyme extension. The PCR products were purified with magnetic beads Agencourt AMPure XP (Beckman Coulter), following manufacturer instructions. Purified amplicons (1 µL) were submitted to a Sanger sequencing reaction with the kit BigDye Terminator v3.1 (ThermoFisher), with 1 µM of one of the primers, according to manufacturer's protocol, and followed to the FIOCRUZ platform of DNA sequencing. Each sample was sequenced in both strands. Sequences were analyzed with Geneious v. Prime90 and submitted to GenBank (accession numbers: MT740753-MT740765 and MT707209-MT707210). We compared our sequences with those available in the GenBank (NCBI Blast) to check their similarity with sequences from worldwide populations.
Expression analysis of genes related to detoxification of insecticides
We evaluated the expression profiles of genes previously associated with resistance in Aedes populations from South-east Asia in PAg populations: three were from the CYP9 family (CYP9M6, CYP9J10, and CYP9J28) and one from CYP6 (CYP6BB2). The expression profile of one carboxylesterase (CCEAe3a) and one sensory appendage protein (SAP2) was also investigated31,91,92. The expression levels were relative to the housekeeping gene of the ribosomal protein S14 (RpS14)93.
Five-day-old female mosquitos were pooled in a tube. For each population, we used four pools as biological replicates per population. In order to isolate RNA, these mosquitoes were macerated in 300 µL TRIzol (Invitrogen, CA, USA) and then homogenized with glass beads in TissueLyser II (Qiagen, Venlo, Netherlands). RNA was precipitated with TRIzol (Invitrogen, CA, USA) and chloroform and then washed with ethanol to remove any debris of DNA and protein, according to the manufacturer's protocol. The pellet obtained was air-dried and eluted with RNase-free water. The isolated RNA was quantified with Qubit RNA HS Assay kit (Invitrogen), and cDNA was then synthesized in an RT-PCR reaction with SuperScript Vilo MasterMix (Invitrogen), using 5 µL RNA and molecular grade water qi 20 µL. The reagents were incubated at room temperature for 10 min, 42 °C for 1 h, and 85 °C for 10 min. We used the Qubit dsDNA HS Assay kit (Invitrogen) to quantify this cDNA and diluted it to 4 ng/µL for qPCR use.
The qPCR reactions were performed in a 96-Well MicroAmp reaction (Invitrogen) plate, with the kit KAPA SYBR FAST qPCR Master Mix (1x) and ROX LOW (KAPA Biosystems) kit, 0.2 µM of each forward and reversed primers (Table 5), 4 ng/µL cDNA and molecular grade water q.s. 10 µL. Each pool (biological replicate) was divided into four technical replicates. Thermocycling conditions in a QuantStudio 6 Flex Real-Time PCR system (ThermoFisher Scientific) consisted of enzyme activation and initial denaturation at 50 °C and 95 °C for 2 and 3 min, respectively, followed by 40 cycles of denaturation (95 °C for 3 s) and annealing/extension of primers (60 °C for 1 min), with an additional standard melting curve analysis step.
Analysis of gene expression
We obtained the Ct values of four replicates for each gene and calculated their means. Replicates presenting different Ct from Grubbs values (outliers)98 or with an unexpected peak in the melting analysis were excluded. Relative quantification analyses were performed using the ΔΔCt method99, where the RpS14 was taken as the reference gene and Rock, reared alongside test population, as the reference strain. For all genes, the Ct threshold was set at 0.2. The 2(−ΔΔCt) equation was applied to define the relative fold-change expression of each gene in PAg compared to Rock. The ΔCt values of each gene were compared between Rock and PAg by unpaired t-test with Welch's correction, performed with GraphPad Prism 8 v 8.4.2 for Mac (GraphPad Software, San Diego, California USA).
References
Khanani, M. R., Arif, A. & Shaikh, R. Dengue in Pakistan: Journey from a disease free to a hyper endemic nation. Editorial Board 5, 81 (2011).
Javed, F. T. et al. Prevalence of all four dengue virus serotypes confirmed by using real time RT-PCR among population of Lahore Pakistan. Int. J. Agric. Vet. med. Sci 3, 1–3 (2009).
Idrees, M. et al. Dengue virus serotype 2 (DEN-2): The causative agent of 2011-dengue epidemic in Pakistan. Am. J. Biomed. Sci. 4, 307–315 (2012).
OCHA, U. Outbreak update – Dengue in Pakistan, 19 November 2019, <https://reliefweb.int/report/pakistan/outbreak-update-dengue-pakistan-19-november-2019> (2019).
