Abstract
Sedentary life styles coupled with high-calorie diets and unhealthy social habits such as smoking, have put an ever-increasing number of people at risk of cardiovascular disorders (CVD), worldwide. A concomitant increase in the prevalence of type 2-diabetes (hyperglycemia), a risk factor for CVD, has further contributed towards escalating CVD-related mortalities. The increase in number of cases of type 2-diabetes underscores the importance of early diagnosis of cardiovascular disease in those with diabetes. In this work, we have evaluated the sensitivity and specificity of dyslipidemia and proinflammatory cytokines to be used as biomarkers for predicting the risk of CVD in those with diabetes. We hypothesize that interplay between dyslipidemia and diabetes-induced low-grade inflammation in those with type 2-diabetes increases the risk of CVD. A total of 215 participants were randomly recruited from the Cameron County Hispanic Cohort (CCHC). Of these, 99% were Mexican Americans living on Texas-Mexico border. Levels of cytokines, adipokines and lipid profile were measured. Cardiovascular disease (CVD) for this study was defined as prior diagnosis of heart attack, angina and stroke, while diabetes was defined by fasting blood glucose (FBG) of > 100 mg/dL and HbA1c of > 6.5, in accordance with American Diabetes Association (ADA) guidelines. Depending on type and distribution of data, various statistical tests were performed. Our results demonstrated higher rates of heart attack (14% vs 11.8%) and stroke (19.8% vs 10%) in those with diabetes as compared to non-diabetes. The odds of having a heart attack were eight times higher in the presence of elevated triglycerides and pro-inflammatory markers (TNFα and IL6) as compared to presence of pro-inflammatory markers only. The odds for heart attack among those with diabetes, increased by 20 fold in presence of high levels of triglycerides, TNFα, and IL6 when coupled with low levels of high-density lipid cholesterol (HDL-C). Lastly, our analysis showed that poorly controlled diabetes, characterized by HbA1c values of > 6.5 increases the odds of stroke by more than three fold. The study quantifies the role of lipid profile and pro-inflammatory markers in combination with standard risk factors towards predicting the risk of CVD in those with type 2-diabetes. The findings from the study can be directly translated for use in early diagnosis of heart disease and guiding interventions leading to a reduction in CVD-associated mortality in those with type 2-diabetes.
Similar content being viewed by others
Background
Sedentary and unhealthy lifestyles have put an ever-increasing number of people at the risk of developing cardiovascular disorders (CVD)1, which is indicated by an alarming rise in incidence of heart failure, cardiomyopathy, coronary heart disease, and stroke2. American National Health and Nutrition Examination Survey (NHANES) shows that incidence of heart failure (HF) increased by about 9% (from 5.7 million to 6.2 million) in adults ≥ 20 years of age (2.2% of population) from 2013–2016 to 2009–20123. The Global Burden of Disease Study reports that approximately 90% of stroke incidents can be attributed to modifiable risk factors emerging from sedentary and unhealthy life styles. Specifically, smoking, high body mass index (BMI), sub-optimal physical activity coupled with high calorie diets, high cholesterol, elevated blood pressure (BP), diabetes (hyperglycemia) and renal dysfunction have been reported as key determinants of cardio vascular disease (CVD)3,4. Furthermore, the surge in prevalence of type 2-diabetes (hereafter referred to as diabetes) has significantly contributed to escalation in CVD-related mortalities. Hence, diabetes with uncontrolled fasting glucose ≥ 6.5 mmol/L1,5 has also emerged as an important independent risk factor for cardiovascular disease (CVD)6,7,8,9. In 2007, citing the axis between diabetes and CVD, American Heart Association (AHA)and American Diabetes Association (ADA) issued a combined set of recommendations focusing on prevention of CVD in those with diabetes10.
Diabetes was identified as a major risk factor for CVD after conducting a 20 year surveillance study on Framingham cohort aimed at identifying risk factors for cardiovascular disease11. Krauss et al. evaluated the association of type 2 diabetes with lipid and lipoprotein abnormalities12 while Athyros et al. highlighted the high prevalence of dyslipidemia (DD)13 amongst the patients with type 2 diabetes. Cannon et al. reported that metabolic syndrome superimposed on type 2 diabetes augments the risk of CVD14 while Cao et al. stressed upon the increased risk of CVD as a result of elevated non-HDL-C levels amongst type 2 diabetes population15. Recently, Pagidipati et al.further reported hypertriglyceridemia’s involvement in giving rise to CVD related mortalities in individuals with diabetes16,17,18,19. In concert, numerous research reports have classified lipid profile and blood pressure amongst major risk-factors that exhibit a stronger association with CVD than poorly controlled glucose in those with diabetes20. Besides the lipid profile, Tuttle et al.have shown that increased levels of the TNFα and IL6 in diabetes is also associated with elevated morbidity and mortality rates in CVD21. Inflammatory markers such as IL6, TNFα, C-reactive protein (CRP) and IL8-β are well-established mediators in the induction of cardiovascular diseases22,23,24,25,26,27,28. Ridker et al. demonstrated that C-reactive protein (CRP) (an inflammatory marker in the blood) and lipid profile (total cholesterol to HDLC) increased the relative risk of cardiovascular events in women29. Epidemiological studies have also reported associations between inflammatory markers and type 2 diabetes through elevated plasma concentration of inflammatory mediators, such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6)30,31,32. Summarily, meta-analysis of literature does not demonstrate that diabetes is a CVD risk equivalent33,34 and that there is a lack of consensus on which individual risk factors35,36 or their combinations37,38 are the most crucial in giving rise to CVD amongst those with a history of type 2 diabetes. In particular, the interplay of type 2 diabetes-induced low-grade inflammation with lipid abnormalities in the risk of CVD, remains poorly understood.
