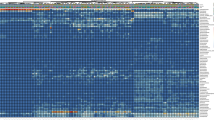
Abstract
Antibiotic resistance in bacterial pathogens or several indicator bacteria is commonly studied but the extent of antibiotic resistance in bacterial commensals colonising the intestinal tract is essentially unknown. In this study, we aimed to investigate the presence of horizontally acquired antibiotic resistance genes among chicken gut microbiota members in 259 isolates with known whole genomic sequences. Altogether 124 isolates contained at least one gene coding for antibiotic resistance. Genes coding for the resistance to tetracyclines (detected in 101 isolates), macrolide-lincosamide-streptogramin B antibiotics (28 isolates) and aminoglycosides (25 isolates) were the most common. The most frequent tetracycline resistance genes were tet(W), tet(32), tet(O) and tet(Q). Lachnospiraceae and Ruminococcaceae frequently encoded tet(W). Lachnospiraceae commonly coded also for tet(32) and tet(O). The tet(44) gene was associated with Erysipelotrichaceae and tet(Q) was detected in the genomes of Bacteroidaceae and Porphyromonadaceae. Without any bias we have shown that antibiotic resistance is quite common in gut commensals. However, a comparison of codon usage showed that the above-mentioned families represent the most common current reservoirs but probably not the original host of the detected resistances.
Similar content being viewed by others
Introduction
The spread of antibiotic resistance via horizontal gene transfer belongs among one of the most serious challenges in current medicine. Due to increasing resistance to antibiotics in pathogens like Klebsiella, Staphylococcus, Pseudomonas or Salmonella1,2, diseases caused by these pathogens, although possible to control when caused by antibiotic-sensitive strains, become serious threats when caused by antibiotic-resistant clones. Antibiotics are not strictly selective against only pathogens, thus whenever antibiotics are used, not only the target pathogen but also commensal microbiota is affected and subjected to selection for resistant clones. Any use of antibiotics to control infection caused by a single bacterial pathogen therefore inevitably leads to the selection of hundreds of commensal species resistant to their action as well. The resistant commensals may later act as reservoirs of antibiotic resistance genes, quite extensive reservoirs, since microbiota of distal parts of intestinal tract consists of approx. one thousand different species with a population density of around 1010 bacterial cells per gram of digesta.
Studies on the distribution of antibiotic resistance among gut microbiota are limited due to their specific culture requirements. This is the reason why antibiotic resistance of gut colonisers has been mainly studied in E. coli, lactobacilli and bifidobacteria for which selective culture conditions are known3,4,5. Unfortunately, specific and selective culture conditions are not known for other gut microbiota members and information on the distribution of antibiotic resistance in Bacteroides, Parabacteroides, Faecalibacterium, Butyricicoccus, Blautia or Sutterella etc. is much more limited.
Since selective culture conditions for the majority of gut microbiota are not known, alternative protocols for characterisation of the gut microbiota resistome have been used. One of the most frequently used protocols includes shotgun sequencing of DNA purified from faecal material or intestinal digesta. Those performed in chickens demonstrated that chicken gut microbiota represents an important source of antibiotic resistance genes with the most abundant genes encoding different drug efflux pumps, resistances to fluoroquinolones and tetracyclines6,7,8.
Despite shedding light on total ARG content, metagenomic sequencing fails to determine the original bacterial host of the detected antibiotic resistance since some of the antibiotic resistance genes spread as a single gene cassette9. Following shotgun sequencing and metagenomic assembly, such cassettes form separate contigs, which are impossible to associate with the rest of the host bacterial genome. Antibiotic resistance genes are also commonly part of mobile genetic elements that can be present in a wide variety of organisms10,11. The detection of such contigs after metagenomic assembly, e.g. a plasmid DNA sequence, again is unable to determine in which bacterial species such a plasmid was present.
The above-mentioned limits can be overcome by culture of gut anaerobes followed by whole genome sequencing. Although anaerobic culture still represents a limiting step, significant advances in the culture of gut anaerobes have been reported recently12,13,14,15. Since we have cultured and sequenced hundreds of chicken gut microbiota members15,16, in this study we searched their genomic sequences for the presence of antibiotic resistance genes. This enabled us to address questions like (i) which antibiotic resistance genes are the most widespread among chicken gut microbiota, (ii) which taxa behave as the most important reservoirs of antibiotic resistance among gut commensals, (iii) which genes are tightly associated with a limited number of taxa and (iv) which genes can be spread among distantly related bacterial species.
