Abstract
Hepatocellular carcinoma (HCC) is one of the most common and most lethal malignant tumors in the world. Microvascular invasion (MVI) is a major risk factor for survival outcomes and intrahepatic metastasis after resection in patients with HCC. Relevant English literatures retrieved using PubMed on the research progress of MVI in patients with HCC were reviewed. For HCC patients, especially those with MVI, it is very important to develop a comprehensive and sequential treatment plan to support the long-term survival of patients. This manuscript reviewed and analyzed the risk factors for MVI; the preoperative prediction of MVI, which informs the selection of surgical strategies; and the current situation and future direction of comprehensive postoperative treatment strategies; to provide a basis for the comprehensive treatment of HCC patients with MVI. For HCC patients with MVI, the preoperative prediction of MVI may play a certain guiding role in planning procedures, and the comprehensive sequential postoperative pathological detection of HCC MVI may provide a basis for treatment decisions.
Similar content being viewed by others
Introduction
HCC is the fifth most common cancer worldwide1 and the third most common cause of cancer-related death, with more than half of the new cases and deaths occurring in China every year2. In the past two decades, substantial progress has been made in the treatment of HCC with advances in medical technology, although surgical resection and liver transplantation are still the most effective treatments. However, the prognosis of patients with HCC after surgical resection is very poor. A high recurrence rate and a high mortality rate are important factors affecting the long-term survival status of patients after surgical treatment. The 5-year recurrence rates after surgical treatment and liver transplantation are as high as 70% and 35%, respectively3. Current research advances suggest that there are many risk factors for tumor recurrence after surgical resection, including residual lesions, vascular invasion, HBV/HCV replication, degree of liver cirrhosis, the presence and the degree of portal hypertension (HP) and surgical methods4. Among them, vascular invasion is considered a key factor in the early recurrence (within 24 months of surgery) of HCC based on the results of multiple retrospective studies3,5,6. Therefore, it is very clinically important to explore the selection of treatment strategies after the surgical resection of HCC, especially in patients with vascular invasion.
Vascular invasion is considered either macroscopic or microscopic. The former is determined by gross tissue evaluation or diagnostic imaging, whereas the latter, microvascular invasion (MVI), is determined by a histopathological examination of the tumor and surrounding hepatic tissue7. Although multiple recent retrospective analyses have found close correlations between MVI and adverse tumor biology and a poor prognosis, the choice of the appropriate treatment for HCC patients complicated with MVI is still worthy of an in-depth discussion.
Definition of MVI
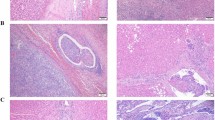
Strictly speaking, MVI is a pathological diagnosis and there are many definitions and standards for MVI. Microscopically, MVI appears as nests of malignant cells lining the vascular cavities of endothelial cells or portal and hepatic venous systems (Fig. 1)8. MVI can be evidenced by the identification of tumor cells within endothelial-lined spaces on standard hematoxylin and eosin stained slides7. Previous studies have shown that the incidence of MVI fluctuates between 15 and 57.1% in HCC patients9. This difference may be related to the sampling site and the non-uniform diagnostic criteria. Cong et al. drew conclusions from previous research results, and they recommend that MVI be evaluated in all tissue sections and graded according to the level of risk based on the number and distribution of sites as follows: M0, no MVI; M1 (low-risk), MVI < 5 and at ≤ 1 cm away from the adjacent liver tissue; and M2 (high-risk), MVI > 5 or at > 1 cm away from the adjacent liver tissue (Fig. 2)10. This was also the first detailed description of the definition of MVI in a pathology guide in China.
MVI classification and its presence in HCC. MVI be evaluated in all tissue sections and graded according to the level of risk based on the number and distribution of sites as follows: M0, no MVI; M1 (low-risk), MVI < 5 and at ≤ 1 cm away from the adjacent liver tissue; and M2 (high-risk), MVI > 5 or at > 1 cm away from the adjacent liver tissue.
Hepatocyte canceration usually occurs on the basis of chronic inflammatory stimulation, biological factors, toxic exposure and metabolic abnormalities, activating proto-oncogenes, inactivating tumor suppressor genes through multiple signaling pathways, leading to inhibition of cell apoptosis and triggering uncontrolled cell proliferation. Tumor cells can secrete cathepsin H to break down extracellular matrix, and at the same time reduce the synthesis of cadherin E to reduce the adhesion between cells, so as to facilitate tumor metastasis. Tumor-induced neoangiogenesis, proliferation and inhibition of apoptosis are the prime reasons of MVI11. Among them, degradation of the basal membrane and herniation of the tumor cells to the capillary lumen is the key step in the process of MVI. In this process, circulating tumor cells are coated with endothelial cells and form microemboli in the capillary lumen. When MVI occurs, it indicates that HCC cells have invaded blood vessels and may begin to metastasize, and the tumor has progressed to a new stage. Small blood vessels in the liver are composed of vascular endothelial cells, fibronectin and fibrinogen basement membrane. The cluster of hepatocellular carcinoma cells can be embedded in the vascular endothelial cells and surrounded by the vascular endothelial cells to survive in the blood, so as to escape the immune cell clearance of the body and avoid activating the coagulation mechanism to achieve distant metastasis12.