To address this gap, we aim to estimate the association of lipid profiles and inflammatory markers with the risk of developing CVD, in Mexican Americans. Next, we investigate the combinatorial utility of these modifiable risk factors in predicting the risk of CVD in those with type 2-diabetes, by clustering lipid profiles and inflammatory markers. Lastly, we validate the effect of superimposition of diabetes-induced inflammatory cytokines on lipid profile on increasing the odds of developing CVD. Our results show that lipid profile and inflammatory cytokines are predictive biomarkers of cardiovascular disease especially in those with diabetes, which can be employed in clinical settings towards early diagnosis of heart disease. We further report that the odds of having heart attack in presence of elevated triglycerides and pro-inflammatory parameters (TNFα and IL6) were approximately eight times higher as compared to heart attack in individuals with only elevated levels of pro-inflammatory markers. However, the odds of having a heart attack in participants with diabetes increased by 20 fold in presence of high levels of triglycerides, TNFα and IL6 if coupled with low levels of HDL-C. Lastly, for stroke, our analysis showed that uncontrolled diabetes increases its odds by over three times.
Methods
Study participants and demographics
We measured level of cytokines and adipokines in 215 participants from the Cameron County Hispanic Cohort (CCHC). The Cameron County Hispanic Cohort (CCHC) was formed in 2004 and comprised of Mexican Americans inhabiting the United States-Mexico border39,40,41,42. Previous studies performed on the border communities have indicated that Latinos have 1.4 times higher risk of diabetes than those reported nationally for Mexican Americans43. Therefore, CCHC randomly recruited 2000 residents from City of Brownsville for onward investigations. Since earlier studies on CCHC reported higher levels of inflammatory markers (TNF-alpha and IL6) amongst Mexican Americans participants with diabetes32, the current study was designed to further investigate the crosstalk between inflammation, diabetes and the risk of CVD.
The American Diabetes Association goal for glycemic control of HbA1c < 6.5% in the Standards of Medical Care in Diabetes-American Diabetes Association, Diabetes Care 2010, Supplement 1-S111 was used to identify diabetes. Moreover, each participant had a clinical diagnosis of diabetes or impaired fasting blood glucose for at least 2 years prior to the start of the study while some of them were also on medication for diabetes. None of the participants had any hemoglobinopathies nor had undergone blood transfusion. The selected participants were selected on the following criteria, (i) diabetes no cardiovascular disease, (ii) diabetes with cardiovascular disease, (iii) no diabetes and no cardiovascular disease. The study was approved by the Institutional Review Board of University of Texas, Health Science Center, School of Public Health and all methods were performed in accordance with the relevant guidelines and regulations. The study was explained to participants in both English and Spanish and informed consent was obtained. After signing of the consent form, participants were requested for 10mls of blood for onward cytokine analysis as well as for quantitation of metabolic markers. Demographic and anthropometric data were collected using a questionnaire.
Study population and selection criteria
Self-report of a previous clinical diagnosis or a random blood glucose test were used to determine the diabetes status of participants in the study. The criteria defined by the American Diabetes Association (ADA) in their “standards of medical care in diabetes—2010”1 was used to classify diabetes. Specifically, participants were said to have type 2-diabetes if they fulfilled any one of the following criteria; Fasting blood glucose (FBG) > 100 mg/dl and HbA1c > 6.5; a doctor’s diagnosis of diabetes and/or on medication for diabetes44. Absence of diabetes was defined as no history of diabetes and FBG < 100 mg/dl. Diabetes control over three month period was measured by using glycated hemoglobin (HbA1c), where a value of < 6.5 was considered well-controlled diabetes, whereas HbA1c > 6.5 indicated poor glucose control44. Cardiovascular disease was also self-reported based on history of congestive heart failure, coronary heart disease, angina, myocardial infarction or hypertension. Three sub-cohorts were henceforth defined with (i) participants having type 2-diabetes with any of the following cardiovascular issues: hypertension, angina, heart attack, and high cholesterol, (ii) cardiovascular disease but no diabetes, and (iii) no cardiovascular disease and no diabetes.
Measurement of analytes in serum samples
Blood was kept at room temperature until it was transported to the laboratory at the School of Public Health University of Texas, Brownsville Campus. Blood was allowed to clot followed by centrifugation and separation of serum. Serum was divided in 500 µl aliquots and kept frozen at − 80 °C. Levels of cytokines and adipokines, were measured using a multiplex ELISA technology (Milliplex Map, Millipore CA), followed by reading of results in Luminex 200 system (Luminex corp. Austin TX). Measurement of cytokines and adipokines was made using two panels, wherein panel-A comprised of adiponectin and resistin, panel-B contained probes for pro-inflammatory cytokines IL-6, TNF-alpha, IL-1beta, and leptin. Plasma samples stored at − 80 °C were thawed on ice, diluted as per manufacturer’s instructions, and incubated with beads coated with antibodies for corresponding analytes. Reaction was read in the Luminex 200 system.