Results
Bacterial strains and identification of acquired resistance genes
Altogether 259 bacterial isolates obtained from the chicken caeca in pure culture were included in this study. The whole genome sequences were determined and based on the 16S rDNA sequences, the isolates were classified to eight different phyla; Firmicutes (159 isolates), Bacteroidetes (50 isolates), Actinobacteria (38 isolates), Proteobacteria (6 isolates), Fusobacteria (3 isolates), Verrucomicrobia (1 isolate), Elusimicrobia (1 isolate) and Synergistetes (1 isolate).
A comparison using the ResFinder database showed that 124 isolates (47.9% out of all) harboured at least one antibiotic resistance gene (Supplementary Fig. S1). Resistance genes coding for a single antibiotic were detected in 87 isolates. Genes responsible for resistance to two different antibiotics were detected in 32 isolates and additional 5 isolates encoded genes responsible for resistance to three different antibiotics. The recorded genes coded for resistance to tetracyclines (detected in 101 isolates), macrolide-lincosamide-streptogramin B antibiotics (28 isolates), aminoglycosides (25 isolates), nitroimidazole (4 isolates), β-lactams (2 isolates), sulphonamides (2 isolates), and phenicols, fosfomycins, glycopeptides, and trimethoprim resistance, each found in a single isolate (Fig. 1). In 135 isolates (52.1% out of all), no gene encoding antibiotic resistance was identified using ResFinder database. Low frequency of antibiotic resistance genes was recorded mainly in the isolates belonging to phylum Actinobacteria and to family Veillonellaceae (Supplementary Fig. S1).
Acquired antibiotic resistance genes in chicken gut anaerobes. Whole genomic sequences of 259 gut anaerobes were searched using ResFinder for the presence of horizontally acquired genes responsible for antibiotic resistance. See Supplementary Fig. S1 for the distribution of these resistances among individual isolates.
Identification of tetracycline resistance genes
Since genes coding for resistance to tetracyclines were the most common, we analysed their distribution in greater detail. Due to the fact that some of the isolates harboured more than one gene responsible for tetracycline resistance, altogether 114 tetracycline resistance genes were found in 101 isolates. Three of them, tetA(P), tet(L) and tet(40), encoded efflux pump proteins, eight of them, tet(M), tet(S), tet(44), tet(O), tet(32), tet(Q), tet(W) and tetB(P) encoded ribosomal protection proteins (RPP) and one mosaic gene tet(O/32/O) of RPP type of resistance was also recorded (Table 1).
Taxonomic distribution of tetracycline resistance genes
Tetracycline resistance genes were detected among isolates belonging to three different phyla: Firmicutes (82 isolates with tetracycline resistance genes, i.e. 51.6% of all isolates from this phylum); Bacteroidetes (14 isolates, 28%); and Actinobacteria (5 isolates, 13.2%). In isolates belonging to the remaining phyla, no genes coding for tetracycline resistance were identified (Fig. 2a).
Presence of genes coding for resistance to tetracycline in isolates belonging to different phyla and families. (a) All isolates included in this study were aligned according to their 16S rRNA gene sequence. The phylogenetic tree was performed in iTOL v5.5.1, http://itol.embl.de17. The presence of particular tetracycline resistance genes is indicated by dots external to the dendrogram. (b) Distribution of tetracycline resistance genes among different families of chicken gut anaerobes. The alluvial diagram was produced in R v4.0.2, https://www.R-project.org/18.
Some of the tetracycline resistance genes were restricted to a certain taxonomic unit. The tet(Q) gene was present only in isolates from phylum Bacteroidetes and tetA(P) was associated exclusively with family Clostridiaceae. The opposite extreme was represented by the tet(W) gene, which was detected in isolates belonging to 5 different families and two phyla. By analysing the same data set from a taxonomic perspective, Actinobacteria harboured only the tet(W) gene and Bacteroidetes encoded only tet(Q). On the other hand, high variability in tetracycline resistance genes was found among isolates from Firmicutes and families Lachnospiraceae and Erysipelotrichaceae, each of them encoding 4 different tet genes (Table 1, Fig. 2).
Real-time PCR quantification of tetracycline resistance genes in complete chicken microbiota
Since the frequency of different tetracycline resistance genes and their association with particular taxa was determined in a rather small number of genomes, which could affect conclusions, we subsequently verified the frequency of distribution and association of the major taxa harbouring selected tetracycline resistance genes in 70 chicken caecal samples. In these samples, the abundance of tet(W), tet(32), tet(Q), tet(O), tet(44) and tetA(P) genes was determined by real-time PCR and microbiota composition was determined by 16S rRNA sequencing.