Risk factors associated with MVI
Tumor classification (Table 1)
According to the classification criteria of Eggel, HCC can be roughly classified into four categories: type I, single nodular (SN) type; type II, single nodular type with extranodular growth (SNEG); type III, confluent multinodular type (CMN); and type IV, infiltrative type13. According to previous studies, the incidence of type I fluctuates between 7.7% and 45.8%, which is lower than the incidences of type II (25–93.4%) and type III (12.5 ~ 100%) MVI14,15. He et al. found that the incidence of type I, type II and type III MVI were 36%, 32.4% and 30.3%, respectively, while the incidence of type IV MVI was 63.5%, which was substantially higher than the incidences of the first three types16. The above data indicate that the incidence of MVI may be correlated with the general classification of HCC.
Tumor diameter
Tumor diameter is correlated with the incidence of MVI because the larger the liver tumor diameter is, the higher the incidence of MVI and intrahepatic metastasis, which often predicts a poor prognosis17. A cohort of 245 patients who underwent resection for HCC were enrolled in a clinical study. Twenty-five percent of patients with tumors smaller than < 2 cm had MVI, whereas 31% and 50% of patients with tumors 2–4 cm in size or larger than 4 cm, respectively, had MVI18. Eguchi et al. found that 127 of 229 patients with HCC who underwent curative liver resection had HCC without microscopic portal vein invasion, and 52 had HCC with microscopic portal vein invasion. Among them, the mean diameters of the tumors in the MVI positive group and the MVI negative group were 5.2 cm and 3.0 cm, respectively19.
Degree of tumor differentiation
According to the Edmondson-Steiner pathological classification system, tumor differentiation can be divided into the high differentiation type (I), medium differentiation type (II) and low differentiation type (III–IV)20. There are relationships between the invaded vasculature and MVI and the pathological grade of the tumor. Regarding the association of vascular invasion with histologic grade, when stratified by tumor stage, patients with Edmondson grade I and tumor size < 5 cm had no MVI, while those with Edmondson grade II or higher had relatively higher incidences of MVI, even if they had small tumors (< 2 cm)21. Furthermore, previous research results have shown that epithelial to mesenchymal transition (EMT)-related genes, including SNAIL, FoxC1, and vimentin, are upregulated in HCC with MVI, implying that EMT is associated with the development of MVI22. Zhang et al. demonstrated the important prognostic value of the FoxM1-KIAA0101 axis in HCC patients and highlighted the essential role of KIAA0101 in FoxM1-driven HCC invasion and metastasis based on the regulation of genes involved in EMT23.
Hepatitis B virus (HBV) and steroid hormone
HBV is associated with 70–90% of HCC cases, and more than 70% of all newly diagnosed HCC cases occur in highly endemic areas for HBV in the Asia–Pacific region, particularly in China24,25. Chronic HBV infection is a major risk factor for the development of liver cirrhosis and HCC and is also associated with an increased recurrence rate and a decreased survival rate of HCC after surgical resection26. Previous studies have confirmed that the HBV-initiated tumorigenic process may play a role in the development of MVI in HCC27,28. Lei et al. established a novel prediction model for the preoperative prediction of the presence of MVI in HBV-related HCC, in which a high HBV DNA load (> 104 IU/ml) was independently associated with the development of MVI24. The results from Wei et al. also showed that compared with patients without HBV infection, HBV-infected patients with HCC had a significantly higher incidence of MVI29.
As a sexually dimorphic organ, the liver can express the estrogen receptors (ERs) ERα and ERβ, regulating the expression of various genes involved in the cell cycle, proliferation, apoptosis and inflammation30. Patients could be protected from HCC by estrogen, which is considered to be a preventive factor, and achieve better recovery after HCC treatment31. The findings of one study indicated that males express significantly higher levels of ERα than females but comparable levels of ERβ. The ERα: ERβ expression ratio in males is also significantly higher than that in females32. However, whether these differences in ER subtype expression predispose males to rapid HCC disease progression needs further investigation.
Tumor capsule
The integrity of the tumor envelope is associated with MVI, and the risk of MVI also increases with the decrease in the integrity of the tumor envelope. The tumor envelope is affected by distensible tumor growth, compressing the surrounding normal liver tissue and allowing fibrous tissue to proliferate and form around the tumor. Most scholars believe that the integrity of the tumor envelope can prevent contact between tumor cells and surrounding tissues and limit the exogenous invasive growth of tumors, while an incomplete or absent envelope can result in the tumor being more likely to invade the surrounding tissues. Chou et al. analyzed the preoperative CT data of 102 patients with HCC including tumor size, tumor capsule, tumor margins and peritumoral enhancement, and the postoperative pathological results33. They found that nonsmooth margins (presenting as the focal outgrowth of nodules protruding into the nontumor parenchyma) and nonsmooth margins with the multinodular type were associated with MVI. They thus thought that nonsmooth margins detected on multiphasic CT may be predictive of MVI in HCC. Renzulli et al34 have indicated that peritumoral enhancement is a significant marker of histologic MVI and that radiographic tumor margins may be radiological indicators for predicting MVI in patients with HCC. It is possible to assess peritumoral enhancement by using noninvasive techniques, such as CT or dynamic MR imaging, which are recommended for diagnosing HCC. In particular the newly introduced MRI contrast agent gadoxetic acid (gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid, Gd-EOB-DTPA) has enabled the concurrent assessment of tumor vascularity and hepatocyte-specific contrast enhancement during the hepatobiliary phase (HBP), which can help to detect and characterize smaller HCCs and their precursors35,36. The organic anion transporter peptides (OATP) 1B1 and 1B3 are liver-specific molecules, and they are transporters of cholephilic organics, playing an important role in the uptake of the MRI contrast media Gd-EOB-DTPA. Therefore, OATP, identified by this unique technique (MRI) in HCC, could be used together with K7/19 to identify a phenotypical spectrum of HCC progression37. In summary, whether it CT or MRI is used, there are significant associations between MVI and peritumoral enhancement and peritumoral hypointensity38.