Statistical analysis
International Business Machine (IBM)—Statistical Package for Social Sciences (SPSS) version 2345 and Stata/MP version 13 were used for data analysis46. Data was evaluated for normality using Kolmogorov–Smirnov47 and Shapiro–Wilk tests48. In accordance with the distribution of independent variables, non-parametric methods including Mann Whitney U test and parametric tests including independent two-sample t-test were employed for analysis of data. Anthropometric measures, lipid profile, adipokines and inflammatory markers were compared by diabetes status, (HbA1c) status, CVD (heart attack, stroke and angina) status by stratification of the cohort using Mann Whitney U test49. Prevalence of CVD was assessed with 95% confidence intervals (CI) amongst those with and without diabetes. Relative risk, after adjusting for age and BMI, was computed for CVD in those with diabetes. Chi-square50 and Cochran–Mantel–Haenszel51 tests were used to calculate odds of CVD as well as the association of lipid profile and inflammatory markers with CVD in those with and without diabetes. Multilayer Perceptron neural network52 was trained to test for CVD risk factors. Analyses were declared significant for P-value < 0.05.
Results
Evaluation of baseline anthropometric and metabolic characteristics in those with and without diabetes
Participants with diabetes mellitus (DM) and without diabetes (nDM) were evaluated for anthropometric and metabolic characteristics (Table 1). From amongst the cohort, a sample of 91 participants were self-reported to have had one or more of the following; angina, heart attack or stroke which were collectively termed as “cardiovascular diseases”. Out of 215 participants, 44.7% had diabetes of which 66.7% were females and 33.3% were males while for those without diabetes (55.4% of 215 participants), 58% were female and 42% were male. The ratio between genders in those with and without diabetes was found to be comparable by comparison of frequency of valid (non-missing) observations in both DM and nDM groups. Next, we compared the DM and nDM groups across 14 different factors. These included age, body mass index (BMI), mean fasting blood glucose (FBG), glycated hemoglobin (GHB), triglycerides, high-density lipoprotein (HDL), leptin, systolic and diastolic blood pressure, receptor for advanced glycation end (RAGE), C-reactive protein (CRP), cholesterol, resistin and adiponectin. Non-parametric Mann–Whitney U test reported no significant difference between both genders amongst those with and without diabetes (P < 0.05). Age and BMI were significantly higher in diabetes as compared to non-diabetes (P = 0.0014, and P = 0.0007), respectively. A significant increase was observed in levels of HbA1c (5.5 vs. 7.4%), mean FBG (94 vs. 127.5 mg/dl), triglycerides (169.5 vs. 152 mg/dl, P = 0.0007), and systolic blood pressure (125.5 vs. 119 mm/Hg, P = 0.0003) in diabetes. However, a low HDL-C (45 vs. 48 mg/dl P = 0.01) was observed in DM as compared to nDM. Further, no significant difference was observed in adipokines (including leptin, adiponectin and resistin), in participants with and without diabetes.
Measurement of pro and anti-inflammatory markers by diabetes status in absence of CVD
To evaluate the inflammatory response in diabetes, we compared cytokine expressions between those with and without diabetes using Mann–Whitney U test. The cytokines evaluated included both pro-inflammatory (IL-6, TNFα, and IL1β) and anti-inflammatory (IL8) markers. Our results show that DM in comparison to nDM, exhibits a significant increase in levels of pro-inflammatory IL-6 (4.1 vs 2.45, P = 0.007) and TNFα (5.385 vs 5.03, P = 0.0013) (Table 1). To understand the role of glucose control on inflammation we compared levels of cytokines among participants with HbA1c < 6.5% (controlled) and > 6.5 (poorly controlled). Non-parametric Mann–Whitney U test was used for these comparisons, however, no significant differences were found within cytokines and adipokines (data not shown).
Comparison of baseline anthropometric, inflammatory and metabolic characteristics by diabetes status in absence of cardiovascular disorders
To further analyze baseline parameters in participants without any cardiovascular disease (CVD), we stratified the cohort by CVD status (n = 123). The Mann–Whitney U test was employed and results are shown in Table 2. The results obtained were consistent with our previous findings (Table 1) except for HDL-C and triglycerides, which were not significant in those with diabetes and no CVD. This analysis helped identify factors that might be contributing significantly towards cardiovascular in the presence of diabetes.
Evaluation of baseline anthropometric and metabolic characteristics in participants with cardiovascular diseases with and without diabetes
Next, we set out to analyze the full subset (n = 215) for cardiovascular diseases regardless of diabetes status. Specifically, anthropometric, metabolic characteristics and pro-inflammatory characteristics were evaluated by heart attack and stroke status. Mann Whitney U test was performed and our results (Table 3-A) indicate that participants with a history of a heart attack showed a significant increase in the levels of triglycerides (226.5 mg/dL) as compared to patients without any history of heart attack (138 mg/dL). Glycated hemoglobin (GHB) showed a significant increase from 5.8% to 7% in individuals with history of stroke as compared to those who never had a stroke (Table 3-B).