Among caecal samples originating from chickens younger than one month of age, tet(W), tet(32) and tet(O) genes were the most abundant tetracycline resistance genes with an abundance ranging from 1 to 5% of the bacterial population. The tet(44) and tet(Q) genes were present at an abundance of 0.01 to 0.1% and tetA(P) was the least abundant tetracycline resistance gene in microbiota of chickens younger than 1 month with an abundance of around 0.001% (Fig. 3a). On the other hand, when caecal samples originating from chickens older than 1 month were tested, the tet(Q) gene was the most frequently detected gene with an abundance ranging from 1 to 5%. The tet(W) gene was present at an abundance of 0.5 to 1% followed by tet(32), tet(44) and tet(O) that were present at an abundance of around 0.1%. The tetA(P) gene was the least abundant tetracycline resistance gene in microbiota of chickens older than 1 month with an abundance below 0.001% (Fig. 3a).
(a) Abundance of selected tetracycline resistance genes and (b) microbiota composition in chickens younger or older than 1 month. *, p < 0.05. In agreement with predictions from genomic analysis (Table 1 and Fig. 2), microbiota of chickens under 1 month of age was dominated by Firmicutes and tet genes characteristic for Firmicutes were commonly detected in these samples. On the other hand, Bacteroidetes formed approx. 47% of total microbiota of adult hens and tet(Q) gene, predicted as associated with Bacteroidetes, was significantly more abundant in the samples from adult chickens in comparison with the chickens younger than 1 month. (c) Correlation heat map of microbiota composition at family level and frequency of selected genes coding for tetracycline resistance. *, p < 0.05. The heat map was produced in R v4.0.2, https://www.R-project.org/18.
When the microbiota composition was determined in the same samples, those originating from chickens under 1 month of age were dominated by Firmicutes (79.0 ± 8.65%) and Proteobacteria (19.14 ± 7.81%). Bacteroidetes and Actinobacteria were present in less than 1% of total microbiota (Fig. 3b). On the other hand, caecal samples originating from chickens older than 1 month were dominated by Bacteroidetes and Firmicutes, which were present at 46.96 ± 18.79% and 40.28 ± 21.19%, respectively. Proteobacteria formed 7.4 ± 6.59% of the total bacterial population and Actinobacteria formed less than 1% of total microbiota (Fig. 3b). Correlation analysis confirmed predictions from genomic analyses and indicated that Lachnospiraceae currently represents the most likely reservoir of tet(W), tet(32) and tet(O), Eubacteriaceae and Erysipelotrichaceae represent the reservoir of tet(44), and Bacteroidaceae, Porphyromonadaceae, Prevotellaceae or Flavobacteriaceae, all from phylum Bacteroidetes, are the most likely reservoirs of tet(Q). The frequency of tetA(P) correlated the most with abundance of family Peptostreptococcaceae (Fig. 3c).
Origin of tetracycline resistance genes
Current reservoirs may not necessarily represent the original host. Finally, we therefore attempted to identify the most likely original source of each of the tetracycline resistance genes. To address this, GC content and codon usage in all tet resistance genes were compared with GC content and codon usage of genomes of analysed bacterial isolates (Table 1, Fig. 4). tet(W) gene had the highest GC content (53% GC content). The GC content of all the remaining tetracycline resistance genes ranged from 29 to 43%. According to hierarchical clustering of codon usage, tet(M), tet(S), tet(44), and tet(O), tet(32), tet(O/32/O), tet(40) formed two clusters of genes with similar codon usage, respectively, indicating their common origin. The tet(L) and tet(Q) genes each formed a separate lineage suggesting their independent origin. However, none of these genes clustered closely with any of the included genomes. On the other hand, tetA(P) and tetB(P) clustered together with Tyzzerella sp. An114 from family Lachnospiraceae, Fusobacterium mortiferum, Clostridium saudiense and Clostridium perfringens pointing to their potential original reservoir. When we assessed the origin of tet genes with bacteria at family level, except for tet(W), the origin of all tested tet genes could be traced to low GC content bacteria such as Erysipelotrichaceae, Lactobacillaceae, Enterococcaceae, Peptostreptococcaceae or Clostridiaceae.