Although a number of clinical features, including tumor size, capsule integrity, pathological grade, and imaging manifestations are correlated with the occurrence of MVI, the accurate prediction of MVI cannot be evaluated by a single indicator39. Instead, a comprehensive analysis of multiple factors is needed for a valuable clinical prediction scheme. A study conducted in China, that included 1004 samples of HBV-related HCC found that preoperative factors, including multiple tumors, a large tumor diameter, an incomplete tumor capsule, a high serum α-fetoprotein level, an HBV DNA load greater than 104 IU/ml, a platelet count less than 100 × 103/µl, and the presence of a typical dynamic pattern on contrast-enhanced MRI, were significantly associated with MVI24. Based on the above seven indicators, they constructed a prediction model that performed well and was supported by C-index values of 0.81 and 0.80 in the training and validation cohorts, respectively. The optimal calibration curves demonstrated good agreement between the predictions and actual observations. As a pathological indicator, MVI is often difficult to measure accurately before surgery. Therefore, researchers have attempted to explore methods of preoperatively predicting MVI in a large number of studies to enable the more accurate evaluation of tumor invasion, and the formulation of comprehensive targeted treatment plans during and after surgery.
Clinical significance of MVI
Preoperative prediction of MVI guides surgical planning (Table 2)
For malignant tumors, adequate surgical margins are guaranteed for radical surgery. However, for liver resection, due to the particularity of the tumor location or degree of liver sclerosis, the scope of surgical resection is often limited, so it is sometimes difficult to ensure adequate surgical margins40. In addition, the width of the surgical resection margin has always been controversial, especially in patients with cirrhosis. Some scholars believe that excessive resection of normal liver tissue may not affect the prognosis of patients but may increase the incidence of postoperative complications. Some scholars argue that anatomic resection of HCC can simultaneously remove both the lesion and micrometastases that may have invaded the portal vein or flowed along the branch of the portal vein into the liver segment beyond the main tumor. If this is true, the anatomic resection of HCC could effectively guarantee an adequate scope of resection and theoretically reduce the postoperative recurrence due to micrometastases in the residual tissues that is observed after nonanatomic resection41,42. A systematic review and meta-analysis from Moris et al41 indicated no difference in perioperative complications or perioperative mortality but a higher level of blood loss among patients undergoing anatomic resection versus those undergoing nonanatomic resection; these findings were due to the fact that the resection margin was slightly wider following anatomic resection than after nonanatomic resection. Anatomic resection was associated with an obvious disease-free survival (DFS) benefits at 1-, 3- and 5 years. In addition, anatomic resection seemed to offer an advantage over nonanatomic resection in terms of DFS and overall survival (OS) among patients undergoing resection of HCC, especially among those without cirrhosis. Portolani N et al. also found that for patients undergoing nonanatomic resection, the early recurrence ratio could reach as high as 76.2% in the first 24-month period43. Therefore, multiple meta-analyses and current mainstream research suggest that anatomic resection appears to benefit the DFS and OS of patients with HCC, especially those without cirrhosis41,44. MVI is not only an important predictor of the risk of the postoperative recurrence of liver cancer but also an important pathological indication of the efficacy of antitumor therapy of clinical HCC after an operation. According to clinical data presented by Feng et al7, 41.9% of patients with HCC were pathologically positive for MVI, and the surrounding area near the tumor (< 1 cm) was found to be a high-incidence area for MVI. Studies45 have also found that compared with the M1 and M0 groups, the M2 group had significantly lower OS and DFS rates. In addition, the liver resection method and tumor diameter of the M2 group were independent risk factors for OS and DFS after hepatectomy. Therefore, caution should be exered when considering the choice of surgical methods for liver cancer patients with MVI. As mentioned above, although a number of studies have used individual factors to predict preoperative MVI, including tumor size, capsule integrity, CT/MRI tumor characteristics, or multiple indicators to establish evaluation models, it is still difficult to accurately predict the occurrence of MVI before surgery at this time. A tumor diameter greater than 2 cm, multiple tumors, an incomplete tumor capsule, the presence of a typical dynamic pattern and a high serum α-fetoprotein (AFP) level are independently associated with MVI. Therefore, for patients with these characteristics, anatomic resection may be a relatively better option if their liver function is preoperatively assessed as being adequate and sufficient remaining liver tissue will exist. If the hepatic functional reserve of the patient is poor or the remaining liver volume is insufficient, nonanatomical resection can be selected, but the surgical margin must be greater than 1 cm. Only in this manner can the liver function of the patient be guaranteed and the risk of early local recurrence be reduced.