Prevalence estimates of CVD and its risk assessment in those with diabetes
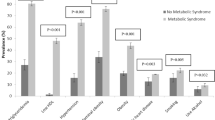
Estimation of prevalence of CVD (heart attack, stroke and angina) shows an increased rate of heart attack (14% vs 11.8%) and stroke (19.8% vs 10%) in DM as compared to nDM (Table 4). Next, to evaluate whether diabetes is a risk factor for developing CVD, we performed relative risk (RR) analysis. These RR were adjusted for Age and BMI with 95% confidence interval (CI) (Fig. 1). Our results indicate an increased relative risk of developing CVD in participants with diabetes for heart attack (RR: 1.22, 95% CI: [0.616, 2.45]), stroke (RR: 1.96, 95% CI: [1.00, 3.83]), and angina (RR: 1.09, 95% CI: [0.65–1.80]). The adjusted relative risk (ARR) were computed to be 1.15 with 95% CI: [0.547, 2.44], 2.02 with 95% CI: [0.98, 4.14], and 1.23 with 95% CI: [0.70, 2.16], respectively. However these results were not statistically significant (p-value > 0.05). These results are consistent with literature reports that diabetes increases the risk of developing CVD53. This also aligns with our aforementioned finding that reported significantly higher levels of GHB in individuals who had suffered stroke thus indicating diabetes as a risk factor of CVD (Table 3).
Association between heart attack, triglycerides, TNFa, IL6 and HDL-C in those with diabetes
To identify the key determinants of heart attack amongst those with diabetes, we segregated our subset on the basis of heart attack status in diabetes (n = 96). We evaluated each parameter using Mann–Whitney U test and identified a significant association between triglycerides and heart attack amongst those with diabetes (P = 0.02*). Next, we set out to determine the underlying association between heart attack and triglycerides while controlling for TNFα, IL6 and HDL-C. For that, we converted triglycerides, TNFα, IL6 and HDL-C data to dichotomous categories, high and low. The thresholds for “Low” category were set at triglycerides < 200 mg/dL, TNFα ≤ 8.7 pg/mL, IL6 < 5.0 pg/ml, and HDL-C < 40 mg/dL while triglycerides > 200 mg/dL, TNFα > 8.7 pg/mL, IL6 > 5.0 pg/ml and HDL-C > 40 mg/dL were classified as “High”54,55,56,57. Chi-square statistic and Odds ratio were calculated using Cochran–Mantel–Haenszel test and results are shown in Table 5. Our results indicate that participants with diabetes and elevated levels of triglycerides were 12.68 times more likely to have a heart attack. Further, the odds of heart attack increased up to 15 times for participants with elevated levels of TNFα in presence of high triglyceride expression. Taken together, our results show that the odds of having a history of heart attack in presence of elevated triglycerides and pro-inflammatory parameters (TNFα and IL6) are approximately eight fold higher as compared to heart attack with elevation only in the levels of TNFα and IL6 (Table 5).
In order to validate these findings, we further used TNFα, IL6, and triglycerides to train a neural network for predicting heart attack. The classification table of test and training datasets for the neural network is provided in Table 6. The results indicate that from among the metabolic markers that were used in this study, triglycerides levels are the most important metabolic marker for predicting heart attack (Fig. 2-A). The ROC was constructed and area under the curve was estimated at 0.803 (Fig. 2-B).
Normalized importance for three independent variables and ROC curve for dependent variable. (A) Showing the importance of each independent variable in predicting the occurrence of heart attack hence proving to be important risk factors in DM. (B) Shows the ROC curve for the predictive model of heart attack for the neural analysis. Area under the curve (AUC) of 0.803 is indicative of the accuracy of sensitivity analysis.
Next, we assessed the association between high levels of triglycerides, low levels of HDL-C and heart attack in those with diabetes in presence of pro-inflammatory markers (IL6, TNFα). Cochran Mantel–Haenszel test was employed to the effect. Our results shows that odds of heart attack increase by 20 fold if diabetes is associated with high levels of triglycerides, TNFα and IL6 in addition with low levels of HDL-C, (Table 5).
We validated these findings by training a neural network of four factors TNFα, IL6, and triglycerides to predict heart attack. The classification table of test and training datasets for the neural network is provided in Table 7. The results again indicated that triglycerides levels are the most important metabolic marker for predicting heart attack (Fig. 3-A). The ROC was constructed and area under the curve was estimated at 0.817 (Fig. 3-B).
Normalized importance for four independent variables and ROC curve for dependent variable. (A) Showing the importance of each independent variable in predicting the occurrence of heart attack hence proving to be important risk factors among DM. (B) Shows the ROC curve for the predictive model of heart attack for the neural analysis. Area under the curve (AUC) of 0.817 is indicative of the accuracy of sensitivity analysis of a good model.
Association between poorly controlled diabetes and stroke in those with diabetes
Our results revealed that GHB levels were significantly elevated in participants with a history of stroke (Table 3-B). To evaluate this association between poorly controlled diabetes and stroke, we computed odds ratio. The results show that uncontrolled diabetes increases the odds of stroke by over 3 times (Table 8). Further, to assess this relationship in presence of high levels of TNFα and IL6, we applied Cochran Mantel–Haenszel test. Consistent odds were observed for stroke while controlling for different combinations of uncontrolled diabetes, TNFα and IL6 (Table 8).