Powell, J. R., Gloria-Soria, A. & Kotsakiozi, P. Recent history of Aedes aegypti: Vector genomics and epidemiology records. Bioscience 68, 854–860 (2018).
Powell, J. R. Mosquito-borne human viral diseases: Why Aedes aegypti?. Am. J. Trop. Med. Hyg. 98, 1563–1565 (2018).
Moyes, C. L. et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 11, e0005625. https://doi.org/10.1371/journal.pntd.0005625 (2017).
Akhtar, M. S., Aihetasham, A., Saeedb, M. & Abbassa, G. Aedes survey following a dengue outbreak in Lahore, Pakistan, 2011. Dengue 36, 87 (2012).
Aamir, U. B., Badar, N., Salman, M., Ahmed, M. & Alam, M. M. Outbreaks of chikungunya in Pakistan. Lancet. Infect. Dis 17, 483 (2017).
Mallhi, T., Khan, Y., Khan, A., Tanveer, N. & Qadir, M. First chikungunya outbreak in Pakistan: A trail of viral attacks. New Microb. New Infect. 19, 13–14 (2017).
Governament of Punjab, P. Standard operation protocol for dengue control and prevention, <https://phc.org.pk/downloads/GoPb%20SOPs%20for%20Dengue%20Control%202014.pdf> (2014).
Khan, H. A. A., Akram, W., Shehzad, K. & Shaalan, E. A. First report of field evolved resistance to agrochemicals in dengue mosquito, Aedes albopictus (Diptera: Culicidae), from Pakistan. Parasit. Vect. 4, 146 (2011).
Khan, G. Z. et al. Monitoring of resistance status in dengue vector Aedes albopictus (Skuse) (Culicidae: Diptera) to currently used public health insecticides in selected districts of Khyber Pakhtunkhwa-Pakistan. Int. J. Mosquito Res. 4, 123–127 (2017).
Kushwah, R. B. S., Dykes, C. L., Kapoor, N., Adak, T. & Singh, O. P. Pyrethroid-resistance and presence of two knockdown resistance (kdr) mutations, F1534C and a novel mutation T1520I, in Indian Aedes aegypti. PLoS Negl. Trop. Dis. 9, 2 (2015).
Khan, H. A. A. & Akram, W. Resistance status to deltamethrin, permethrin, and temephos along with preliminary resistance mechanism in aedes aegypti (Diptera: Culicidae) from Punjab, Pakistan. J. Med. Entomol. 56, 1304–1311 (2019).
Jahan, N., Razaq, J. & Jan, A. Laboratory evaluation of chitin synthesis inhibitors (Diflubenzuron and Buprofezin) against Aedes aegypti larvae from Lahore, Pakistan. Pak. J. Zool. 43, 2 (2011).
Silver, K. S. et al. Advances in Insect Physiology 389–433 (Elsevier, Amsterdam, 2014).
Dong, K. et al. Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem. Mol. Biol. 50, 1–17 (2014).
Narahashi, T., Ginsburg, K. S., Nagata, K., Song, J.-H. & Tatebayashi, H. Ion channels as targets for insecticides. Neurotoxicology 19, 581–590 (1998).
Mian, L. S. & Mulla, M. S. Residue Reviews 27–112 (Springer, Berlin, 1982).
Scott, J. G. Life and death at the voltage-sensitive sodium channel: Evolution in response to insecticide use. Annu. Rev. Entomol. 64, 243–257 (2019).
Kushwah, R. B. S., Dykes, C. L., Kapoor, N., Adak, T. & Singh, O. P. Pyrethroid-resistance and presence of two knockdown resistance (kdr) mutations, F1534C and a novel mutation T1520I, in Indian Aedes aegypti. PLoS Negl. Trop. Dis. 9, e3332 (2015).
Cosme, L. V., Gloria-Soria, A., Caccone, A., Powell, J. R. & Martins, A. J. Evolution of kdr haplotypes in worldwide populations of Aedes aegypti: Independent origins of the F1534C kdr mutation. PLoS Negl. Trop. Dis. 14, e0008219 (2020).
Bingham, G., Strode, C., Tran, L., Khoa, P. T. & Jamet, H. P. Can piperonyl butoxide enhance the efficacy of pyrethroids against pyrethroid-resistant Aedes aegypti?. Trop. Med. Int. Health 16, 492–500 (2011).
