Abstract
The mechanical muscular oscillations are rarely the objective of investigations regarding the identification of a biomarker for Parkinson’s disease (PD). Therefore, the aim of this study was to investigate whether or not this specific motor output differs between PD patients and controls. The novelty is that patients without tremor are investigated performing a unilateral isometric motor task. The force of armflexors and the forearm acceleration (ACC) were recorded as well as the mechanomyography of the biceps brachii (MMGbi), brachioradialis (MMGbra) and pectoralis major (MMGpect) muscles using a piezoelectric-sensor-based system during a unilateral motor task at 70% of the MVIC. The frequency, a power-frequency-ratio, the amplitude variation, the slope of amplitudes and their interlimb asymmetries were analysed. The results indicate that the oscillatory behavior of muscular output in PD without tremor deviates from controls in some parameters: Significant differences appeared for the power-frequency-ratio (p = 0.001, r = 0.43) and for the amplitude variation (p = 0.003, r = 0.34) of MMGpect. The interlimb asymmetries differed significantly concerning the power-frequency-ratio of MMGbi (p = 0.013, r = 0.42) and MMGbra (p = 0.048, r = 0.39) as well as regarding the mean frequency (p = 0.004, r = 0.48) and amplitude variation of MMGpect (p = 0.033, r = 0.37). The mean (M) and variation coefficient (CV) of slope of ACC differed significantly (M: p = 0.022, r = 0.33; CV: p = 0.004, r = 0.43). All other parameters showed no significant differences between PD and controls. It remains open, if this altered mechanical muscular output is reproducible and specific for PD.
Similar content being viewed by others
Introduction
There is plenty of research dealing with the investigation of pathomechanisms or the search for a possible biomarker for Parkinson’s disease (PD). Currently, the diagnosis is based on the UK Parkinson’s Disease Society Brain Bank Diagnostic Criteria, which especially covers clinical examinations using the Unified Parkinson’s Disease Rating Scale (UPDRS)1,2,3,4. Clinical examinations are critisised to be subjective, although some investigations found the UPDRS to be at least sufficient reliable5,6,7,8,9. The clinical findings can be supplemented by various additional examinations (SPECT, midbrain sonography, odor test, polysomnography). Several researchers point out the need for an objective diagnostic tool for PD10,11,12, which can be quickly and easily applied in addition to the routine procedures. Potential biomarkers for diagnosis of PD, which are currently investigated, are presented in Kalia and Lang10. A biomarker using the motor output is not considered therein. In a recent paper, Evers et al.11 pointed out to record sensor-based parameters of motor output as gait parameters and tremor-related items to monitor PD. However, not each PD patient exhibits a tremor, especially not in the early stages. A clinical diagnosis in prodromal stages is difficult, since the cardinal symptoms rigor, resting tremor and bradykinesis are often extremely discreet. A challenge arises from the task to identify persons at risk even prior to the manifestation of the cardinal symptoms (premotor stage). If the motor stage is reached, a significant degeneration of at least 50% decline of the dopaminergic neurons has already occured13,14. Therefore, a main objective is to identify an early diagnosis to probably prevent further loss through an early started intervention15.
Kalia & Lang10 stated that “Parkinson’s disease is now viewed as a slowly progressive neurodegenerative disorder that begins years before diagnosis can be made” (p. 896). Therefore, it is assumed that discreet changes in motor output might exist in the preclinical phase. According to McAuley and Marsden16 the mechanical muscle oscillations might serve as a “’window’ into the function of central oscillations”. They pointed out the obvious limitations to directly record the central oscillations. So, why not use the motor output to provide information of central abnormalities? Several researchers examined gait parameters as stride duration, arm and leg swing as well as step time in PD, which partly showed changes already in prodromal stages17,18,19,20,21,22,23. Deviation in those parameters seem not to be specific for PD, since they do not only occur in PD, but also in diseases like Alzheimer’s or Huntington’s diesaese23,24,25.
A further parameter to monitor the motor output is the mechanomyography (MMG), which is used to record the muscular micro-oscillations. The research group around Marusiak et al. 26,27 investigated MMG in PD patients with tremor. However, in PD patients with apparent tremor, the detection via MMG or a common tremor measurement is not necessary because a visual diagnosis can be performed easily. Of course, it can provide information about the characteristics of the tremor; however, it is not of great value for diagnosis. The muscular micro-oscillations might probably show deviations already before the tremor is apparent. Therefore, the objective of this study was to investigate, whether or not Parkinson's patients without a clinical tremor show altered oscillation patterns in the mechanical muscular output.
Methods
The aim of this exploratory study was to examine how the mechanical myofascial oscillations behave during a unilateral isometric motor task in patients with Parkinson’s disease without tremor compared to healthy controls. A similar investigation on the same population was already done in a specific bilateral isometric motor task, whereby the subject was interacting with itself28. Therefore, some parts of this methods section are overlapping with the methods in Schaefer & Bittmann28.
Participants
Controls
There were 29 healthy subjects who volunteered to participate in the study. They were recruited from the Club Aktiv of the Brandenburgischer Verein für Gesundheitsförderung e.V. (BVfG; Brandenburg Association of Health Promotion; Potsdam, Germany) or from relatives of the included patients. Exclusion criteria were complaints of the upper extremities, the shoulder girdle and cervical spine within the last six month, a malignant hypertension and any hints for a neurological disease. The healthy controls passed a neurological examination performed by neurologists of the Neurological Clinic for Movement Disorders and Parkinson’s Disease (Beelitz-Heilstätten, Germany). Four participants had to be excluded because of the results of that examination. The anthropometric data and the averaged maximal voluntary isometric contraction (MVIC) during the isometric tasks for the armflexors of the remaining 25 healthy subjects (n = 12 male, n = 13 female) are displayed in Table 1. Two subjects were left-handed. The remaining 23 controls were right-handed28.
Patients with Parkinson’s disease
There were 28 patients diagnosed with Parkinson’s disease (PD) without a tremor who volunteered to be part of the PD group. The patients were recruited from the Neurological Clinic for Movement Disorders and Parkinson’s Disease in Beelitz-Heilstätten (Germany; Chief physician: Prof. Dr. G. Ebersbach) and were measured in the clinical routine during the medical off phase (~ 12 h after medication intake). Exclusion criteria included the appearance of a clinical tremor, neurological symptoms beyond PD, a manifest polyneuropathy, a malignant hypertension, a coronary heart disease of NYHA III or higher, brain pacemaker, brain aneurysms, glaucoma and hemorrhagic apoplex. Relative exclusion criteria were orthopedic symptoms of the upper extremities, the shoulder girdle and cervical spine within the last six months.