Clustering of all isolates analysed in this study and all tetracycline resistance genes based on GC content and codon usage. Frequency of codon usage in each of the tetracycline resistance genes and frequencies of codon usage averaged across all genes in the genomes of each of the analysed isolates were used for mutual clustering. Since tet proteins consisted of 408 to 657 amino acids, the distribution of each of the 61 codons coding for a particular amino acid could be considered as random and representative. However, since only a single stop codon may appear in each tet gene, frequency of stop codon usage was not considered. The heat map was constructed in ClustVis, https://biit.cs.ut.ee/clustvis/19.
Discussion
In this study, we were interested in the dissemination of antibiotic resistance genes among chicken gut commensals, i.e. in bacterial species which have never been the target of antibiotic therapy. Our results show that nearly half of the 259 selected isolates possessed at least one of the horizontally acquired genes conferring resistance to antibiotics. In fact, the real number might be even higher because we could identify only the genes which were present in the ResFinder database. Even with this limit, genes coding for resistance to 10 different classes of antibiotics were recorded. Of these, genes coding for resistance to tetracyclines were the most common likely due to the broad use of tetracyclines as feed additives and growth promoters20.
Twelve different genes increasing resistance to tetracyclines were recorded with tet(W), tet(Q), tet(32) and tet(O) being the most frequent. This conclusion obtained after analysis of bacterial genomes was also confirmed by quantitative PCR in chicken caecal samples. The tet(W), tet(Q), tet(32) and tet(O) genes were identified among the most common tetracycline resistance genes also in pig manure21. The same genes were also found as common in human gut samples22. However, when performing such comparisons, it should be kept in mind that the frequency of a particular gene is affected by the sample type collected. We detected the tet(M) gene in Lactobacillus and Enterococcus. If we sampled ileal contents, which are rich in lactobacilli and enterococci23, we could expect a much broader tet(M) distribution. Similarly, if sampling faecal material, which mostly represents discharges of ileal digesta and less frequently caecal excretions24, we would also expect a much broader tet(M) prevalence as previously proposed25. Alternatively, if collecting samples from young chickens, e.g. broilers, the tet(Q) gene would be underrepresented since this gene is present in Bacteroidetes26,27 and isolates of this phylum usually appear in chicken gut microbiota later during life28.
The tet(W) gene has been previously reported as one of the most widespread tetracycline resistance genes, present in anaerobic bacteria from geographically distant locations25. The tet(W) gene has been found in Clostridium spp., Roseburia spp., Selenomonas spp., Mitsuokella spp., Megasphaera elsdenii, Bifidobacterium longum from bovine and sheep rumen, and porcine and human faeces9,29. The tet(W) gene can therefore spread among phyla (Firmicutes and Actinobacteria). Within Firmicutes, it can be found in Veillonellaceae (Selenomonas, Mitsuokella, and Megamonas funiformis in this study), which express an outer membrane similar to Gram-negative bacteria30. Perhaps not surprising, conserved sequences flanking tet(W) gene are quite short, only 657 bp upstream and 43 bp downstream of the tet(W) gene9. The genetic context of tet(W) therefore varies widely thus enabling this gene to successfully spread among distantly related bacteria. Despite this, tet(W) genes from different isolates formed a separate cluster in codon usage analysis and their common origin is likely.
The tet(32) gene was originally detected in two isolates of Streptococcus spp. and one isolate of Eubacterium saburreum from the oral cavity31. This agrees with our observation when we recorded this gene in genomes of Lachnospiraceae, Ruminococcaceae, and Erysipelotrichaceae, all common gut microbiota members from phylum Firmicutes.
We detected the tet(O) gene only in Lachnospiraceae and Erysipelotrichaceae. However, exactly the same gene has been reported also in Campylobacter32,33 and we have found the same gene also in Fusobacterium mortiferum and Fusobacterium perfoetens from pig gut microbiota (unpublished data). This makes the tet(O) gene phylogenetically the most widespread, capable of crossing the barrier between Gram-positive and Gram-negative bacteria.