Discussion
In this study, we have investigated the association between lipid profiles and inflammatory markers in Mexican-Americans with diabetes towards estimating the odds of having CVD. Although hyperlipidemia can be classified as major risk factor for CVD but different ethnicities can exhibit disparities in its prevalence. According to previous cross-sectional and retrospective studies conducted on Asian Americans, Mexican Americans and Blacks, distinct patterns of dyslipidemia have been reported58,59. Hence, risk factors in addition to ethnic/racial minority-specific single nucleotide polymorphism (SNPs) can help in better understanding and prevention of CVD risk and associated mortality60. Our results from the current study show that diabetes is a major contributor to inflammatory changes in Mexican-Americans, which in addition to the dyslipidemia can culminate in CVD outcomes.
Pro-inflammatory cytokine profile is associated with diabetes
Insulin resistance underlying type-2 diabetes (T2DM) superimposed on obesity is known to elevate plasma levels of inflammatory markers such as IL-6, TNFα. Two different underlying mechanisms have been proposed for this inflammatory response. Firstly, high glucose intake and over-nutrition can lead to oxidative stress besides inducing an imbalanced inflammatory response30. Secondly, higher levels of inflammatory mediators (IL-6 and TNFα) lead to disruption of insulin action due to suppression of insulin transduction61. The results from our study are consistent with these literature reports as a significant elevation was observed in inflammatory markers amongst the DM (Table 1). Further, data from the European nested case–control prospective study showed no change in IL-1β between the DM and non-disease participants besides higher levels of IL-6 and TNFα62. This finding is also in agreement with our pro-inflammatory profile analysis (Table 1).
Relative risk of CVD amongst DM
Amongst all the risk factors associated with CVD, diabetes contributes significantly towards CVD related mortalities63. A prospective study from the Framingham cohort also concluded higher incidence of CVD in men and women with diabetes8. Our results exhibit a similar trend with a higher prevalence of heart attack and stroke in DM as compared to nDM (Fig. 1).
Uncontrolled glucose and CVD
Diabetes leads to changes at micro- and macro-vascular levels that culminate into a variety of clinical complications. Specifically, diabetes increases the susceptibility of an individual to cerebral small and large vessel diseases64,65. Thus, increased glucose levels (hyperglycemia) lead to elevated risk for outcomes such as ischemic stroke etc. Our study revealed that the relative risk for stroke was highest amongst individuals with diabetes after adjusting for age and BMI (Fig. 1). In an earlier multiethnic prospective study, the relationship between fasting blood glucose and ischemic stroke was evaluated along with the importance of how strict glucose control can prevent stroke66. These findings are coherent with our study, where a significant increase in HbA1c was observed amongst participants with a history of stroke (Table 3B). Moreover, the odds of stroke were observed to increase by up to 3 times in patients with poorly controlled diabetes (Table 8) further supporting the association between stroke and poorly controlled diabetes.
Triglycerides and CVD
Elevated levels of triglyceride are known to cause impairment in lipoprotein metabolism which leads to an increase in the risk of CVD67,68. Our statistical analysis shows that triglyceride levels are significantly higher in DM thus linking them closely to T2DM (Table 1). High levels of triglycerides are considered as an epiphenomenon of the metabolic syndrome and insulin resistance68,69, which can help identify CVD susceptible candidates with low levels of HDL-C and small LDL particles70. Furthermore, analysis performed by National Health and Nutrition Examination Survey (NHANES) showed strongest association of triglycerides with risk of CVD in comparison to other components of metabolic syndrome71. Here, we showed that participants with a history of heart attack patients exhibit a significant increase in triglyceride expressions (Table 3-A). Further, our risk analysis shows that the odds of heart attack increases by up to 5.7 fold in DM as compared nDM (Table 5).
HDL-C and CVD
Benitez et al. reported the relationship between HDL-C and CVD all-cause mortality72. Prospective and epidemiological investigations also show an inverse association between HDL-C and CVD with the risk of coronary heart disease (CHD) lowered by 2–3 percent for every 1 mg/dl increase in HDL-C73. In the current study, this association between high levels of triglycerides, while controlling for the low levels of HDL-C, showed that the odds of heart attack increase by 7.36 times in DM as compared to nDM (Table 5).
Association of lipid profile and inflammatory markers with CVD
Elevated triglycerides and lowered HDL-C levels in serum are common metabolic abnormalities associated with insulin resistance68,69. Hence, hypertriglyceridemia can act as an indicator of the metabolic syndrome and type-2 DM. Further, the ratio of triglyceride levels to HDL-C can be employed as a clinical marker for insulin resistance which can then be a surrogate for measuring the impact of diabetes on the CVD incidence70. Importantly, previous prospective studies on triglycerides have also indicated a strong association between CVD risk and lowered levels of HDL-C, LDL-C in individuals with T2DM71. The current study evaluates different combinations of lipid profile (high triglycerides and low HDL-C levels) and increased levels of the hyperglycemia-induced inflammatory mediators in increasing the odds of heart attack (Table 5). Moreover, the proposed neural network model of these major risk factors can assist in accurately predicting heart attacks in DM (Figs. 2 and 3).