In total, six patients had to be excluded due to their medical history (n = 4) or signal quality (n = 2). The anthropometric data and the MVIC of the armflexors are displayed in Table 128.
Setting
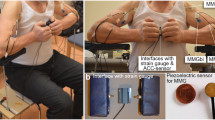
In the setting-up (Fig. 1) the subjects sat on a specially customised chair with 90° hip and knee angle. A plate was attached to the chair to fix the strain gauge either on the left or on the right side of the subject with the possibility to adjust the position of the strain gauge in the depth. The strain gauge (model: ML MZ 2000 N 78, 2000 N, modified by biovision) was fixed in a rail of the plate and was connected to a strap. The strap enclosed the distal forearm of the participant, so that the subject was able to pull on the strain gauge. Thereby, the arm was adducted with 90° flexion in the elbow joint, the hand was in neutral position. The acceleration sensor with a sensitivity of 312 mV/g (range ± 2 g, linearity: ± 0.2%; comp. biovision) was fixed on the strap to detect the accelerations along the longitudinal acting force vector. The mechanical oscillations of the biceps brachii (MMGbi), the brachioradialis (MMGbra) and of the pectoralis major muscles (MMGpect) were recorded using piezoelectric sensors (Shadow SH 4001). They were fixed on the skin above the muscle bellies with ECG-tape. The MMG-signals were conducted across an amplifier (Nobels preamp booster pre-1). All signals were converted by an ADC (National Instruments, 14-bit, USB 6009; modified by Biovision) and subsequently were recorded by the software NI DIAdem 2012 (National Instruments) on a measurement notebook (Toshiba Satellite Pro L500-1T2; Windows 7). Sampling rate was set at 1000 Hz28.
Setting for the unilateral isometric motor task. The MMG-sensors were fixed above the biceps brachii, brachioradialis and pectoralis major pars clavicularis muscles and amplified using the Nobels pre-amp booster pre-1. The strain gauge, which was fixed by a strap to the forearm of the participants, recorded the force and the ACC-sensors captured the motion and accelerations of the forearm. All signals were transmitted through an AD-converter to the measuring notebook.
Measuring procedure
Prior to measurements, the patients were examined by various neurologists using the UPDRS. For organisational reasons, the controls were examined neurologically in an extra appointment. Afterwards, the subject was introduced to the system and procedure. The subject was then seated on the chair and was equipped with the sensors. The unilateral isometric task (in total 10 trials) was performed randomized (coin toss) for the left and right side: The first trial was performed in a resting position to indicate a potential resting tremor. Thereby, the subject had its hands on lap. The second trial was performed in the starting position (without pulling on the strap) to detect a potential tremor. Afterwards, the MVIC was identified in two trials, in which the subject had to pull maximally on the strap. The higher of the two maximal values was then used to calculate 70% of the MVIC for the further five trials. Thereby, the subject had to pull on the strap until 70% of the MVIC was reached and maintain this force level for 8 s. The intensitiy could be controlled via biofeedback on a screen (pointer display). The resting period between the trials of MVIC and of 70% of the MVIC was set at 90 s. The last trial was done in the resting position again to once more determine a possible tremor. Subsequently, the same procedure was performed with the contralateral side.
Data processing and statistical analysis
The software NI DIAdem 14 was utilised for the data processing and partly for the analysis. Excel (Microsoft Office, 2013) was used for further processing and SPSS Statistics 25 (IBM) for statistical analyses. The isometric plateau at 70% of the MVIC was cut from the raw data of each trial (reference: force signal; deviations of ± 10% were accepted) and was used for further analyses. Various parameters were taken into account: (1) MVIC (force signal); and the following parameters concerning the oscillating signals of MMGs and ACC within one trial: (2) a specific ratio (QREL) of the Power Spectral Density (PSD); (3) the slope of the amplitude maxima; (4) the variation of the amplitude maxima and (5) the mean frequency28.
For the analysis of oscillating signals, the raw signals must have a signal-to-noise-ratio (SNR) of at least 10 dB29. Signals with a lower SNR were excluded (Table 2). This is the reason why some comparisons are based on lower sample sizes. The following explanations concerning the five parameters are based on the description in Schaefer & Bittmann28.
-
(1)
Maximal voluntary isometric contraction (MVIC)
The maximum value of the filtered force signal (filter type: Butterworth; filtering degree 10, cutoff frequency 3) was determined in NI DIAdem. The higher value of both MVIC trials was transmitted in SPSS for group comparisons28.
-
(2)
Specific power ratio QREL
The idea behind this parameter is to get the percentage of the power in the low frequency range of 3 to 7 Hz (Interval 1; I1) on the power in the frequency range of 3 to 12 Hz (Interval 2; I2). This was compiled since in descriptive analysis two peaks were identified in PSD of PD patients: one in a lower and one in a higher frequency range. By calculating the mean frequency those peaks would be eliminated28.
The raw data (Fig. 2) were used to estimate the PSD, which is the basis for calculating QREL:
Exemplary raw signals of one PD patient and one control. The left panels display the raw signals of force, ACC, MMGbi, MMGbra, MMGpect of the left side of a PD patient during one trial of the unilateral task at 70% of the MVIC, the right panels illustrate the same signals of one participant of the control group. The dark area in the force signals indicates the isometric plateau.
For each trial and each MMG-/ACC-signal one value QREL results. For further analysis the following variables were calculated out of the five power ratios QREL:28
-
I.
Arithmetic mean (M) of the five QREL for each signal (MMG, ACC) and side:
-
(1)
MQREL = absolute value of MQREL
-
(2)
Relative asymmetrie [percentage points (pp)] of left (le) and right (ri) side of MQREL:
$${\mathbf{Asym}}-{\mathbf{MQ}}_{{{\mathbf{REL}}}} = \left| {\left( {\frac{{MQ_{REL} le}}{{MQ_{REL} le + MQ_{REL} ri}} \cdot 100} \right) - 50} \right|$$
-
(1)
-
II.
Coefficient of variation (CV) of the five QREL for each signal (MMG, ACC) and side:
-
(1)
CVQREL = absolute value of the CV
-
(2)
Asym-CVQREL = relative asymmetrie [pp] of left and right side of CVQREL: (Analogue to I.2).
-
(1)
Data processing for parameters (3) to (5)
The MMG and ACC-signals (isometric plateau) were filtered (Butterworth, filtering degree 5, cutoff frequency 20 Hz) and the maxima of each amplitude were determined28.