Murcia, O. et al. Presence of the point mutations Val1016Gly in the voltage-gated sodium channel detected in a single mosquito from Panama. Parasit. Vect. 12, 62 (2019).
Fan, Y. & Scott, J. G. The F1534C voltage-sensitive sodium channel mutation confers 7-to 16-fold resistance to pyrethroid insecticides in Aedes aegypti. Pest Manag. Sci. 76, 2251–2259 (2020).
Essandoh, J., Yawson, A. E. & Weetman, D. Acetylcholinesterase (Ace-1) target site mutation 119S is strongly diagnostic of carbamate and organophosphate resistance in Anopheles gambiae ss and Anopheles coluzzii across southern Ghana. Malar. J. 12, 404 (2013).
Hemingway, J., Hawkes, N. J., McCarroll, L. & Ranson, H. The molecular basis of insecticide resistance in mosquitoes. Insect Biochem. Mol. Biol. 34, 653–665 (2004).
Strode, C. et al. Genomic analysis of detoxification genes in the mosquito Aedes aegypti. Insect Biochem. Mol. Biol. 38, 113–123 (2008).
Marcombe, S. et al. Distribution of insecticide resistance and mechanisms involved in the arbovirus vector Aedes aegypti in Laos and implication for vector control. PLoS Negl. Trop. Dis. 13, e0007852 (2019).
Ingham, V. A. et al. A sensory appendage protein protects malaria vectors from pyrethroids. Nature 577, 376–380 (2020).
Chatterjee, M. et al. Polymorphisms in voltage-gated sodium channel gene and susceptibility of Aedes albopictus to insecticides in three districts of northern West Bengal India. PLoS Negl. Trop. Dis. 12, e0006192 (2018).
Abbas, S., Nasir, S., Fakhar-e-Alam, M. & Saadullah, M. Toxicity of different groups of insecticides and determination of resistance in Aedes aegypti from different habitats. Pak. J. Agric. Sci. 56, 3 (2019).
Jahan, N. & Shahid, A. Evaluation of resistance against Bacillus thuringiensis israelensis WDG in dengue vector from Lahore, Pakistan. Pak. J. Zool. 44, 2 (2012).
Jahan, N. & Shahid, A. Evaluation of resistance against deltamethrin and cypermethrin in dengue vector from Lahore, Pakistan. J. Anim. Plant Sci. 23, 1321–1326 (2013).
Mohsin, M. et al. Susceptibility status of Aedes aegypti and Aedes albopictus against insecticides at eastern Punjab, Pakistan. MMJ 3, 41–46 (2016).
Rathor, H., Toqir, G. & Reisen, W. Status of insecticide resistance in anopheline mosquitoes of Punjab Province, Pakistan. Southeast Asian J. Trop. Med. Public Health 11, 332–340 (1980).
Rathor, H. R., Nadeem, G. & Khan, I. A. Pesticide susceptibility status of Anopheles mosquitoes in four flood-affected districts of South Punjab Pakistan. Vector Borne Zoon. Dis. 13, 60–66. https://doi.org/10.1089/vbz.2012.1055 (2013).
Rowland, M. Location of the gene for malathion resistance in Anopheles stephensi (Diptera: Culicidae) from Pakistan. J. Med. Entomol. 22, 373–380 (1985).
Mahmood, M. A. Bio-efficacy and persistancy of Pyriproxyfen against Aedes aegypti: A control trial at district Lahore, Punjab, Pakistan.
Hemingway, J. The genetics of malathion resistance in Anopheles stephensi from Pakistan. Trans. R. Soc. Trop. Med. Hyg. 77, 106–108 (1983).
Reisen, W. K., Mahmood, F. & Parveen, T. Anopheles culicifacies Giles: A release-recapture experiment with cohorts of known age with implications for malaria epidemiology and genetical control in Pakistan. Trans. R. Soc. Trop. Med. Hyg. 74, 307–317 (1980).
Rathor, H. R. & Toqir, G. Malathion resistance in Anopheles stephensi liston in Lahore Pakistan. Mosq. News 40, 526–531 (1980).
Valle, D., Bellinato, D. F., Viana-Medeiros, P. F., Lima, J. B. P. & Martins Junior, A. J. Resistance to temephos and deltamethrin in Aedes aegypti from Brazil between 1985 and 2017. Memórias do Inst. Oswaldo Cruz 114, 2 (2019).
Rahman, R. U., Cosme, L. V., Costa, M. M., Carrara, L., Lima. J. B. P. & Martins, A. J. Insecticide resistance and genetic structure of Aedes aegypti populations from Rio de Janeiro State, Brazil. PLoS Negl Trop Dis 15(2), e0008492 https://doi.org/10.1371/journal.pntd.0008492 (2021).