Finally, we attempted to define current reservoirs and possible original sources of individual tetracycline resistance genes. We have shown recently that the addition of antibiotics into growth media positively selected for Erysipelotrichaceae15. In agreement, we found 4 different tetracycline resistance genes in the genomes of 23 isolates from family Erysipelotrichaceae. Additional important reservoirs should be sought in family Lachnospiraceae since 34 isolates from this family encoded 32 tetracycline resistance genes of 4 different types (tet(W), tet(32), tet(O/32/O), tet(40)). On the other hand, numerically the most represented family Ruminococcaceae (n = 55 in this study) encoded only 3 different tetracycline resistance genes. Differential distribution of tetracycline resistance genes among gut microbiota members can be explained by GC content and codon usage in tetracycline resistance genes and analysed genomes as can be seen in Table 1 in which tetracycline resistance genes and bacterial families are arranged according to descending GC content. In Coriobacteriaceae (66.3% average genomic GC content), only the tet(W) gene with the highest GC content of all tet genes (53.2% GC content) was detected, similar to a previous report5. In Ruminococcaceae, with 59.5% average genomic GC content, tet(W) with 53.2% GC content was the most frequent tet gene followed by tet(32) and recombinant tet(O/32/O) genes with 41–42% GC content. The tet genes with lower than 40% GC content were not detected in Ruminococcaceae. Erysipelotrichaceae, with an average genomic GC content of 33.9%, encoded only tet genes with a GC content between 30.4 and 40.4%. Finally, 5 strains of family Clostridiaceae with average genomic GC content 30.1% encoded only tetA(P) and tetB(P) with GC content 29.6 and 31.8%, respectively. This dependence was present also in Bacteroidetes since tet(Q) (40.0% GC content) was common in Bacteroidaceae (46.1% GC content) and Porphyromonadaceae (48.2% GC content) but rare in Rikenellaceae (60.8% GC content). Currently, Lachnospiraceae therefore represent the most important reservoir of tet(W), tet(32), tet(O/32/O), tet(40) and tet(O), Erysipelotrichaceae of tet(44), Clostridiaceae of tetA(P) and tetB(P), and Bacteroidaceae, and Porphyromonadaceae of tet(Q).
Although we were able to identify currently the most important reservoirs of the antibiotic resistance genes, predicting the original host of the tested tet genes was more difficult. Except for tetA(P) and tetB(P), all the remaining tetracycline resistance genes exhibited codon preferences different from the bacteria included in this study. The tested bacteria therefore do not represent the original source of tetracycline resistance and this resistance has been introduced to these species by horizontal gene transfer. Only tetA(P) and tetB(P) exhibited codon usage similar to Tyzzerella sp., Fusobacterium mortiferum, Clostridium saudiense and Clostridium perfringens. Any of these species may therefore represent an original source of this tetA(P) and tetB(P). The tetA(P) and tetB(P) genes are common to soil microbiota and Blau et al. reported an increased abundance of tetA(P) in manure-treated soil concurring with an enrichment of clostridia34. We therefore favour Clostridium saudiense and Clostridium perfringens and related low GC content clostridia as possible original source of this gene. However, we cannot exclude other species not included in this study as the original host of tetA(P) and tetB(P) since these genes were detected also in Clostridium difficile35 and we correlated its presence with Peptostreptococcaceae, i.e. the family to which Clostridium difficile belongs. The low GC content clostridia or Peptostreptococcaceae may represent the possible original source of tetA(P) and tetB(P).
Methods
Bacterial sample collection, whole genome sequencing and data availability
Altogether 259 isolates originating from chicken caecal contents were included in this study (Supplementary Table S1). The isolates were obtained from healthy chickens or hens as described previously16. DNA isolation, whole genome sequencing and bioinformatic analysis was performed according to Medvecky et al.16 and genomic sequences are deposited in NCBI under accession number PRJNA377666.
Identification of acquired resistance genes
On-line version of the ResFinder available at https://cge.cbs.dtu.dk/services/ResFinder/ was used to identify acquired antibiotic resistance genes36. The threshold for a match between genes in the ResFinder database and the input genome sequence was set to 90% identity over 60% of the length of the resistance gene.
GC content and codon usage
GC content and codon usage were calculated for all protein coding genes of all investigated genomes and all tetracycline resistance genes using CodonUsage python script embedded in BioPython37. All 3 stop codons were excluded from codon usage analysis since each antibiotic resistance gene uses only a single stop codon which does not allow for any variation.