(3) Slope of the amplitude maxima
To calculate the mean slope of all maxima of one signal, the slope function in Excel was used. In order to create a linear parameter for further consideration, this was converted into degree. One slope value resulted per trial for each signal and side28.
(4) Amplitude variation (VAmp)
For the variation of amplitude within one trial, the absolute difference between the y-values of two consecutive maxima was calculated in Excel. The resulting differences were averaged per trial and were relativized to the arithmetic mean of the amplitudes. One value VAmp resulted per trial for each signal and side28.
(5) Mean frequency
The frequency of one signal was calculated by, firstly, determining the reciprocal of the time distances (x-values) between two consecutive maximum data points and, secondly, averaging these values of one signal. This method is rather unusual for stochastically distributed variables as captured here. However, concerning Pikovsky et al.30 it is possible to use the reciprocal of period duration in almost periodic oscillations in chaotic systems, as the neuromuscular system. The authors regard this technique as appropriate for the present investigation to get information about the mean frequency, considering that, thereby, the amplitudes are not taken into account. The latter is done by parameter (2)28.
For further statistical analyses of the parameters (3), (4) and (5), the arithmetic mean (M) and coefficient of variation (CV) were calculated for all signals for the left and right side using the values of the five trials. Furthermore, the relative asymmetrie (Asym) of the left and the right side was calculated (interlimb-asymmetry). Hereby, the sides of PD patients were divided into ‘not or less affected’ and ‘more affected’ on the base of the results of the UPDRS28.
UPDRS
The scores of the UPDRS concerning motor control (items 18–31) were estimated by the neurologists. A maximal amount of 108 points may be obtained thereof. A healthy person should have a value of zero. In the course of data analysis, the estimation of the more affected side using the UPDRS was compared to the own perception of the patient.
The differentiation of sides (not-less affected/more affected) are based on the UPDRS scores. A difference between UPDRS values of left and right side of more than 2 points are considered as real side difference (proposed by the neurologists). However, the analysis of interlimb-asymmetry included all participants. The consideration of the less and more affected side of PD patients within the PD group was performed additionally by including exclusively patients with an UPDRS difference of more than 2 pts.
Statistical considerations
Each parameter in each group (PD vs. Con) was checked for normal distribution by using a Shapiro-Wilk test. For the group comparisons of anthropometric data and MVIC a one-way ANOVA was performed, including the Bonferroni post-hoc test. The gender differences were analysed using the Chi-squared test28.
For the parameters (2) to (5) an unpaired t-test for parametric data or a Mann-Whitney-U test for non-parametric data were utilised to compare the groups PD and Con. For ANOVA and t-test the Levène test of variance homogeneity was performed and required. If variance homogenity was not fulfilled, a Welch correction was performed. For the comparisons of less affected and more affected side of the PD, the Wilcoxon (non-parametric data) or the t-test for paired samples (parametric data) were used.
The effect size was determined either with the Pearson’s correlation coefficient (r) for parmetric data or with the Cohen’s r for non-parametric data. Furthermore, the 95% confidence intervals (CI) were calculated for the parameters (2) to (5). For correlation of UPDRS and MVIC the Spearman correlation coefficient was utilised. Significance level was set at α = 0.0528.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on request.
Ethical approval
The study was approved by the ethic committee of the University of Potsdam (Germany; approval no. 60/2016) and by the State Chamber of Medicine in Brandenburg (Germany). It was conducted in accordance with the declaration of Helsinki. All subjects/participants were informed in detail and gave their informed written consent to participate.
Informed consent
Informed written consent was obtained from all individual participants included in the study. Furthermore, the participant in Fig. 1 approved to be published.
Results
Anthropometric data and MVIC
The anthropometric data did not differ statistically significant between the groups regarding to age (t(41) = − 2.045; p = 0.063) and BMI (t(41) = 1.229, p = 0.229). The proportion of female and male over the whole sample (PD and Con) was statistically not significant (χ2 = 7.739, p = 0.052). The amount of male participants (n = 17) was significantly higher than the amount of females (n = 4) in the PD group (χ2 = 8.048, p = 0.005) compared to the controls (n = 12 male, n = 13 female; χ2 = 0.040, p = 0.841) (Table 1).
All MVIC data were normally distributed and the variance homogeneity was fulfilled for both groups. As displayed in Table 1, the arithmetic mean of the MVIC of male controls were approximately 11 Nm (left) and 12 Nm (right), respectively, higher compared to the PD-group. Female controls showed an approximately 5 Nm (left) and 6 (right) Nm higher MVIC compared to the PD group. However, the difference of MVIC between the whole PD and control groups was not significant (left: t(43) = − 0.043, p = 0.966; right: t(43) = − 0.155, p = 0.878). The ANOVA was, as expected, found to be significant for MVIC between the gender independent of the groups control or PD (left: F(41,3) = 0.922, p = 0.000; right: F(41,3) = 11.219, p = 0.000). The post-hoc Bonferroni test displayed no significant differences between the groups PD and controls within the females or males (female left: padj = 1.0; right: padj = 1.0; male left: padj = 0.530; right: padj = 0.270). Therefore, the following analyses are based on similar force intensities between the groups PD and Con. The Spearman correlation coefficient showed no significant correlation between UPDRS score and MVIC (left: p = 0.287, right: p = 0.443).
Specific ratio QREL
The 95% CIs of the variable MQREL for the MMG and ACC signals compared between PD and Con are displayed in Fig. 3, the related statistical parameters are given in Table 3. The MQREL of MMGbi and MMGbra differed not significantly between PD and Con (p > 0.05), whereas the MMGpect showed a significantly higher MQREL in Con compared to PD (t(58) = -3.652, p = 0.001, r = 0.432).
Specific ratio MQREL. Displayed are the 95% confidence intervals of the arithmetic mean of the specific ratio Qrel (MQREL) of the MMGs and the ACC signals. As can be seen in the MMG-signals, the PD have a lower ratio compared to controls. However, only concerning the MMGpect significant differences of p = 0.001 occur. The CIs of the ACC signal are overlapping completely.