Bellinato, D. F. et al. Resistance status to the insecticides temephos, deltamethrin, and diflubenzuron in Brazilian Aedes aegypti populations. BioMed Res. Int. 20, 16 (2016).
Bisset, J., Rodriguez, M., Soca, A., Pasteur, N. & Raymond, M. Cross-resistance to pyrethroid and organophosphorus insecticides in the southern house mosquito (Diptera: Culicidae) from Cuba. J. Med. Entomol. 34, 244–246 (1997).
Andrighetti, M. T. M., Cerone, F., Rigueti, M., Galvani, K. C. & Macoris, M. d. L. d. G. Effect of pyriproxyfen in Aedes aegypti populations with different levels of susceptibility to the organophosphate temephos. (2008).
Campos, K. B. et al. Assessment of the susceptibility status of Aedes aegypti (Diptera: Culicidae) populations to pyriproxyfen and malathion in a national wide monitoring of insecticide resistance in Brazil, 2017–2018. (2020).
Lau, K. W., Chen, C. D., Lee, H. L., Norma-Rashid, Y. & Sofian-Azirun, M. Evaluation of insect growth regulators against field-collected Aedes aegypti and Aedes albopictus (Diptera: Culicidae) from Malaysia. J. Med. Entomol. 52, 199–206 (2015).
Kawada, H. et al. Nationwide investigation of the pyrethroid susceptibility of mosquito larvae collected from used tires in Vietnam. PLoS Negl. Trop. Dis. 3, e391 (2009).
Smith, L. B., Kasai, S. & Scott, J. G. Pyrethroid resistance in Aedes aegypti and Aedes albopictus: Important mosquito vectors of human diseases. Pestic. Biochem. Physiol. 133, 1–12 (2016).
Smith, L. B., Tyagi, R., Kasai, S. & Scott, J. G. CYP-mediated permethrin resistance in Aedes aegypti and evidence for trans-regulation. PLoS Negl. Trop. Dis. 12, e0006933 (2018).
Kasai, S. et al. Mechanisms of pyrethroid resistance in the dengue mosquito vector, Aedes aegypti: Target site insensitivity, penetration, and metabolism. PLoS Negl. Trop. Dis. 8, 2 (2014).
Chan, H. H. & Zairi, J. Permethrin resistance in Aedes albopictus (Diptera: Culicidae) and associated fitness costs. J. Med. Entomol. 50, 362–370 (2013).
Ishak, I. H. et al. Pyrethroid resistance in Malaysian populations of dengue vector Aedes aegypti is mediated by CYP9 family of cytochrome P450 genes. PLoS Negl. Trop. Dis. 11, e0005302 (2017).
Marcombe, S. et al. Exploring the molecular basis of insecticide resistance in the dengue vector Aedes aegypti: A case study in Martinique Island (French West Indies). BMC Genom. 10, 494 (2009).
Reid, W. R. et al. Transcriptional analysis of four family 4 P450s in a Puerto Rico strain of Aedes aegypti (Diptera: Culicidae) compared with an Orlando strain and their possible functional roles in permethrin resistance. J. Med. Entomol. 51, 605–615 (2014).
Costa, M. d. M. Avaliação da resistência a inseticidas e mecanismos selecionados em populações de Aedes aegypti Linnaeus 1762 (Diptera, Culicidae) da fronteira entre Brasil e Guiana Francesa, (2017).
Pavlidi, N. et al. Transgenic expression of the Aedes aegypti CYP9J28 confers pyrethroid resistance in Drosophila melanogaster. Pestic. Biochem. Physiol. 104, 132–135 (2012).
Poupardin, R., Srisukontarat, W., Yunta, C. & Ranson, H. Identification of carboxylesterase genes implicated in temephos resistance in the dengue vector Aedes aegypti. PLoS Negl. Trop. Dis. 8, 2 (2014).
Ishak, I. H., Jaal, Z., Ranson, H. & Wondji, C. S. Contrasting patterns of insecticide resistance and knockdown resistance (kdr) in the dengue vectors Aedes aegypti and Aedes albopictus from Malaysia. Parasit. Vect. 8, 181 (2015).
Wuliandari, J. R. et al. Association between three mutations, F1565C, V1023G and S996P, in the voltage-sensitive sodium channel gene and knockdown resistance in Aedes aegypti from Yogyakarta Indonesia. Insects 6, 658–685 (2015).