Prophylactic treatment with transarterial chemoembolization (TACE) after liver resection
The mechanism of underlying early recurrence after resection of HCC is very complex. The postoperative recurrence of HCC is primarily driven by intrahepatic diffusion and is most common near the surgical resection margin; this finding may be related to a compensatory increase in blood supply or incomplete surgical resection resulting in residual MVI46. TACE, which is used to treat intermediate-stage HCC, is currently recognized as one of the most commonly used nonsurgical HCC treatment methods47,48. TACE can decrease blood flow to the tumor, and ischemic tumor necrosis can be induced via the arterial injection of chemotherapeutic drugs and embolizing agents49. The effectiveness of TACE as an adjuvant therapy in HCC has been documented in clinical studies50,51,52; however, whether all HCC patients need routine prophylactic use of TACE after surgery remains controversial. Because TACE may reduce remnant liver function and immune function and because recurring tumors usually have different clonal origins from primary tumors53,54, Jiang JH et al. believed that postoperative adjuvant TACE does not improve OS or reduce recurrence in HCC patients55. However, that study was a retrospective analysis that was likely subject to subtle selection biases, even after propensity score matching. Nevertheless, a randomized controlled trial (RCT) by Li JQ et al. showed postoperative adjuvant TACE to be beneficial for patients with HCC lesions larger than 5 cm in diameter, multiple nodules, macroscopic vascular invasion56 or macroscopic portal vein tumor thrombus57. Another study58 reached a similar conclusion: adjuvant TACE (two or three cycles) after radical resection is beneficial for HCC patients with poor differentiation and MVI, especially for those with a tumor diameter > 5 cm. Similarly, postoperative adjuvant TACE significantly increased RFS and OS and reduced the early recurrence rate of HCC patients with MVI, and postoperative adjuvant TACE was found to be an independent risk factor for postoperative RFS and OS59. The significant effect of postoperative adjuvant TACE on HCC patients with high risk factors for recurrence may be attributed to the following reasons60. Tumor cells in HCC patients with MVI tend to form micrometastases that travel through the blood system to normal tissues surrounding the tumor, resulting in poor DFS and OS61,62. Postoperative adjuvant TACE after liver resection could kill tumor cells and improve the survival rate of patients63. Furthermore, micrometastatic lesions could also be addressed in this way64. In conclusion, for liver cancer patients with high-risk recurrence factors, especially those with MVI, postoperative adjuvant TACE treatment has gradually become the recommended treatment method.
Future of HCC treatment with molecular targeted therapy and immunotherapy
Over the past decade, the molecular-targeted drug sorafenib has been introduced and has been shown to inhibit tumor growth through multiple mechanisms. Sorafenib65 is a multitargeted, orally active small molecule tyrosine kinase inhibitor (TKI) that inhibits Raf kinase and the vascular endothelial growth factor receptor (VEGFR) intracellular kinase pathway; this drug is promising for the treatment of patients with advanced HCC66. The multicenter European SHARP trial67 and Asian-Pacific trial68 established sorafenib monotherapy as the new reference standard systemic treatment for advanced HCC and formed the basis for the approval of sorafenib for treatment of unresectable HCC. Lenvatinib is an inhibitor of VEGFR-1, VEGFR-2, and VEGFR-3, as well as fibroblast growth factor receptors (FGFRs), platelet-derived growth factor receptor (PDGFR) alpha, RET, and KIT. In the REFLECT study69, lenvatinib was compared with sorafenib in 954 patients with unresectable HCC, and it was considered noninferior to sorafenib. Based on the REFLECT trial, lenvatinib received approval for the first-line treatment of unresectable HCC in 2018. A benefit associated with regorafenib in patients progressing after first-line treatment with sorafenib was suggested in the RESORCE trial70. Regorafenib is also promising in patients with oral drug resistance to sorafenib and has become the first approved second-line treatment for HCC. Checkpoint inhibitor immunotherapy is an important research direction in generalized immunotherapy. Nivolumab and pembrolizumab are human monoclonal antibodies that target the programmed cell death 1 receptor (PD-1), restoring T cell immune activity directed against tumor cells. The median OS times in advanced HCC patients given nivolumab and perbrolizumab were 15.6 months and 12.9 months, respectively, and they are currently approved for second-line HCC treatment71,72.
Many studies have shown significantly better survival outcomes by combining sorafenib with radiofrequency ablation or TACE to treat HCC73,74. However, due to the high degree of biological heterogeneity of HCC, the benefits of adjuvant sorafenib in the subgroup of HCC patients with MVI need to be further explored. A total of 728 HCC patients with MVI in the resected specimens after R0 resection were enrolled in a propensity score matching analysis75. In that study, the OS and RFS in HCC patients with MVI who underwent R0 resection and were given postoperative adjuvant therapy with sorafenib were significantly better than those in the patients who underwent liver resection alone. This indicates that patients with MVI after R0 resection can benefit from postoperative adjuvant sorafenib. Peng et al76 also found that the combination of TACE and sorafenib might play an important role in the improvement in the survival of patients with MVI-positive lesions. The above conclusions also indicate that patients with MVI after the resection of HCC may benefit from postoperative adjuvant oral sorafenib combination therapy. Multicenter studies need to be conducted. However, regarding lenvatinib, regorafenib and immunotherapy checkpoint inhibitors, their clinical applications are still supported by data from clinical trials due to their short clinical application time.
Future directions
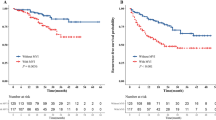
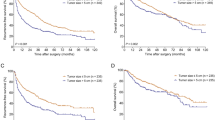
MVI is an important pathological predictor of the risk of the postoperative recurrence of HCC. MVI is an important factor guiding surgical planning and the formulation of comprehensive postoperative treatment plans. We can make preoperative predictions based on the AFP level, des-gamma carboxyprothrombin (DCP) level, tumor size, circular RNA77, imaging characteristics and PIVKAII78 or postoperative MVI determined through a postoperative histological examination. The preoperative prediction of MVI can reduce the risk of postoperative recurrence by indicating the use of antiviral drugs in advance and the need for the expansion of the scope of surgical resection. Postoperative adjuvant TACE combined with sorafenib could improve DFS and OS in HCC patients with MVI. However, the correlation between MVI and prognosis also has certain limitations in HCC. In addition to MVI, there are a series of interfering factors affecting the prognosis of patients with intermediate-advanced HCC, such as the presence of satellite lesions, a heavy tumor burden and persistent HBV replication. Similarly, the presence of MVI can also be detected in early-advanced stage HCC patients with good liver function; however, the prognostic importance of MVI is not strong at this stage of HCC79. Traditionally speaking, when stratified by BCLC stages, MVI is a powerful predictor of outcomes in BCLC HCC stages A and B. However, after propensity score matching (PSM) analysis, MVI was found to independently predict OS in BCLC stage A HCC and not BCLC stage B80. Therefore, in MVI-positive HCC patients, the choice of postoperative therapy, such as TACE and targeted therapy, and BCLC stage are also important reference factors.