The relative asymmetry of MQREL (Asym-MQREL[pp]) between left and right side was found to be significant for MMGbi and MMGbra between PD and Con, but not for the MMGpect (Fig. 4; MMGbi: U = 67.00, p = 0.008, r = 0.335; (nPD = 15; ncon = 19); MMGbra: t(24) = 2.079, p = 0.048, r = 0.39 (nPD = 13; ncon = 13); MMGpect: p = 0.171; (nPD = 12; ncon = 13). Thereby, the more affected side in PD patients with an UPDRS side difference of more than 2 pts indicated a rather lower MQREL compared to the less or not affected side (Fig. 5) (MMGbi: t(11) = 1.470, pMMGbi = 0.170, nless = 7, nmore = 6; t(6) = 0.884, pMMGbra = 0.411, nless = 4, nmore = 4; t(8) = 0.858, pMMGpect = 0.416 , nless = 5, nmore = 5). In those PD patients, the side asymmetries concerning the ratio MQREL in MMGbi were the highest with an amount of 19.4 ± 13.5 pp (range: 7.5 – 42.89) between the more and the less affected limb. The patients with a UPDRS difference of less or equal 2 points showed a relative side asymmetry of 13.7 ± 7.6 pp (range: 6.7 – 26.8). The relative side asymmetry of controls was found to be 8.4 ± 6.2 pp (range: 0.3 – 22.93) (Fig. 6).
Interlimb asymmetries of ratio MQREL. Displayed are the 95% confidence intervals of the side differences of the arithmetic mean of the specific ratio Qrel (Asym-MQREL) of the MMGs and the ACC signals. As can be seen in the MMG-signals of the elbow flexors, the PD have a higher side difference compared to the controls (p = 0.008; p = 0.048). In the MMGpect, a tendency of higher side asymmetry in PD is visible, whereas the ACC-signal show no difference between PD and Con.
Comparison of less and more affected side in PD with UPDRS-difference > 2 pts. Displayed are the 95%-CIs of the arithmetic mean of Qrel (MQREL) of the MMG-signals comparing the more affected and the not or less affected side in PD. Only PD patients with a difference of > 2 pts in UPDRS were included. As can be seen, the more affected side tend to have a lower ratio compared to the less affected side. However, the data are not significant.
Relative interlimb asymmetry of MQrel. Displayed are the relative differences between left and right MMG of biceps brachii muscle according to the parameter MQREL between the PD patients and the controls sorted by size. The dotted line shows a possible boarder according to the 95%-CI at approximately 11 pp difference. The dark filled circles represent the PD patients with a side difference in UPDRS of > 2 pts.
The coefficient of variation of the QREL (CVQREL) of the MMG-signals showed no significant result concerning MMGbi (p = 0.268; nPD = 33; ncon = 39) and the MMGpect (p = 0.171, nPD = 29; ncon = 32), respectively. The CVQREL was significant for the MMGbra in comparing the total sample of PD and Con (U = 601.00, p = 0.048; r = 0.25; nPD = 29; ncon = 32), whereby the PD group showed higher CVs compared to the controls.
The CIs for the M and CV of the QREL of the ACC-signals were markedly overlapped in the unilateral trials and no significance was to be found (Fig. 3).
Further parameters (3) to (5) of oscillatory signals
MMG-signals
The MMG-signals of biceps brachii and brachioradialis muscles showed no significant differences between PD and controls concerning mean frequency, amplitude variation and slope (p = 0.088 – 0.964) (Table 4). The arithmetic mean of VAmp of the MMGpect differed significantly between PD and Con (t(73) = 3.081, p = 0.003, r = 0.34, nPD = 33, nCon = 42) (Fig. 7, Table 4). The side asymmetry was found to be higher in the PD group compared to controls with an effect size of r = 0.37 (Asym-M-VAmp: U = 201.00, p = 0.033) (Table 4).
Oscillatory parameters of MMGpect. Displayed are the 95%-CIs of the parameters VAmp and frequency of the MMGpect (above) and of the related interlimb asymmetries (below). The M of VAmp differs significantly with p = 0.003, r = 0.34 and the CV of frequency shows no significant difference between the total group of PD and Con. The side differences, however, are significantly different with p = 0.033 (r = 0.37) for Asym-M-VAmp and p = 0.004 (r = 0.48) for Asym-CV-Freq.
The frequency of MMGpect varied slightly more in Con (n.s., p = 0.781), but the relative interlimb asymmetry of the CV of the frequency was significantly higher in PD with an effect size of r = 0.48 (Asym-CV-Freq: t(31) = -2.739, p = 0.004, nPD = 14, nCon = 20). (Fig. 7, Table 4).
ACC-signal
The slope of amplitude maxima of the ACC-signal differed significantly between PD and Con with regard to the M and CV of the five trials. The M of slope of PD was significantly higher compared to the Con group (M slope: U = 2.297, p = 0.022, r = 0.33, n = 48), whereas the CV of slope was significantly lower in PD (U = -2.876, p = 0.004, r = 0.42) (Fig. 8). Significant interlimb asymmetries did not exist (Table 4).
Slope of the amplitude maxima of ACC. Displayed are the 95% CIs of the arithmetic mean (left) and the coefficient of variation (right) of the slope of the amplitude maxima of the ACC signal. Both show significant results (M: p = 0.022, CV: p = 0.004; nPD = 22, ncon = 26). The PD show higher mean slope than the controls, whereas the PD have less variation between the five trials.
UPDRS
The UPDRS scores for the healthy controls were 0 in all cases. In PD patients they ranged from 0 to 17 points for the right and from 0 to 11 points for the left side and showed no statistical differences between left and right side (W = 31.5, p = 0.103). The interlimb differences ranged from 0 to 8 points. Eight out of the 22 patients showed a difference of more than 2 points. Six patients exhibited severer symptoms in the neurological examination on the right side, two on the left side.
In comparing the UPDRS and the own assessment of patients with respect to the more affected side, nine cases indicated a different estimation. However, only in three of those patients the UPDRS difference between left and right side was higher than 2 points.
In the remaining five patients with an UPDRS difference higher than 2 pts, the own assessment and the estimation using the UPDRS agreed. The UPDRS score had no significant influence on all considered parameters (p > 0.05).
Discussion
The objective of the study was to evaluate the oscillatory patterns of the armflexors and pectoralis muscle during a unilateral motor task in PD patients without tremor. The presented results show that the majority of comparisons are not to be found significant. This is not in accordance with the corresponding investigation using a bilateral motor task in the same participants28. However, the consideration of interlimb asymmetries seem to be relevant in the unilateral task: the PD patients showed higher side differences for the arithmetic mean of power ratio MQREL in MMGbi and MMGbra as well as for the arithmetic mean of amplitude variation and the CV of mean frequency of MMGpect. Furthermore, the ACC signal shows differing behaviour of slope of amplitude maxima, whereby the mean of slope is higher in PD, but the PD patients show lower variability between the trials (CV). Before presenting a content-related discussion of the results, the limitations have to be considered.