Plernsub, S. et al. Temporal frequency of knockdown resistance mutations, F1534C and V1016G, in Aedes aegypti in Chiang Mai city, Thailand and the impact of the mutations on the efficiency of thermal fogging spray with pyrethroids. Acta Trop. 162, 125–132 (2016).
Koou, S.-Y., Chong, C.-S., Vythilingam, I., Lee, C.-Y. & Ng, L.-C. Insecticide resistance and its underlying mechanisms in field populations of Aedes aegypti adults (Diptera: Culicidae) in Singapore. Parasit. Vect. 7, 471 (2014).
Kawada, H. et al. Co-occurrence of point mutations in the voltage-gated sodium channel of pyrethroid-resistant Aedes aegypti populations in Myanmar. PLoS Negl. Trop. Dis. 8, 2 (2014).
Muthusamy, R. & Shivakumar, M. Involvement of metabolic resistance and F1534C kdr mutation in the pyrethroid resistance mechanisms of Aedes aegypti in India. Acta Trop. 148, 137–141 (2015).
Fernando, S. D. et al. First report of V1016G and S989P knockdown resistant (kdr) mutations in pyrethroid-resistant Sri Lankan Aedes aegypti mosquitoes. Parasit. Vect. 11, 526 (2018).
Zhou, X. et al. Knockdown resistance (kdr) mutations within seventeen field populations of Aedes albopictus from Beijing China: first report of a novel V1016G mutation and evolutionary origins of kdr haplotypes. Parasit. Vect. 12, 180 (2019).
Chen, H. et al. First identification of kdr allele F1534S in VGSC gene and its association with resistance to pyrethroid insecticides in Aedes albopictus populations from Haikou City, Hainan Island China. Infect. Dis. Poverty 5, 31 (2016).
Al Nazawi, A. M., Aqili, J., Alzahrani, M., McCall, P. J. & Weetman, D. Combined target site (kdr) mutations play a primary role in highly pyrethroid resistant phenotypes of Aedes aegypti from Saudi Arabia. Parasit. Vect. 10, 161 (2017).
Haddi, K. et al. Detection of a new pyrethroid resistance mutation (V410L) in the sodium channel of Aedes aegypti: A potential challenge for mosquito control. Sci. Rep. 7, 46549 (2017).
Stenhouse, S. A. et al. Detection of the V1016G mutation in the voltage-gated sodium channel gene of Aedes aegypti (Diptera: Culicidae) by allele-specific PCR assay, and its distribution and effect on deltamethrin resistance in Thailand. Parasit. Vect. 6, 253 (2013).
Fan, Y. et al. Evidence for both sequential mutations and recombination in the evolution of kdr alleles in Aedes aegypti. PLoS Negl. Trop. Dis. 14, e0008154 (2020).
Moyes, C. L. et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl. Trop. Dis. 11, 2 (2017).
Amelia-Yap, Z. H., Chen, C. D., Sofian-Azirun, M. & Low, V. L. Pyrethroid resistance in the dengue vector Aedes aegypti in Southeast Asia: Present situation and prospects for management. Parasit. Vect. 11, 332 (2018).
Powell, J. R. & Tabachnick, W. J. History of domestication and spread of Aedes aegypti—a review. Mem. Inst. Oswaldo Cruz 108, 11–17 (2013).
Rueda, L. M. Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with dengue virus transmission. (Walter Reed Army Inst Of Research Washington Dc Department Of Entomology, 2004).
Kuno, G. Early history of laboratory breeding of Aedes aegypti (Diptera: Culicidae) focusing on the origins and use of selected strains. J. Med. Entomol. 47, 957–971 (2014).
WHO. Vol. WHO/VBC/81.805 4 (World Health Organization, Geneva, 1981).
Organization, W. H. Monitoring and managing insecticide resistance in Aedes mosquito populations: interim guidance for entomologists. (2016).
Brito, L. P., Carrara, L., Freitas, R. M., Lima, J. B. P. & Martins, A. J. Levels of resistance to pyrethroid among distinct kdr alleles in Aedes aegypti laboratory lines and frequency of kdr Alleles in 27 natural populations from Rio de Janeiro Brazil. BioMed Res. Int. 20, 18 (2018).
Brogdon, W. & Chan, T. Guideline for evaluating insecticide resistance in vectors using the CDC bottle bioassay. (2011).
Organization, W. H. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. (2016).