Although there are various ways to treat HCC, each treatment has its own advantages and limitations. More importantly, when determining the appropriate therapy for HCC, we must consider both tumor-related factors and background liver-related factors. For BCLC stage A HCC patients with MVI positivity, anatomic resection is recommended based on the prediction model. For these patients, postoperative adjuvant TACE combined with sorafenib is also a very important treatment. Liver resection and TACE are recommended for BCLC intermediate stage HCC patients without MVI and with MVI, respectively81. For HCC patients with MVI, we can formulate more individualized programs, such as shortening the review time, to reduce as much as possible in clinical practice the early recurrence rate caused by high-risk factors.
Worldwide, in the past 20 years, although the research field of HCC has made remarkable progress, there are still many difficulties worth studying and solving. Surgical treatment is still the preferred treatment for HCC. For HCC patients with MVI, the preoperative prediction of MVI may play a certain guiding role in planning procedures, and the comprehensive sequential postoperative pathological detection of HCC MVI may provide a basis for treatment decisions. The methods of combining molecular targeted therapies and immunotherapies in personalized diagnosis and treatment plans for patients while at the same time reducing the side effects of drug therapy will become be the focus of future HCC research.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- TACE:
-
Transarterial chemoembolization
- TKI:
-
Tyrosine-kinase inhibitors
- MVI:
-
Microvascular invasion
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- OS:
-
Overall survival
- RFS:
-
Relapse free survival
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. https://doi.org/10.3322/caac.21590 (2020).
Chen, W. et al. Cancer incidence and mortality in China, 2014. Chin. J. Cancer Res. 30, 1–12. https://doi.org/10.21147/j.issn.1000-9604.2018.01.01 (2018).
Mazzaferro, V. et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 10, 35–43. https://doi.org/10.1016/S1470-2045(08)70284-5 (2009).
Marasco, G. et al. Role of liver and spleen stiffness in predicting the recurrence of hepatocellular carcinoma after resection. J. Hepatol. 70, 440–448. https://doi.org/10.1016/j.jhep.2018.10.022 (2019).
Lauwers, G. Y. et al. Prognostic histologic indicators of curatively resected hepatocellular carcinomas: A multi-institutional analysis of 425 patients with definition of a histologic prognostic index. Am. J. Surg. Pathol. 26, 25–34. https://doi.org/10.1097/00000478-200201000-00003 (2002).
Lim, K. C. et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann. Surg. 254, 108–113. https://doi.org/10.1097/SLA.0b013e31821ad884 (2011).
Feng, L. H. et al. Novel microvascular invasion-based prognostic nomograms to predict survival outcomes in patients after R0 resection for hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 143, 293–303. https://doi.org/10.1007/s00432-016-2286-1 (2017).
Roayaie, S. et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 137, 850–855. https://doi.org/10.1053/j.gastro.2009.06.003 (2009).
Rodriguez-Peralvarez, M. et al. A systematic review of microvascular invasion in hepatocellular carcinoma: Diagnostic and prognostic variability. Ann. Surg. Oncol. 20, 325–339. https://doi.org/10.1245/s10434-012-2513-1 (2013).
Evidence-based practice guidelines for standardized pathological diagnosis of primary liver cancer in China: 2015. Zhonghua gan zang bing za zhi 23, 321–327 (2015).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: The next generation. Cell 144, 646–674. https://doi.org/10.1016/j.cell.2011.02.013 (2011).
Erstad, D. J. & Tanabe, K. K. Prognostic and therapeutic implications of microvascular invasion in hepatocellular carcinoma. Ann. Surg. Oncol. 26, 1474–1493. https://doi.org/10.1245/s10434-019-07227-9 (2019).
Kanai, T. et al. Pathology of small hepatocellular carcinoma. A proposal for a new gross classification. Cancer 60, 810–819 (1987). https://doi.org/10.1002/1097-0142(19870815)60:4<810::aid-cncr2820600417>3.0.co;2-1.
Hui, A. M. et al. Predictive value of gross classification of hepatocellular carcinoma on recurrence and survival after hepatectomy. J. Hepatol. 33, 975–979. https://doi.org/10.1016/s0168-8278(00)80131-2 (2000).
Tsujita, E. et al. The clinicopathological impact of gross classification on solitary small hepatocellular carcinoma. Hepatogastroenterology 60, 1726–1730 (2013).
He, J. et al. The clinicopathologic and prognostic significance of gross classification on solitary hepatocellular carcinoma after hepatectomy. Medicine 94, e1331. https://doi.org/10.1097/MD.0000000000001331 (2015).
Cho, E. S. & Choi, J. Y. MRI features of hepatocellular carcinoma related to biologic behavior. Korean J. Radiol. 16, 449–464. https://doi.org/10.3348/kjr.2015.16.3.449 (2015).
Esnaola, N. F. et al. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 6, 224–232 (2002). https://doi.org/10.1016/s1091-255x(01)00015-4.