Methodological limitations
Proportion of gender
The amount of female PD patients was clearly lower than the proportion of male PD patients (n = 4 vs. n = 17). This is justified by the clinical practice. Female Parkinson patients, who meet the inclusion criteria, were rare. With regard to the pilot character, we decided to accept this difference in gender proportion in the groups. Since MMG is gender-neutral31 and the investigated parameters did not differ statistically with respect to gender, the potential for error is assessed to be minor.
The force level is more relevant with regard to a probable influencing effect on the amplitude of the oscillatory signals. The force differences between the groups PD and Con were not to be found significant. Especially in male, however, the PD group had a lower MVIC compared to controls (left ≈ -11 Nm, p = 0.530; right ≈ -12 Nm, p = 0.270). Although the differences were not statistically relevant, this still might have influenced the signal quality. Since only relative parameters were considered, the outcome should not be influenced thereby.
Signal quality
Several signals had to be excluded due to the signal quality. According to Husar29 the SNR has to amount at least 10 dB for analysing the oscillatory behaviour of data. Therefore, all signals with a lower SNR were excluded. Due to the exclusions, the sample sizes are reduced for some comparisons (Table 2).
Multiple testing
Within this explorative study, a large amount of comparisons were performed (n = 64), because the results should be compared to the results of the measurements in a bilateral motor task, which were conducted in the same population. This was done to get insights into different characteristics of the muscular oscillations in this explorative study design28. Some authors stated that in explorative studies or non-clinical studies, a correction for multiple testing is not mandatory32,33,34. However, it has to be mentioned, that using the Simes procedure for multiple testing no comparison turned out to be significant anymore.
The setting of the unilateral motor task might have also influenced the results. One has to take into account that the oscillation pattern might be affected by the fixation of the force sensor on the plate of the chair. This might probably have dampened the oscillations.
The results have to be considered with caution in any way, due to the explorative character, the small sample sizes and the multiple comparisons. However, the results still reveal insights into probable mechanisms and characterisations of the mechanical muscle oscillations in PD patients without tremor.
Content-related discussion
Power frequency distribution in patients with Parkinson’s disease
It was primarily hypothesized that the frequency of mechanical muscular oscillations would be shifted to lower frequency bands in PD patients due to the commonly appearing tremor of ~ 5 Hz in motor state35. It was assumed that this shift might be visible in the muscular micro-oscillations in PD patients without tremor, thus, prior to the manifestation of the Parkinsonion tremor. The presented results indicate that the shift seems to be diametrical. Considering the MMG signals, the arithmetic mean of power in I1 (3 to 7 Hz) amounts approximately 28 ± 6% of the entire frequency range of 3 to 12 Hz in controls, whereas in PD the power of I1 amounts to about 19 ± 10% (p = 0.001 – 0.225) of the total frequency range considered. This indicates that the power in the low-frequency range is relatively higher in controls. Thus, there is a different power proportion, however, not in the hypothesized way. This differing power ratio already was found for the bilateral motor task28. The changed pattern is also visible for the PD patients considering the less and more affected side, although this comparison is not significant (UPDRS difference > 2 pts.): I1 amounts approximately 27 ± 15% for the less affected side and 20 ± 11% for the more affected side (n = 8, p > 0.05). The mechanical muscle oscillations must be an expression of neuronal activity, which reflects the motor control16. Nevertheless, MMG only reflects the mechanical motor output. Thus, if at all, it can provide indirect insights into central processes. A lot of research has been performed regarding oscillations of neuronal structures in PD patients with tremor, e.g. of the subthalamic nucleus as part of the basal ganglia. Even if concrete details of the activity in the involved neuronal networks still remain widely unclear36,37,38, the beta band oscillations seem to play an important role in the pathophysiology of PD39,40,41,42. Probably, the changed power frequency pattern reflects those changes of central processes. A more detailed discussion concerning the central oscillations with respect to the mechanical muscular oscillations was provided in the article of the bilateral tasks28. It would be conceivable that the shift of the power frequency pattern to higher frequency ranges might reflect a counter regulation in PD patients prior to the manifestation of the tremor. Probably, the neuromuscular system up-regulates the frequency of motor control as a last opportunity to prevent the low-frequency tremor. Therefore, we suspect that this shifted pattern might probably be specific for PD patients without tremor.
Changed variability in PD patients as a rather unspecific change of motor control
In several other neuromusculoskeletal diseases, changes in variability are detectable between healthy people and patients. A lower variability in patients was shown for gait-lines in herniated disc of lumbar spine43 or gait lines in patients with orthopedic complaints of the lower limbs44.
A higher variability in patients with Parkinson’s and Huntington’s disease was shown for parameters as stride duration18,19,23 as well as swing and step time17,19,20,21, stride length45 or other postural adjustements during gait46. Obviously, it is crucial, which parameter is examined. The parameters which are necessary to balance or to adapt adequately to external forces (balance during one step, amplitude variation during isometric muscle action, etc.) seem rather to decrease, whereas the variation of parameters of a specific motor programme (as swing and step time etc.) seem rather to increase.
In the presented study, the variation of the amplitude maxima of the MMGpect within one trial is lower in PD with 68 ± 16% compared to Con with 78 ± 14% (p = 0.003), whereby the interlimb asymmetry of this parameter is higher in PD (PD: Asym-VAmp = 7.6 ± 5.2 pp; Con: Asym-VAmp = 3.9 ± 2.7 pp; p = 0.033). The reason why this pattern is not apparent in the armflexors only can be assumed. Probably, the setting up also influences this parameter. It has to be taken into account, too, that the results might be random.
Interlimb asymmetries might reflect the unilateral onset of motor symptoms in PD
The relative interlimb differences between left and right side appear to be the most recurring significant parameter in the unilateral measures. Concerning the mean of the ratio QREL of the MMG of armflexors as well as concerning the mean of amplitude variation and the CV of mean frequency of the MMGpect the side asymmetries differed significantly between PD and Con (p = 0.003 – 0.048). Thereby, the PD always showed a higher interlimb asymmetry. It is assumed that the side difference concerning neuromuscular parameters should be low in healthy persons as indicated for the controls in the presented study. This is supported by other investigations, in which non-significant side differences have occured in healthy controls concerning neuromuscular parameters as reflex latencies of the semitendinosus tendon47 or concerning the stride-to-stride variability23. While in diseases as Parkinson’s, the stride-to-stride variability23, the arm swing during walking48 or the balance control49 showed a higher interlimb asymmetry. Furthermore, PD patients exhibited deficits in interlimb coordination25, which is related to the interlimb asymmetry.