Ethics approval and consent to participate
All randomly recruited participants were informed and provided a written consent. Institutional review board (IRB) approval was obtained before conducting the study by The Committee for the Protection of Human Subjects Office of Research Support Committees, UT-Houston—SPH—Brownsville Regional Camp/RAHC (Reference No. HSC-SPH-09-0276).
Consent for publication
Consent was provided by all authors.
Conclusion
This study concludes that diabetes is associated with low-grade inflammation and that raised levels of triglycerides and low levels of HDL-C in DM increases the risk of cardiovascular disease (CVD) outcomes. Prevalence analysis of CVD in diabetes mellitus (DM) in comparison to nDM is indicative of DM as a major risk factor in CVD. Moreover, elevation of the inflammatory markers resulting from DM with concomitant increase in the lipid profile amongst DM contributes to heart attack.
Data availability
The datasets used and/or analyzed during the current study are available from the authors on reasonable request.
Change history
05 July 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41598-021-93445-9
Abbreviations
- CVD:
-
Cardiovascular disorders
- CCHC:
-
Cameron County Hispanic Cohort
- FBG:
-
Fasting blood glucose
- ADA:
-
American Diabetes Association
- TNFα:
-
Tumor necrosis factor alpha
- IL6:
-
Interleukin-6
- HDL-C:
-
High density lipid cholesterol
- LMIC:
-
Low and middle income countries
- WHO:
-
World health organization
- NHANES:
-
American National Health and Nutrition Examination Survey
- HF:
-
Heart failure
- AHA:
-
American Heart Association
- JNC:
-
Seventh Joint National Committee
- BMI:
-
Body mass index
- CRP:
-
C-reactive protein
- RAGE:
-
Receptor for advanced glycation end
- IBM:
-
International Business Machine
- DM:
-
Diabetes
- nDM:
-
Without diabetes
- RR:
-
Relative risk
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- T2DM:
-
Type-2 diabetes
References
Association, A. D. Standards of medical care in diabetes-2010. Diabetes Care 33, S11 (2010).
Kilkenny, M. F. et al. Knowledge of risk factors for diabetes or cardiovascular disease (CVD) is poor among individuals with risk factors for CVD. PLoS ONE 12, e0172941 (2017).
Benjamin, E. J. et al. Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association. Circulation 139, (2019).
Tun, N. N., Arunagirinathan, G., Munshi, S. K. & Pappachan, J. M. Diabetes mellitus and stroke: a clinical update. World J. Diabetes 8, 235–248 (2017).
The Emerging Risk Factors Collaboration et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375, 2215–2222 (2010).
Raghavan, S. et al. Diabetes mellitus-related all-cause and cardiovascular mortality in a national cohort of adults. J. Am. Heart Assoc. 8, e011295 (2019).
Tuomilehto, J., Rastenytė, D., Jousilahti, P., Sarti, C. & Vartiainen, E. Diabetes mellitus as a risk factor for death from stroke. Stroke 27, 210–215 (1996).
Kannel, W. B. & McGee, D. L. Diabetes and glucose tolerance as risk factors for cardiovascular disease: the framingham study. Diabetes Care 2, 120–126 (1979).
Juutilainen, A., Lehto, S., Rönnemaa, T., Pyörälä, K. & Laakso, M. Type 2 diabetes as a coronary heart disease equivalent: an 18-year prospective population-based study in Finnish subjects. Diabetes Care 28, 2901–2907 (2005).
Fox, C. S. et al. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 38, 1777–1803 (2015).
Hajar, R. Risk factors for coronary artery disease: historical perspectives. Heart Views 18, 109–114 (2017).
Krauss, R. M. Lipids and lipoproteins in patients with type 2 diabetes. Diabetes Care 27, 1496–1504 (2004).
Athyros, V. G. et al. Diabetes and lipid metabolism. Hormones 17, 61–67 (2018).
Cannon, C. P. Mixed dyslipidemia, metabolic syndrome, diabetes mellitus, and cardiovascular disease: clinical implications. Am. J. Cardiol. 102, 5L-9L (2008).
Cao, Y. et al. Non-high-density lipoprotein cholesterol and risk of cardiovascular disease in the general population and patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res. Clin. Pract. 147, 1–8 (2019).
Pagidipati, N. et al. 1472-P: association between triglycerides and residual cardiovascular (CVD) risk in patients with type 2 diabetes and established CVD: an analysis of the BARI2D trial. Diabetes 68, 1472P (2019).
Ye, X., Kong, W., Zafar, M. I. & Chen, L.-L. Serum triglycerides as a risk factor for cardiovascular diseases in type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Cardiovasc. Diabetol. 18, 48 (2019).
Sone, H. et al. Comparison of various lipid variables as predictors of coronary heart disease in Japanese men and women with type 2 diabetes: subanalysis of the Japan Diabetes Complications Study. Diabetes Care 35, 1150–1157 (2012).
Liu, J. et al. Effects of blood triglycerides on cardiovascular and all-cause mortality: a systematic review and meta-analysis of 61 prospective studies. Lipids Health Dis. 12, 159 (2013).