Eguchi, S. et al. Predictor for histological microvascular invasion of hepatocellular carcinoma: A lesson from 229 consecutive cases of curative liver resection. World J. Surg. 34, 1034–1038. https://doi.org/10.1007/s00268-010-0424-5 (2010).
Zhou, L. et al. Edmondson-Steiner grade: A crucial predictor of recurrence and survival in hepatocellular carcinoma without microvascular invasio. Pathol. Res. Pract. 213, 824–830. https://doi.org/10.1016/j.prp.2017.03.002 (2017).
Kim, B. K. et al. Prediction of microvascular invasion before curative resection of hepatocellular carcinoma. J. Surg. Oncol. 97, 246–252. https://doi.org/10.1002/jso.20953 (2008).
Xu, Z. Y. et al. FOXC1 contributes to microvascular invasion in primary hepatocellular carcinoma via regulating epithelial-mesenchymal transition. Int. J. Biol. Sci. 8, 1130–1141. https://doi.org/10.7150/ijbs.4769 (2012).
Zhang, T. et al. KIAA0101 is a novel transcriptional target of FoxM1 and is involved in the regulation of hepatocellular carcinoma microvascular invasion by regulating epithelial-mesenchymal transition. J. Cancer 10, 3501–3516. https://doi.org/10.7150/jca.29490 (2019).
Lei, Z. et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg. 151, 356–363. https://doi.org/10.1001/jamasurg.2015.4257 (2016).
Yuen, M. F., Hou, J. L., Chutaputti, A. & Asia Pacific Working Party on Prevention of Hepatocellular, C. Hepatocellular carcinoma in the Asia pacific region. J. Gastroenterol. Hepatol. 24, 346–353 (2009). https://doi.org/10.1111/j.1440-1746.2009.05784.x.
Wu, C. Y. et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA 308, 1906–1914. https://doi.org/10.1001/2012.jama.11975 (2012).
Chen, L. et al. Viral and host inflammation-related factors that can predict the prognosis of hepatocellular carcinoma. Eur. J. Cancer 48, 1977–1987. https://doi.org/10.1016/j.ejca.2012.01.015 (2012).
Li, L., Li, B. & Zhang, M. HBV DNA levels impact the prognosis of hepatocellular carcinoma patients with microvascular invasion. Medicine 98, e16308. https://doi.org/10.1097/MD.0000000000016308 (2019).
Wei, X. et al. Hepatitis B virus infection and active replication promote the formation of vascular invasion in hepatocellular carcinoma. BMC Cancer 17, 304. https://doi.org/10.1186/s12885-017-3293-6 (2017).
Heldring, N. et al. Estrogen receptors: How do they signal and what are their targets. Physiol. Rev. 87, 905–931. https://doi.org/10.1152/physrev.00026.2006 (2007).
Sukocheva, O. A. Estrogen, estrogen receptors, and hepatocellular carcinoma: Are we there yet?. World J. Gastroenterol. 24, 1–4. https://doi.org/10.3748/wjg.v24.i1.1 (2018).
Iyer, J. K., Kalra, M., Kaul, A., Payton, M. E. & Kaul, R. Estrogen receptor expression in chronic hepatitis C and hepatocellular carcinoma pathogenesis. World J. Gastroenterol. 23, 6802–6816. https://doi.org/10.3748/wjg.v23.i37.6802 (2017).
Chou, C. T. et al. Prediction of microvascular invasion of hepatocellular carcinoma: Preoperative CT and histopathologic correlation. AJR Am. J. Roentgenol. 203, W253-259. https://doi.org/10.2214/AJR.13.10595 (2014).
Renzulli, M. et al. Can current preoperative imaging be used to detect microvascular invasion of hepatocellular carcinoma?. Radiology 279, 432–442. https://doi.org/10.1148/radiol.2015150998 (2016).
Golfieri, R., Garzillo, G., Ascanio, S. & Renzulli, M. Focal lesions in the cirrhotic liver: Their pivotal role in gadoxetic acid-enhanced MRI and recognition by the Western guidelines. Dig. Dis. 32, 696–704. https://doi.org/10.1159/000368002 (2014).
Ariizumi, S. et al. A non-smooth tumor margin in the hepatobiliary phase of gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging predicts microscopic portal vein invasion, intrahepatic metastasis, and early recurrence after hepatectomy in patients with hepatocellular carcinoma. J Hepato-Biliary-Pancreatic Sci. 18, 575–585. https://doi.org/10.1007/s00534-010-0369-y (2011).
Vasuri, F. et al. OATP 1B1/1B3 expression in hepatocellular carcinomas treated with orthotopic liver transplantation. Virchows Archiv. Int. J. Pathol. 459, 141–146. https://doi.org/10.1007/s00428-011-1099-5 (2011).
Hu, H. T. et al. Peritumoral tissue on preoperative imaging reveals microvascular invasion in hepatocellular carcinoma: A systematic review and meta-analysis. Abdominal Radiol. 43, 3324–3330. https://doi.org/10.1007/s00261-018-1646-5 (2018).
Ryu, T. et al. A clinical scoring system for predicting microvascular invasion in patients with hepatocellular carcinoma within the Milan criteria. J. Gastrointest. Surg. Offic. J. Soc. Surg. Aliment. Tract 23, 779–787. https://doi.org/10.1007/s11605-019-04134-y (2019).
Liu, Y. et al. Comparative analysis of anatomic and non-anatomic hepatectomy for single small hepatocellular carcinoma with microvascular invasion. Zhonghua yi xue za zhi 98, 1937–1940. https://doi.org/10.3760/cma.j.issn.0376-2491.2018.24.009 (2018).