Especially the balance control seems to be closely related to the performed unilateral motor task in the presented study, since both tasks—balance control and controlling a given force intensity—refer to closed-loop systems. Both rely on proprioception and on feedback control to make adjustments during the tasks. The pertubations during the balance tasks of Boonstra et al.49 might have required higher demands on neuromuscular adjustments compared to the presented study. Thereby, the participants just had to control the given force level by pulling on a strip and using a visual feedback. However, we assume that the neuromuscular control processes in the close loop system might be affected in PD.
The interlimb asymmetry concerning the parameter MQREL in the MMG signals of armflexors might be a precursor of the later inserting tremor, which is characterised by a unilateral onset10,50. We hypothesise that the side differences of the ratio might be specific for PD, whereas the other oscillatory parameters are potentially reflecting a more general change in motor control and might also be present in other diseases of the neuromusculoskeletal system.
Difference of limb accelerations and mechanical muscular micro-oscillations
The parameter slope of amplitude maxima is significant for the ACC signal, which reflects the changes of amplitude extents of limb accelerations within one trial. The mean of slope is higher in PD patients compared to healthy control, whereby the CV between the five trials is lower in PD. That indicates that the amplitudes are increasing in the course of one trial, but between the trials the slope is rather steady. An increasing amplitude is known for fatiguing trials. Probably, the task was more exhausting for the PD patients and, therefore, the slope was higher.
Taking the results of MMGs and ACC together, a relevant methodological conclusion is that the ACC showed different results compared to the MMG signals. This indicates that monitoring the mechanical muscular oscillations could provide different results than recording the accelerations of an extremity. Moreover, the MMGs seem to provide further insights into the motor control due to the above mentioned results.
Classification of results concerning the UPDRS
The sample size was very small considering only patients with a side difference in UPDRS of more than 2 pts and in which the signals of both sides were suitable (MMGbi: n = 6, MMGbra: n = 3, MMGpect: n = 3). Therein the reason for the non-significant differences between the less and more affected limb might be found. However, the interlimb asymmetries concerning the ratio MQREL were the highest in the PD patients with an UPDRS side difference of more than 2 pts. with an amount of averagely 19.4 ± 13.5 pp (range: 7.5 – 42.89 pp) between the more and the less affected limb in MMGbi signals. In the patients with an UPDRS difference of less or equal 2 points the relative interlimb asymmetry of MQREL reduced to averagely 13.7 ± 7.6 pp (range: 6.7 – 26.8 pp). In controls this trend continued and the interlimb asymmetries reduced further to 8.4 ± 6.2 pp (range: 0.3 – 22.93 pp). However, the sample size is too small to draw conclusions from this result. Nevertheless, the estimation using the UPDRS and the measurements seem to still rather agree.
Another point, which has to be considered critically concerning the UPDRS, is the dissonance between the scores taken by the physicians and the assessment of the patients. However, this was the case in only three patients with a UPDRS side difference of 3, 5 and 6 pts, respectively. The other patients, in which the dissonance appeared, had an UPDRS side difference of equal or lower than 2 pts. Probably, the differences are so marginal that the own perception and the estimation by UPDRS might differ. Nevertheless, one would expect an accordance between perception and estimation using the UPDRS. The reason for the discordance cannot be determined appropriately here. There might be a misjudgment on both sides. The patients may have mixed up the sides. However, the main problem of the UPDRS is the lack of objectivity, even though in studies with exercised testers, the reliability of the UPDRS is stated to be reliable and valid5,6. In several other studies, it was postulated that the inter rater reliability is only acceptable7,8,9. Evers et al.11 conclude that there is the need of a more reliable instrument. Probably, the assessment using the oscillatory behaviour of muscles during an isometric uni or bilateral motor task might support the diagnosis in the future. Further investigations have to re-examine the found results on a larger group of PD patients. Additionally, the participant groups have to be extended to other neurodegenerative diseases to assess the specifity of the found results. Thereby, it is suggested to only measure the MMG of pectoralis major and biceps brachii muscle, since the signals of brachioradialis muscle did not reveal further information.
Comparison of the bilateral and the unilateral motor task
Almost all investigated parameters showed a clearer difference between PD and Con during the bilateral task (recently presented in28) compared to the unilateral task presented here. In the bilateral task, the participant pushed with both hands against the measuring device including a strain gauge and accelerometer infront of its chest. Probably, the bilateral task enhances the potentially pathological patterns of motor control, which are reflected in the mechanical muscle oscillations. Especially, the power frequency ratio and the amplitude variation within one trial showed altered patterns in the bilateral task in PD patients. It would be conceivable that the neuromuscular adjustments in the close loop system are more demanding in the bilateral task, since both hemispheres have to mutually adjust to coordinate the action and reaction between both extremities. Since interlimb coordination seems to be reduced in PD patients, this result would be supported by other studies46,48. Therefore, the more complex bilateral task might be more vulnerable with respect to changes in motor control patterns.
The unilateral task probably could be more sensitive for side differences. The bilateral task might transfer the pathological changes to the other side. Hence, the side asymmetries are levelled in the bilateral task. This could indicate that the assumed pathological changes in motor control are dominant or, probably, are enhanced in a bilateral task, like known for the Jendrássik maneuver51,52,53.
For further studies, especially the bilateral task might be promising to indicate changed pattern in PD patients in general, whereby the unilateral task might be appropriate to indicate interlimb asymmetries. However, due to the explorative character of the study, it has to be taken into account, too, that the results might be an effect of multiple testing and, therefore, might be random.
Conclusion and outlook
The need of a more objective diagnostic tool in PD, especially for early stages, is widely accepted. In the present study a potential novel approach was proposed. The novelties are, in particular, that PD patients without tremor were investigated in a loaded motor task. Usually patients with tremor are examined under unloaded conditions. However, the results can only be interpreted as first hints.
The evaluation concerning the wavelet coherence of the MMG and ACC signals between the left and right upper extremity as well as between the muscles of one side is remaining.
Further investigations firstly have to verify the results in healthy persons, patients with PD and with other neurodegenerative diseases. Secondly, it has to be investigated whether or not the changes are specific for PD or if they occur in other or even most neurodegenerative diseases. It might be possible that specific deviation patterns exist for different diseases. Probably, several parameters of motor control as the power-frequency-ratio, the amplitude variation and the interlimb differences during loaded motor tasks might be collectively considered to support the diagnostics of PD in the future.