Henry, R. R. Preventing cardiovascular complications of type 2 diabetes: focus on lipid management. Clin. Diabetes 19, 113–120 (2001).
Tuttle, H. A., Davis-Gorman, G., Goldman, S., Copeland, J. G. & McDonagh, P. F. Proinflammatory cytokines are increased in type 2 diabetic women with cardiovascular disease. J. Diabetes Compl. 18, 343–351 (2004).
Monlun, M., Rigalleau, V., Blanco, L., Mohammedi, K. & Blanco, P. Chronic low grade inflammation in type 2 diabetes—activation of the inflammasomes by circulating metabolites. Diabetes 67, 1726P (2018).
Dunlay, S. M., Weston, S. A., Redfield, M. M., Killian, J. M. & Roger, V. L. Tumor necrosis factor-alpha and mortality in heart failure: a community study. Circulation 118, 625–631 (2008).
Padfield, G. J. et al. Cardiovascular effects of tumour necrosis factor α antagonism in patients with acute myocardial infarction: a first in human study. Heart 99, 1330–1335 (2013).
Feldman, A. M. et al. The role of tumor necrosis factor in the pathophysiology of heart failure. J. Am. Coll. Cardiol. 35, 537–544 (2000).
Collaboration, E. R. F. et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet 375, 132–140 (2010).
Kaptoge, S. et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur. Heart J. 35, 578–589 (2014).
Danesh, J. et al. Low grade inflammation and coronary heart disease: Prospective study and updated meta-analyses. Br. Med. J. 321, 199–204 (2000).
Ridker, P. M., Hennekens, C. H., Buring, J. E. & Rifai, N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 342, 836–843 (2000).
Dandona, P., Aljada, A. & Bandyopadhyay, A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 25, 4–7 (2004).
Lontchi-Yimagou, E., Sobngwi, E., Matsha, T. E. & Kengne, A. P. Diabetes mellitus and inflammation. Curr. Diab. Rep. 13, 435–444 (2013).
Mirza, S. et al. Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: a cross-sectional study. Cytokine 57, 136–142 (2012).
Glovaci, D., Fan, W. & Wong, N. D. Epidemiology of diabetes mellitus and cardiovascular disease. Curr. Cardiol. Rep. 21, 1–8 (2019).
Bulugahapitiya, U., Siyambalapitiya, S., Sithole, J. & Idris, I. Is diabetes a coronary risk equivalent? Systematic review and meta-analysis: original article: epidemiology. Diabet. Med. 26, 142–148 (2009).
Fox, C. S. Cardiovascular disease risk factors, type 2 diabetes mellitus, and the framingham heart study. Trends Cardiovasc. Med. 20, 90–95 (2010).
Marks, J. B. & Raskin, P. Cardiovascular risk in diabetes: a brief review. J. Diabetes Compl. 14, 108–115 (2000).
Nørgaard, C. H., Mosslemi, M., Lee, C. J. Y., Torp-Pedersen, C. & Wong, N. D. The importance and role of multiple risk factor control in type 2 diabetes. Curr. Cardiol. Rep. 21, 1–10 (2019).
McGurnaghan, S. et al. Cardiovascular disease prevalence and risk factor prevalence in Type 2 diabetes: a contemporary analysis. Diabet. Med. 36, 718–725 (2019).
Fisher-Hoch, S. P. et al. Socioeconomic status and prevalence of obesity and diabetes in a Mexican American community, Cameron County, Texas, 2004–2007. Prev. Chronic Dis. 7, A53 (2010).
Fisher-Hoch, S. P., Vatcheva, K. P., Rahbar, M. H. & McCormick, J. B. Undiagnosed diabetes and pre-diabetes in health disparities. PLoS ONE 10, e0133135 (2015).
Fisher-Hoch, S. P. et al. Missed opportunities for diagnosis and treatment of diabetes, hypertension, and hypercholesterolemia in a Mexican American population, Cameron County Hispanic Cohort, 2003–2008. Prev. Chronic Dis. 9, 110298 (2012).
Hispanic Health Research Center - Hispanic Health Research Center - Centers - Research - The University of Texas Health Science Center at Houston (UTHealth) School of Public Health.
Florez, J. C. et al. Strong association of socioeconomic status with genetic ancestry in Latinos: implications for admixture studies of type 2 diabetes. Diabetologia 52, 1528–1536 (2009).
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 29(Suppl 1), S43–S48 (2006).
SPSS Software | IBM.
Stata: Software for Statistics and Data Science.
NCSS & LLC. Normality Tests.
Ghasemi, A. & Zahediasl, S. Normality tests for statistical analysis: a guide for non-statisticians. Int. J. Endocrinol. Metab. 10, 486–489 (2012).
Mann-Whitney U Test in SPSS Statistics | Setup, Procedure & Interpretation | Laerd Statistics.
Hypothesis Testing - Chi Squared Test.
Cochran–Mantel–Haenszel test - Handbook of Biological Statistics.
Sarle, W. S. & Sarle, W. S. Neural Networks and Statistical Models. (1994).