Real-time PCR quantification of tetracycline resistance genes in caecal samples
To verify in silico analyses and predictions, tetracycline resistance genes and microbiota composition were determined in 70 chicken caecal samples. Of these, 37 samples originated from chickens younger than 1 month of age and 33 samples originated from chickens older than 1 month (Supplementary Table S2). Classification into two age categories was adopted due to the known development of chicken caecal microbiota and the appearance of representatives of Bacteroidetes usually in chickens older than one month28. Caecal contents were homogenised in a MagNA Lyser (Roche) and the DNA was extracted using a QIAamp DNA Stool Mini Kit according to the manufacturer’s instructions (Qiagen). Purified DNA was used as a template for real-time PCR quantification of tet(W), tet(32), tet(Q), tet(O), tet(44) and tetA(P) tetracycline resistance genes (Table 2). Amplification of the 16S rRNA gene using Eubacteria specific primers was used as a reference to determine the total amount of eubacterial DNA in each sample. PCR reactions were performed in 3 μl volumes in 384-well microplates using QuantiTect SYBR Green PCR Master mix (Qiagen) as described previously38. After PCR, Ct values of genes of interest were subtracted from the Ct value of bacterial 16S rRNA gene amplification (ΔCt) and the relative abundance of each resistance gene was calculated as 2-ΔCt.
Microbiota composition determined by 16S rDNA sequencing
The DNA extracted from caecal samples was used as a template in PCR with eubacterial primers amplifying the V3/V4 variable region of 16S rRNA genes. Following amplification, the products were processed exactly as described previously23. The amplicon data have been deposited in NCBI under accession number PRJNA673404.
Bioinformatics and statistics
Clustal Omega using sequences of the whole gene for 16S rRNA was applied for clustering of all strains according to their taxonomic relatedness40. Final modification of the phylogenetic tree was performed in iTOL v5.5.117.
Spearman’s correlation was used to calculate correlations between the abundance of a particular family in chicken gut microbiota and the abundance of selected tetracycline resistance genes. A final heat map as well as alluvial diagram were produced in R v4.0.2 using ggplot2 and gplots packages, respectively18.
Average relative frequencies of all codons in protein coding genes within an individual genome and relative frequencies of tetracycline resistance genes were used for heatmap construction and hierarchical clustering using ClustVis19. Data were clustered based on correlation distance and average linkage. For tetracycline resistance genes, only one representative from a group of identical genes (100% identity) was selected.
Differences in the distribution of antibiotic resistance genes in chickens younger or older than 1 month were determined using non-parametric Mann–Whitney U-test. Differences with p < 0.05 were considered as significant.
Approval for animal experiments
Authors declare that not a single chicken has been sacrificed specifically for the purpose of this study and DNA purified from all chicken samples originated from previous studies. The handling of animals in these studies was performed in accordance with current Czech legislation (Animal Protection and Welfare Act No. 246/1992 Coll. of the Government of the Czech Republic) and the specific experiments were approved by the Ethics Committee of the Veterinary Research Institute followed by the Committee for Animal Welfare of the Ministry of Agriculture of the Czech Republic (permit number MZe1922).
References
Faldynova, M. et al. Evolution of antibiotic resistance in Salmonella enterica serovar typhimurium strains isolated in the Czech Republic between 1984 and 2002. Antimicrob. Agents Chemother. 47, 2002–2005. https://doi.org/10.1128/aac.47.6.2002-2005.2003 (2003).
De Oliveira, D. M. P. et al. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. https://doi.org/10.1128/CMR.00181-19 (2020).
Raimondi, S. et al. Antibiotic resistance, virulence factors, phenotyping, and genotyping of E. coli isolated from the feces of healthy s ubjects. Microorganisms https://doi.org/10.3390/microorganisms7080251 (2019).
Dec, M., Urban-Chmiel, R., Stepien-Pysniak, D. & Wernicki, A. Assessment of antibiotic susceptibility in Lactobacillus isolates from chickens. Gut Pathog. 9, 54. https://doi.org/10.1186/s13099-017-0203-z (2017).
Masco, L., Van Hoorde, K., De Brandt, E., Swings, J. & Huys, G. Antimicrobial susceptibility of Bifidobacterium strains from humans, animals and probiotic products. J. Antimicrob. Chemother. 58, 85–94. https://doi.org/10.1093/jac/dkl197 (2006).
Qu, A. et al. Comparative metagenomics reveals host specific metavirulomes and horizontal gene transfer elements in the chicken cecum microbiome. PLoS ONE 3, e2945. https://doi.org/10.1371/journal.pone.0002945 (2008).
Yeoman, C. J. et al. The microbiome of the chicken gastrointestinal tract. Anim. Health Res. Rev. 13, 89–99. https://doi.org/10.1017/S1466252312000138 (2012).
Danzeisen, J. L., Kim, H. B., Isaacson, R. E., Tu, Z. J. & Johnson, T. J. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS ONE 6, e27949. https://doi.org/10.1371/journal.pone.0027949 (2011).