Moris, D. et al. Anatomic versus non-anatomic resection for hepatocellular carcinoma: A systematic review and meta-analysis. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 44, 927–938. https://doi.org/10.1016/j.ejso.2018.04.018 (2018).
Shi, C. et al. Anatomic resection and wide resection margin play an important role in hepatectomy for hepatocellular carcinoma with peritumoural micrometastasis. ANZ J. Surg. 89, E482–E486. https://doi.org/10.1111/ans.15396 (2019).
Portolani, N. et al. Early and late recurrence after liver resection for hepatocellular carcinoma: Prognostic and therapeutic implications. Ann. Surg. 243, 229–235. https://doi.org/10.1097/01.sla.0000197706.21803.a1 (2006).
Zhou, Y., Xu, D., Wu, L. & Li, B. Meta-analysis of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Langenbeck’s Arch. Surg. 396, 1109–1117. https://doi.org/10.1007/s00423-011-0784-9 (2011).
Zhao, H. et al. Prognostic value of a novel risk classification of microvascular invasion in patients with hepatocellular carcinoma after resection. Oncotarget 8, 5474–5486. https://doi.org/10.18632/oncotarget.12547 (2017).
Yoshida, Y., Kanematsu, T., Matsumata, T., Takenaka, K. & Sugimachi, K. Surgical margin and recurrence after resection of hepatocellular carcinoma in patients with cirrhosis. Further evaluation of limited hepatic resection. Annals of surgery 209, 297–301 (1989). https://doi.org/10.1097/00000658-198903000-00008.
Lencioni, R., de Baere, T., Soulen, M. C., Rilling, W. S. & Geschwind, J. F. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology 64, 106–116. https://doi.org/10.1002/hep.28453 (2016).
Lo, C. M. et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 35, 1164–1171. https://doi.org/10.1053/jhep.2002.33156 (2002).
Llovet, J. M. & Bruix, J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 37, 429–442. https://doi.org/10.1053/jhep.2003.50047 (2003).
Lau, W. Y., Lai, E. C., Leung, T. W. & Yu, S. C. Adjuvant intra-arterial iodine-131-labeled lipiodol for resectable hepatocellular carcinoma: A prospective randomized trial-update on 5-year and 10-year survival. Ann. Surg. 247, 43–48. https://doi.org/10.1097/SLA.0b013e3181571047 (2008).
Wang, L., Ke, Q., Lin, N., Zeng, Y. & Liu, J. Does postoperative adjuvant transarterial chemoembolization benefit for all patients with hepatocellular carcinoma combined with microvascular invasion: A meta-analysis. Scand. J. Gastroenterol. 54, 528–537. https://doi.org/10.1080/00365521.2019.1610794 (2019).
Chen, Z. H. et al. Adjuvant transarterial chemoembolization improves survival outcomes in hepatocellular carcinoma with microvascular invasion: A systematic review and meta-analysis. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 45, 2188–2196. https://doi.org/10.1016/j.ejso.2019.06.031 (2019).
Wu, J. C. et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J. Hepatol. 51, 890–897. https://doi.org/10.1016/j.jhep.2009.07.009 (2009).
Otto, G. Pathologic response to preoperative transarterial chemoembolization for resectable hepatocellular carcinoma may not predict recurrence after liver resection. Hepatobiliary Pancreatic Dis. Int. HBPD INT 15, 122–124. https://doi.org/10.1016/s1499-3872(16)60071-1 (2016).
Jiang, J. H. et al. Adjuvant transarterial chemoembolization after curative resection of hepatocellular carcinoma: propensity score analysis. World journal of gastroenterology : WJG 21, 4627–4634. https://doi.org/10.3748/wjg.v21.i15.4627 (2015).
Li, J. Q., Zhang, Y. Q., Zhang, W. Z., Yuan, Y. F. & Li, G. H. Randomized study of chemoembolization as an adjuvant therapy for primary liver carcinoma after hepatectomy. J. Cancer Res. Clin. Oncol. 121, 364–366. https://doi.org/10.1007/bf01225689 (1995).
Zhong, C. et al. A randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for Stage III A hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 135, 1437–1445. https://doi.org/10.1007/s00432-009-0588-2 (2009).
Gao, Z. et al. Adjuvant transarterial chemoembolization after radical resection contributed to the outcomes of hepatocellular carcinoma patients with high-risk factors. Medicine 96, e7426. https://doi.org/10.1097/MD.0000000000007426 (2017).
Sun, J. J. et al. Postoperative adjuvant transcatheter arterial chemoembolization after R0 hepatectomy improves outcomes of patients who have hepatocellular carcinoma with microvascular invasion. Ann. Surg. Oncol. 23, 1344–1351. https://doi.org/10.1245/s10434-015-5008-z (2016).
Wei, W. et al. Adjuvant transcatheter arterial chemoembolization after curative resection for hepatocellular carcinoma patients with solitary tumor and microvascular invasion: A randomized clinical trial of efficacy and safety. Cancer Commun. 38, 61. https://doi.org/10.1186/s40880-018-0331-y (2018).
Shah, S. A. et al. Recurrence after liver resection for hepatocellular carcinoma: Risk factors, treatment, and outcomes. Surgery 141, 330–339. https://doi.org/10.1016/j.surg.2006.06.028 (2007).
Yamanaka, N. et al. Prognostic factors after hepatectomy for hepatocellular carcinomas A univariate and multivariate analysis. Cancer 65, 1104–1110. https://doi.org/10.1002/1097-0142(19900301)65:5%3c1104::aid-cncr2820650511%3e3.0.co;2-g (1990).