A development of a neuromuscular biomarker could have a large and essential impact on the diagnosis and follow-up in Parkinson’s disease. Moreover, the understanding of the underlying pathomechanism might be further investigated by implementing similar uni and bilateral motor tasks.
References
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Review. Mov. Disord. 30(12), 1591–1601. https://doi.org/10.1002/mds.26424 (2015).
Marsili, L., Rizzo, G. & Colosima, C. Diagnostic criteria for parkinson’s disease: from James Parkinson to the concept of prodromal disease. Front. Neurol. 9, 156. https://doi.org/10.3389/fneur.2018.00156 (2018).
Michel, P. P., Hirsch, E. C. & Hunot, S. Understanding dopaminergic cell death pathways in Parkinson disease. Neuron 90, 675–691. https://doi.org/10.1016/j.neuron.2016.03.038 (2016).
Bötzel, K., Tronnier, V. & Gasser, T. The differential diagnosis and treatment of tremor. Dtsch Arztebl. Int. 111(13), 225–236. https://doi.org/10.3238/arztebl.2014.0225 (2014).
Goetz, C. G. et al. Movement disorder society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 23(15), 2129–2170 (2008).
Martinez-Martin, P. et al. Expanded and independent validation of the Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS). J. Neurol. 260(1), 228–236 (2013).
Martinez-Martin, P. et al. Unified Parkinson’s disease Rating Scale characteristics and structure. The Cooperative Multicentric Group. Mov. Disord. 9, 76–83 (1994).
Richards, M. et al. Interrater reliability of the Unified Parkinson’s disease Rating Scale motor examination. Mov. Disord. 9, 89–91 (1994).
Camicioli, R. et al. Discriminating mild parkinsonism: methods for epidemiological research. Mov. Disord. 16, 33–40 (2001).
Kalia, L. V. & Lang, A. E. Parkinson’s disease. Lancet 386, 869–912. https://doi.org/10.1016/S0140-6736(14)61393-3 (2015).
Evers, L. J. W., Krijthe, J. H., Meinders, M. J., Bloem, B. R. & Heskes, T. M. Measuring Parkinson’s disease over time: the real-world within-subject reliability of the MDS-UPDRS. Mov. Disord. 34(10), 1480–1487. https://doi.org/10.1002/mds.27790 (2019).
Izawa, M., Miwa, H., Kajimoto, Y. & Kondo, T. Combination of transcranial sonography, olfactory testing, and MIBG myocardial scintigraphy as a diagnostic indicator for Parkinson’s disease. Eur. J. Neurol. 19, 411–416 (2012).
Gerlach, M., Reichmann, H. & Riederer, P. Die Parkinson-Krankheit: Grundlagen, Klinik, Therapie 2nd edn. (Springer, Berlin, 2001).
Fernley, J. M. & Lees, A. J. Ageing and Parkinson’s disease: substantia nigra regional selectivity. Brain 114, 2283–2301 (1991).
DGN – Deutsche Gesellschaft für Neurologie (Hrsg.). S3 Leitlinien für Diagnostik und Therapie in der Neurologie—Idiopathisches Parkinson-Syndrom. Langversion. AWMF-Register-Nummer: 030-010; https://www.awmf.org/uploads/tx_szleitlinien/030-010l_S3_Parkinson_Syndrome_Idiopathisch_2016-06.pdf (2016).
McAuley, J. H. & Marsden, C. D. Physiological and pathological tremors and rhythmic central motor control. Brain 123, 1545–1567 (2000).
Mirelman, A. et al. Arm swing as a potential new prodromal marker of Parkinson’s disease. Mov. Disord. 31, 1527–1534 (2016).
Del Din, S. et al. Gait analysis with wearables predicts conversion to Parkinson disease. Ann. Neurol. 86, 357–367. https://doi.org/10.1002/ana.25548 (2019).
Ebersbach, G. et al. Interference of rhythmic constraint on gait in healthy subjects and patients with early Parkinson’s disease: evidence for impaired locomotor pattern generation in early Parkinson’s disease. Mov. Disord. 14(4), 619–625 (1999).
Pistacchi, M. et al. Gait analysis and clinical correlations in early Parkinson’s disease. Funct Neurol. 32, 28–34. https://doi.org/10.11138/FNeur/2017.32.1.028 (2017).
Sterling, N. W. et al. Dopaminergic modulation of arm swing during gait among Parkinson’s disease patients. J. Parkinsons Dis. 5, 141–150. https://doi.org/10.3233/jpd-140447 (2015).
Baron, E. I., Miller, K. M., Streicher, M. C., Rosenfeldt, A. B. & Alberts, J. L. Altered kinematics of arm swing in Parkinson’s disease patients indicates declines in gait under dual-task conditions. Parkinsonism Relat. Disord. 48, 61–67. https://doi.org/10.1016/j.parkreldis.2017.12.017 (2018).
Hausdorff, J. M., Cudkowicz, M. E., Firtion, R., Wei, J. Y. & Goldberger, A. L. Gait variability and basal ganglia disorders: stride-to-stride variations of gait cycle timing in Parkinson’s disease and Huntington’s disease. Mov. Disord. 13(3), 428–437. https://doi.org/10.1002/mds.870130310 (1998).
Webster, K. E., Merory, J. R. & Wittwer, J. E. Gait variability in community dwelling adults with alzheimer disease. Alzheimer Dis. Assoc. Disord. 20(1), 37–40. https://doi.org/10.1097/01.wad.0000201849.75578.de (2006).
Daneault, J.-F., Carignan, B., Sadikot, A. F. & Duval, C. Inter-limb coupling during diadochokinesis in Parkinson’s and Huntington’s disease. Neurosci. Res. 97, 60–68. https://doi.org/10.1016/j.neures.2015.02.009 (2015).
Marusiak, J., Jaskólska, A., Kiesel-Sajewicz, K., Yue, G. H. & Jaskólski, A. EMG and MMG activities of agonist and antagonist muscles in Parkinson’s disease patients during absolute submaximal load holding. J. Electromyogr. Kinesiol. 19(5), 903–914 (2009).
Marusiak, J. et al. Electromyography and mechanomyography of elbow agonists and antagonists in Parkinson Disease. Muscle Nerve 40, 240–248 (2009).
Schaefer, L. & Bittmann, F. Parkinson patients without tremor show changed patterns of mechanical muscle oscillations during a specific bilateral motor task compared to controls. Sci. Rep. 10, 1168. https://doi.org/10.1038/s41598-020-57766-5 (2020).
Husar, P. Biosignalverarbeitung (Springer, Berlin, 2010).