Casida, J. E. Mixed-function oxidase involvement in the biochemistry of insecticide synergists. J. Agric. Food Chem. 18, 753–772 (1970).
Abbott, W. S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol 18, 265–267 (1925).
Finney, D. Probit Analysis (Cambridge University Press, Cambridge, 1971).
Linss, J. G. B. et al. Distribution and dissemination of the Val1016Ile and Phe1534Cys Kdr mutations in Aedes aegypti Brazilian natural populations. Parasit. Vect. 7, 25 (2014).
Brito, L. P. et al. Assessing the effects of Aedes aegypti kdr mutations on pyrethroid resistance and its fitness cost. PLoS ONE 8, 2 (2013).
Kearse, M. et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649. https://doi.org/10.1093/bioinformatics/bts199 (2012).
Dusfour, I. et al. Deltamethrin resistance mechanisms in Aedes aegypti populations from three French overseas territories worldwide. PLoS Negl. Trop. Dis. 9, e0004226 (2015).
Faucon, F. et al. Identifying genomic changes associated with insecticide resistance in the dengue mosquito Aedes aegypti by deep targeted sequencing. Genome Res. 25, 1347–1359 (2015).
Lü, J., Yang, C., Zhang, Y. & Pan, H. Selection of reference genes for the normalization of RT-qPCR data in gene expression studies in insects: A systematic review. Front. Physiol. 9, 1560 (2018).
Matthews, B. J. et al. Improved reference genome of Aedes aegypti informs arbovirus vector control. Nature 563, 501–507 (2018).
Bariami, V., Jones, C. M., Poupardin, R., Vontas, J. & Ranson, H. Gene amplification, ABC transporters and cytochrome P450s: Unraveling the molecular basis of pyrethroid resistance in the dengue vector Aedes aegypti. PLoS Negl. Trop. Dis. 6, e1692 (2012).
Kasai, S. et al. Mechanisms of pyrethroid resistance in the dengue mosquito vector, Aedes aegypti: Target site insensitivity, penetration, and metabolism. PLoS Negl. Trop. Dis. 8, e2948 (2014).
Saavedra-Rodriguez, K. et al. Transcription of detoxification genes after permethrin selection in the mosquito Aedes aegypti. Insect Mol. Biol. 21, 61–77 (2012).
Rohlf, F. J. & Sokal, R. R. Statistical Tables (Macmillan, New York, 1995).
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 29, e45–e45 (2001).
Acknowledgements
We would like to thank the World Academy of Sciences (TWAS), the Brazilian National Council for Scientific and Technological Research (CNPq), and the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES). We extend our gratitude to Professor Dr. Muhammad Tahir (Chairperson Zoology department, GC University Lahore) for his permission to use their insectary, Muhammad Asif, for his support during field visits and logistic support. Daniel Marland is thanked for the thorough review of the manuscript and for correcting all the grammatical and textual errors. This study was funded by Instituto Nacional em Ciência e Tecnologia em Entomologia Molecular (INCT-EM) (Grant No. 465678/2014-9) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (Grant No. E-26/202.795/2019).
Author information
Authors and Affiliations
Contributions
Ru.R. and A.J.M. formulated the original idea. Ru.R., I.U., M.A.M. and S.K. collected the samples, identified them, created F1 and helped in the logistics. Ru.R., L.P.B., L.C.S., B.S.S. and M.M.C. carried out biological and molecular assays. A.J.M. and Ru.R. performed the statistical analysis. Ru.R. and A.J.M. compiled the M.S. and M.A.M. and J.B.P.L. made the necessary editions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rahman, R.U., Souza, B., Uddin, I. et al. Insecticide resistance and underlying targets-site and metabolic mechanisms in Aedes aegypti and Aedes albopictus from Lahore, Pakistan. Sci Rep 11, 4555 (2021). https://doi.org/10.1038/s41598-021-83465-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-83465-w
This article is cited by
-
Pyrethroid resistance status and co-occurrence of V1016G, F1534C and S989P mutations in the Aedes aegypti population from two dengue outbreak counties along the China-Myanmar border
Parasites & Vectors (2024)
-
Regionality in vector control: effect of fluctuating temperature in the susceptibility of Aedes aegypti (Diptera: Culicidae) larvae to Pyriproxyfen
Parasitology Research (2024)
-
Cuticular profiling of insecticide resistant Aedes aegypti
Scientific Reports (2023)
-
Genetic structure and kdr mutations in Aedes aegypti populations along a road crossing the Amazon Forest in Amapá State, Brazil
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.