Wang, H., Du, P. C., Wu, M. C. & Cong, W. M. Postoperative adjuvant transarterial chemoembolization for multinodular hepatocellular carcinoma within the Barcelona Clinic Liver Cancer early stage and microvascular invasion. Hepatobiliary surgery and nutrition 7, 418–428. https://doi.org/10.21037/hbsn.2018.09.05 (2018).
Liao, M., Zhu, Z., Wang, H. & Huang, J. Adjuvant transarterial chemoembolization for patients after curative resection of hepatocellular carcinoma: A meta-analysis. Scand. J. Gastroenterol. 52, 624–634. https://doi.org/10.1080/00365521.2017.1292365 (2017).
Huang, Y. et al. Should we apply sorafenib in hepatocellular carcinoma patients with microvascular invasion after curative hepatectomy?. OncoTargets and therapy 12, 541–548. https://doi.org/10.2147/OTT.S187357 (2019).
Liu, L. et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Can. Res. 66, 11851–11858. https://doi.org/10.1158/0008-5472.CAN-06-1377 (2006).
Llovet, J. M. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390. https://doi.org/10.1056/NEJMoa0708857 (2008).
Cheng, A. L. et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 10, 25–34. https://doi.org/10.1016/S1470-2045(08)70285-7 (2009).
Kudo, M. et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 391, 1163–1173. https://doi.org/10.1016/S0140-6736(18)30207-1 (2018).
Bruix, J. et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389, 56–66. https://doi.org/10.1016/S0140-6736(16)32453-9 (2017).
El-Khoueiry, A. B. et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389, 2492–2502. https://doi.org/10.1016/S0140-6736(17)31046-2 (2017).
Zhu, A. X. et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 19, 940–952. https://doi.org/10.1016/S1470-2045(18)30351-6 (2018).
Feng, X. et al. Combination therapy with sorafenib and radiofrequency ablation for BCLC Stage 0–B1 hepatocellular carcinoma: A multicenter retrospective cohort study. Am. J. Gastroenterol. 109, 1891–1899. https://doi.org/10.1038/ajg.2014.343 (2014).
Yao, Q., Zhang, H., Xiong, B. & Zheng, C. Combination of sorafenib and TACE inhibits portal vein invasion for intermediate stage HCC: A single center retrospective controlled study. Oncotarget 8, 79012–79022. https://doi.org/10.18632/oncotarget.20745 (2017).
Zhang, X. P. et al. Postoperative adjuvant sorafenib improves survival outcomes in hepatocellular carcinoma patients with microvascular invasion after R0 liver resection: A propensity score matching analysis. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 21, 1687–1696. https://doi.org/10.1016/j.hpb.2019.04.014 (2019).
Peng, Z. et al. Microvascular invasion as a predictor of response to treatment with sorafenib and transarterial chemoembolization for recurrent intermediate-stage hepatocellular carcinoma. Radiology 292, 237–247. https://doi.org/10.1148/radiol.2019181818 (2019).
Xu, L. et al. The circular RNA ciRS-7 (Cdr1as) acts as a risk factor of hepatic microvascular invasion in hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 143, 17–27. https://doi.org/10.1007/s00432-016-2256-7 (2017).
Pote, N. et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J. Hepatol. 62, 848–854. https://doi.org/10.1016/j.jhep.2014.11.005 (2015).
Shindoh, J. et al. Microvascular invasion does not predict long-term survival in hepatocellular carcinoma up to 2 cm: Reappraisal of the staging system for solitary tumors. Ann. Surg. Oncol. 20, 1223–1229. https://doi.org/10.1245/s10434-012-2739-y (2013).
Shen, J. et al. The prognostic value of microvascular invasion in early-intermediate stage hepatocelluar carcinoma: A propensity score matching analysis. BMC Cancer 18, 278. https://doi.org/10.1186/s12885-018-4196-x (2018).
Wang, H., Qian, Y. W., Wu, M. C. & Cong, W. M. Liver resection is justified in patients with BCLC intermediate stage hepatocellular carcinoma without microvascular invasion. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract https://doi.org/10.1007/s11605-019-04251-8 (2019).
Funding
This work was supported by grant from National Nature Science Foundation of China (Nos. 81802414 and 81802458) and Shandong Provincial Key Research and Development Program (No. 2019GSF108053).
Author information
Authors and Affiliations
Contributions
W.W. and Y.G. contributed equally to this work; W.W., Y.G. and J.Z. drafting of the manuscript; Q.W., X.W. and H.W. retrieved the relevant literatures; J.L. and P.X. designed the structure of this article and reviewed the final manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, W., Guo, Y., Zhong, J. et al. The clinical significance of microvascular invasion in the surgical planning and postoperative sequential treatment in hepatocellular carcinoma. Sci Rep 11, 2415 (2021). https://doi.org/10.1038/s41598-021-82058-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82058-x
This article is cited by
-
Understanding the role of radiologists in complex treatment decisions for patients with hepatocellular carcinoma
Abdominal Radiology (2023)
-
The role of shear wave elastography in differentiation between benign and malignant portal vein thrombosis in hepatocellular carcinoma
Egyptian Journal of Radiology and Nuclear Medicine (2022)
-
Contrast-enhanced CT findings-based model to predict MVI in patients with hepatocellular carcinoma
BMC Gastroenterology (2022)
-
MRI-guided radiotherapy for PVTT in HCC patients: evaluation of the efficacy and safety
Journal of Cancer Research and Clinical Oncology (2022)
-
Preoperative application of systemic inflammatory biomarkers combined with MR imaging features in predicting microvascular invasion of hepatocellular carcinoma
Abdominal Radiology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.