Kazimierczak, K. A., Flint, H. J. & Scott, K. P. Comparative analysis of sequences flanking tet(W) resistance genes in multiple species of gut bacteria. Antimicrob. Agents Chemother. 50, 2632–2639. https://doi.org/10.1128/AAC.01587-05 (2006).
Whittle, G., Shoemaker, N. B. & Salyers, A. A. The role of Bacteroides conjugative transposons in the dissemination of antibiotic resistance genes. Cell Mol. Life Sci. 59, 2044–2054. https://doi.org/10.1007/s000180200004 (2002).
Buckwold, S. L., Shoemaker, N. B., Sears, C. L. & Franco, A. A. Identification and characterization of conjugative transposons CTn86 and CTn9343 in Bacteroides fragilis strains. Appl. Environ. Microbiol. 73, 53–63. https://doi.org/10.1128/AEM.01669-06 (2007).
Lagier, J. C. et al. Microbial culturomics: paradigm shift in the human gut microbiome study. Clin. Microbiol. Infect. 18, 1185–1193. https://doi.org/10.1111/1469-0691.12023 (2012).
Rettedal, E. A., Gumpert, H. & Sommer, M. O. Cultivation-based multiplex phenotyping of human gut microbiota allows targeted recovery of previously uncultured bacteria. Nat. Commun. 5, 4714. https://doi.org/10.1038/ncomms5714 (2014).
Lau, J. T. et al. Capturing the diversity of the human gut microbiota through culture-enriched molecular profiling. Genome Med. 8, 72. https://doi.org/10.1186/s13073-016-0327-7 (2016).
Crhanova, M. et al. Systematic culturomics shows that half of chicken caecal microbiota members can be grown in vitro except for two lineages of Clostridiales and a single lineage of Bacteroidetes. Microorganisms. https://doi.org/10.3390/microorganisms7110496 (2019).
Medvecky, M. et al. Whole genome sequencing and function prediction of 133 gut anaerobes isolated from chicken caecum in pure cultures. BMC Genom. 19, 561. https://doi.org/10.1186/s12864-018-4959-4 (2018).
Letunic, I. & Bork, P. Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 44, W242-245. https://doi.org/10.1093/nar/gkw290 (2016).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. (2017).
Metsalu, T. & Vilo, J. ClustVis: a web tool for visualizing clustering of multivariate data using principal component analysis and heatmap. Nucleic Acids Res. 43, W566-570. https://doi.org/10.1093/nar/gkv468 (2015).
Granados-Chinchilla, F. & Rodriguez, C. Tetracyclines in food and feedingstuffs: From regulation to analytical methods, bacterial resistance, and environmental and health implications. J. Anal. Methods Chem. 2017, 1315497. https://doi.org/10.1155/2017/1315497 (2017).
Zhu, Y. G. et al. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc. Natl. Acad. Sci. U S A 110, 3435–3440. https://doi.org/10.1073/pnas.1222743110 (2013).
Hu, Y. et al. Metagenome-wide analysis of antibiotic resistance genes in a large cohort of human gut microbiota. Nat. Commun. 4, 2151. https://doi.org/10.1038/ncomms3151 (2013).
Kollarcikova, M. et al. Use of 16S rRNA gene sequencing for prediction of new opportunistic pathogens in chicken ileal and cecal microbiota. Poult. Sci. 98, 2347–2353. https://doi.org/10.3382/ps/pey594 (2019).
Rychlik, I. Composition and function of chicken gut microbiota. Animals (Basel) https://doi.org/10.3390/ani10010103 (2020).
Roberts, M. C. Update on acquired tetracycline resistance genes. FEMS Microbiol. Lett. 245, 195–203. https://doi.org/10.1016/j.femsle.2005.02.034 (2005).
Lorenzo, M. et al. Antimicrobial resistance determinants among anaerobic bacteria isolated from footrot. Vet. Microbiol. 157, 112–118. https://doi.org/10.1016/j.vetmic.2011.11.029 (2012).
Veloo, A. C. M., Baas, W. H., Haan, F. J., Coco, J. & Rossen, J. W. Prevalence of antimicrobial resistance genes in Bacteroides spp. and Prevotella spp. Dutch clinical isolates. Clin. Microbiol. Infect. 25(1156), e1159–e1156. https://doi.org/10.1016/j.cmi.2019.02.017 (2019).
Videnska, P. et al. Succession and replacement of bacterial populations in the caecum of egg laying hens over their whole life. PLoS ONE 9, e115142. https://doi.org/10.1371/journal.pone.0115142 (2014).