Pikovsky, A., Rosenblum, M. & Kurths, J. Synchronization. A Universal Concept in Nonlinear Sciences (Cambridge University Press, Cambridge, 2003).
Cramer, J. T. et al. Gender, muscle, and velocity comparisons of mechanomyographic and electromyographic responses during isokinetic muscle actions. Scand. J. Med. Sci. Sp. 14(2), 116–127. https://doi.org/10.1111/j.1600-0838.2003.00317.x (2004).
Bender, R., Lange, S. & Ziegler, A. Multiples Testen (Georg Thieme, 2002). https://www.thieme-connect.com/products/ejournals/pdf/10.1055/s-2007-959035.pdf.
Rothman, K. J. No adjustments are needed for multiple comparisons. Epidemiology. 1(1), 43–46 (1990).
O’Brien, P. C. The appropriateness of analysis of variance and multiple-comparison procedures. Biometrics 39(3), 787–788 (1983).
Baumann, C. R. Epidemiology, diagnosis and differential diagnosis in Parkinson’s disease tremor. Parkinsonism Relat. Disord. 18, 90–92 (2012).
Foffani, G. et al. 300-Hz subthalamic oscillations in Parkinson’s disease. Brain 126, 2153–2163. https://doi.org/10.1093/brain/awg229 (2003).
Priori, A. et al. Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson’s disease. Exp. Neurol. 189(2), 369–379 (2004).
Dirkx, M. F. et al. The cerebral network of Parkinson’s tremor: an effective connectivity fMRI study. J. Neurosci. 36(19), 5362–5372 (2016).
Brown, P. Bad oscillations in Parkinson’s disease. J. Neural Transm. 70, 27–30 (2006).
Yang, A. I., Vanegas, N., Lungu, C. & Zaghloul, K. A. Beta-coupled high-frequency activity and beta-locked neuronal spiking in the subthalamic nucleus of Parkinson’s disease. J. Neurosci. 34(39), 12816–12827 (2014).
Brown, P. Abnormal oscillatory synchronisation in the motor system leads to impaired movement. Curr. Opin. Neurobiol. 17(6), 656–664 (2007).
Whitmer, D. et al. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson’s disease. Front. Hum. Neurosci. 6, 155. https://doi.org/10.3389/fnhum.2012.00155 (2012).
Jelen, P., Wit, A., Dudzinski, K. & Nolan, L. Expressing gait-line symmetry in able-bodied gait. Dyn. Med. 7, 17. https://doi.org/10.1186/1476-5918-7-17 (2008).
Bittmann, F., Badtke, G. & Silbermann, U. Kontrolle des Rehabilitationsverlaufes nach Knie- und Sprunggelenksverletzungen mit Hilfe der Computerdynographie. Poster presentation at the 34th congress of sports medicine 1995 in Saarbrücken, Germany. Deutsche Zeitschrift für Sportmedizin 63(7–8), 200 (2012).
Blin, O., Ferrandes, A. M. & Serratrice, G. Quantitative analysis of gait in Parkinson patients: increased variability of stride length. J. Neurol. Sci. 98, 91–97 (1990).
Lin, C.-C., Creath, R. A. & Rogers, M. W. Variability of anticipatory postural adjustments during gait initiation in individuals with Parkinson’s disease. J. Neurol. Phys. Ther. 40(1), 40–46. https://doi.org/10.1097/NPT.0000000000000112 (2016).
Wijntjes, J., Hilgevoord, A. & Laman, D. M. Normative values of semitendinosus tendon reflex latencies. Clin. Neurophysiol. Pract. 2, 35–37 (2017).
Lin, C.-C. & Wagenaar, R. C. The impact of walking speed on interlimb coordination in individuals with Parkinson’s disease. J. Phys. Ther. Sci. 30, 658–662. https://doi.org/10.1589/jpts.30.658 (2018).
Boonstra, T. A., van Vugt, J. P. P., van der Kooji, H. & Bloem, B. R. Balance asymmetry in Parkinson’s disease and its contribution to freezing of gait. PLoS ONE 9(7), e102493. https://doi.org/10.1371/journal.pone.0102493 (2014).
Yust-Katz, S., Tesler, D., Treves, T. A., Melamed, E. & Djaldetti, R. Handedness as a predictor of side of onset of Parkinson’s disease. Parkinsonism Relat. Disord. 14(8), 633–635. https://doi.org/10.1016/j.parkreldis.2008.01.017 (2008).
Tsuruike, M., Koceja, D. M., Yabe, K. & Shima, N. Age comparison of H-reflex modulation with the Jendrássik maneuver and postural complexity. Clin. Neurophysiol. 144(5), 945–953. https://doi.org/10.1016/S1388-2457(03)00039-7 (2003).
Zehr, E. P. & Stein, R. B. Interaction of the Jendrássik maneuver with segmental presynaptic inhibition. Exp. Brain Res. 123, 474–480 (1999).
Hayes, K. C. Jendrassik maneuver facilitation and fractionated patellar reflex times. J. Appl. Physiol. 32(3), 290–295 (1972).
Acknowledgements
Primarily, we thank the whole staff of the Clinic for Movement Disorders and Parkinson’s Disease in Beelitz-Heilstätten (Germany), especially Prof. Dr. G. Ebersbach (Chief), Dr. D. Gruber and Dr. F. Gandor for their support, the recruiting and examination of the participants and for providing suitable facilities to perform the measurements in the clinic. We also thank the Brandenburgischer Verein für Gesunheitsförderung e.V. for their support and assistance in recruiting controls. Last but not least we are grateful to all subjects for participating in the study and for their interest in our research.
Funding
The study was funded by the German Society for Parkinson’s and movement disorders. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
L.S. and F.B. contributed to the conception and design of the study. J.K. and N.L. perfomed the acquisition and processing of data. L.S. supervised the scientific data acquisition and performed the data analysis. L.S. and F.B. participated in the process of interpretation and discussion of the data. L.S. drafts the manuscript and it was critically revised by all authors. All authors gave their final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no financial or non-financial competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schaefer, L.V., Löffler, N., Klein, J. et al. Mechanomyography and acceleration show interlimb asymmetries in Parkinson patients without tremor compared to controls during a unilateral motor task. Sci Rep 11, 2631 (2021). https://doi.org/10.1038/s41598-021-81672-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-81672-z
This article is cited by
-
Muscle oxygenation and time to task failure of submaximal holding and pulling isometric muscle actions and influence of intermittent voluntary muscle twitches
BMC Sports Science, Medicine and Rehabilitation (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.