Pagidipati, N. et al. 1472-P: association between triglycerides and residual cardiovascular (CVD) risk in patients with type 2 diabetes and established CVD: an analysis of the BARI2D Trial. Diabetes 68, 1472-P (2019).
Miller, M. et al. Triglycerides and cardiovascular disease. Circulation 123, 2292–2333 (2011).
Digel, W. et al. Tumor necrosis factor induces proliferation of neoplastic B cells from chronic lymphocytic leukemia. Blood 73, 1242–1246 (1989).
Sun, A., Chia, J.-S., Chang, Y.-F. & Chiang, C.-P. Serum interleukin-6 level is a useful marker in evaluating therapeutic effects of levamisole and Chinese medicinal herbs on patients with oral lichen planus. J. Oral Pathol. Med. 31, 196–203 (2002).
High HDL Cholesterol: Can It Be a Problem?
Chang, M. H. et al. Racial/Ethnic variation in the association of lipid-related genetic variants with blood lipids in the US adult population. Circ. Cardiovasc. Genet. 4, 523–533 (2011).
Ariel, T. H. et al. Racial/ethnic differences in dyslipidemia patterns. Circulation https://doi.org/10.1161/CIRCULATIONAHA.113.005757 (2014).
Morris, A. & Ferdinand, K. Clinical Lipidology Hyperlipidemia in racial/ethnic minorities: differences in lipid profiles and the impact of statin therapy. Clin. Lipidol. https://doi.org/10.2217/clp.09.70 (2017).
Schmidt, M. I. et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet 353, 1649–1652 (1999).
Spranger, J. et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 52, 812–817 (2003).
Stamler, J., Vaccaro, O., Neaton, J. D., Wentworth, D. & Group, T. M. R. F. I. T. R. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 16, 434–444 (1993).
Chen, R., Ovbiagele, B. & Feng, W. Diabetes and stroke: epidemiology, pathophysiology, pharmaceuticals and outcomes. Am. J. Med. Sci. 351, 380–386 (2016).
Group, D. D. Prevalence of small vessel and large vessel disease in diabetic patients from 14 centres The World Health Organisation Multinational Study of Vascular Disease in Diabetics. Diabetes Drafting Group. Diabetologia 28(Suppl), 615–640 (1985).
Boden-Albala, B. et al. Diabetes, fasting glucose levels, and risk of ischemic stroke and vascular events: findings from the Northern Manhattan Study (NOMAS). Diabetes Care 31, 1132–1137 (2008).
McBride, P. Triglycerides and risk for coronary artery disease. Curr. Atheroscler. Rep. 10, 386–390 (2008).
Eckel, R. H., Grundy, S. M. & Zimmet, P. Z. The metabolic syndrome. Lancet 365, 1415–1428 (2005).
Grundy, S. M. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. Am. J. Cardiol. 83, 25–29 (1999).
Kannel, W. B. & Vasan, R. S. Triglycerides as vascular risk factors: new epidemiologic insights. Curr. Opin. Cardiol. 24, 345–350 (2009).
Miller, M. et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 123, 2292–2333 (2011).
Vazquez-Benitez, G. et al. Preventable major cardiovascular events associated with uncontrolled glucose, blood pressure, and lipids and active smoking in adults with diabetes with and without cardiovascular disease: a contemporary analysis. Diabetes Care 38, 905–912 (2015).
Gordon, D. J. et al. High-density lipoprotein cholesterol and cardiovascular disease four prospective American studies. Circulation 79, 8–15 (1989).
Acknowledgements
We thank Rocio Uribe and her cohort team, who recruited and documented the participants and obtained the specimens. We also thank Marcela Morris and other laboratory staff for their contributions, and Christina Villarreal for administrative support. We thank Valley Baptist Medical Center, Brownsville, Texas for providing us space for our Center for Clinical and Translational Science Clinical Research Unit. We also thank the communities of Brownsville, Laredo and Harlingen and the participants who so willingly gave their time for this study in their cities.
Funding
This work was supported by (i) Lahore University of Management Sciences, Faculty Initiative Fund Grant No. FIF559-1920-BIO, (ii) Grant Number MD000170 P20 from the National Center on Minority Health and Health disparities (NCMHD), and (iii) Centers for Translational Science Award U54RR023417-01 from the National Center for Research Resources (NCRR).
Author information
Authors and Affiliations
Contributions
S.U. and S.M. supervised the study. P.M., S.F., J.M., J.G. and S.M. developed the cohort and obtained the data. A.T., F.A., S.M. and S.U. designed the data analysis. A.T. and S.U. performed the analysis and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: Joseph McCormick was omitted from the author list in the original version of this Article. The Author Contributions section now reads: “S.U. and S.M. supervised the study. P.M., S.F., J.M., J.G. and S.M. developed the cohort and obtained the data. A.T., F.A., S.M. and S.U. designed the data analysis. A.T. and S.U. performed the analysis and wrote the manuscript.”. The original Article has been corrected.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tahir, A., Martinez, P.J., Ahmad, F. et al. An evaluation of lipid profile and pro-inflammatory cytokines as determinants of cardiovascular disease in those with diabetes: a study on a Mexican American cohort. Sci Rep 11, 2435 (2021). https://doi.org/10.1038/s41598-021-81730-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-81730-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.