Ammor, M. S., Florez, A. B., Alvarez-Martin, P., Margolles, A. & Mayo, B. Analysis of tetracycline resistance tet(W) genes and their flanking sequences in intestinal Bifidobacterium species. J. Antimicrob. Chemother. 62, 688–693. https://doi.org/10.1093/jac/dkn280 (2008).
Yutin, N. & Galperin, M. Y. A genomic update on clostridial phylogeny: Gram-negative spore formers and other misplaced clostridia. Environ. Microbiol. 15, 2631–2641. https://doi.org/10.1111/1462-2920.12173 (2013).
Warburton, P. et al. Characterization of tet(32) genes from the oral metagenome. Antimicrob. Agents Chemother. 53, 273–276. https://doi.org/10.1128/AAC.00788-08 (2009).
Poly, F., Threadgill, D. & Stintzi, A. Identification of Campylobacter jejuni ATCC 43431-specific genes by whole microbial genome comparisons. J. Bacteriol. 186, 4781–4795. https://doi.org/10.1128/JB.186.14.4781-4795.2004 (2004).
Elhadidy, M. et al. Antimicrobial resistance patterns and molecular resistance markers of Campylobacter jejuni isolates from human diarrheal cases. PLoS ONE 15, e0227833. https://doi.org/10.1371/journal.pone.0227833 (2020).
Blau, K. et al. Manure and doxycycline affect the bacterial community and its resistome in lettuce rhizosphere and bulk soil. Front. Microbiol. 10, 725. https://doi.org/10.3389/fmicb.2019.00725 (2019).
Kecerova, Z., Cizek, A., Nyc, O. & Krutova, M. Clostridium difficile isolates derived from Czech horses are resistant to enrofloxacin; cluster to clades 1 and 5 and ribotype 033 predominates. Anaerobe 56, 17–21. https://doi.org/10.1016/j.anaerobe.2019.01.005 (2019).
Zankari, E. et al. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 67, 2640–2644. https://doi.org/10.1093/jac/dks261 (2012).
Cock, P. J. et al. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 25, 1422–1423. https://doi.org/10.1093/bioinformatics/btp163 (2009).
Gerzova, L. et al. Characterization of antibiotic resistance gene abundance and microbiota composition in feces of organic and conventional pigs from four EU countries. PLoS ONE 10, e0132892. https://doi.org/10.1371/journal.pone.0132892 (2015).
Aminov, R. I., Garrigues-Jeanjean, N. & Mackie, R. I. Molecular ecology of tetracycline resistance: development and validation of primers for detection of tetracycline resistance genes encoding ribosomal protection proteins. Appl. Environ. Microbiol. 67, 22–32. https://doi.org/10.1128/AEM.67.1.22-32.2001 (2001).
Madeira, F. et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 47, W636–W641. https://doi.org/10.1093/nar/gkz268 (2019).
Acknowledgements
This work was supported by the Czech Science Foundation (grant number 18-15238Y) and by the Czech Ministry of Agriculture (grant number RVO0518). Authors would like to thank Peter Eggenhuizen for English language corrections.
Author information
Authors and Affiliations
Contributions
H.J. and I.R. designated the experiments, analysed the data and wrote the manuscript. H.J. and J.M. performed the real-time quantification. T.K. and D.C. were responsible for genome sequencing. D.C. was responsible for bioinformatics analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Juricova, H., Matiasovicova, J., Kubasova, T. et al. The distribution of antibiotic resistance genes in chicken gut microbiota commensals. Sci Rep 11, 3290 (2021). https://doi.org/10.1038/s41598-021-82640-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82640-3
This article is cited by
-
Metagenomic analysis reveals patterns and hosts of antibiotic resistance in different pig farms
Environmental Science and Pollution Research (2023)
-
Plastic wastes and surface antibiotic resistance genes pollution in mangrove environments
Environmental Monitoring and Assessment (2023)
-
The impacts of viral infection and subsequent antimicrobials on the microbiome-resistome of growing pigs
Microbiome (2022)
-
Occurrence of antibiotics and bacterial resistance genes in wastewater: resistance mechanisms and antimicrobial resistance control approaches
World Journal of Microbiology and Biotechnology (2022)
-
Longitudinal study on the effects of growth-promoting and therapeutic antibiotics on the dynamics of chicken cloacal and litter microbiomes and resistomes
Microbiome (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.