Abstract
Conventional microalgae oil extraction applies physicochemical destruction of dry cell biomass prior to transesterification process to produce fatty acid methyl esters (FAMEs). This report presents a simple and rapid direct transesterification (DT) method for FAMEs production and fatty acid profiling of microalgae using freshly harvested biomass. Results revealed that the FAMEs recovered from Chlorella vulgaris were 50.1 and 68.3 mg with conventional oil-extraction-transesterification (OET) and DT method, respectively. While for Messastrum gracile, the FAMEs recovered, were 49.9 and 76.3 mg, respectively with OET and DT methods. This demonstrated that the DT method increased FAMEs recovery by 36.4% and 53.0% from C. vulgaris and M. gracile, respectively, as compared to OET method. Additionally, the DT method recovered a significantly higher amount of palmitic (C16:0) and stearic (C18:0) acids from both species, which indicated the important role of these fatty acids in the membranes of cells and organelles. The DT method performed very well using a small volume (5 mL) of fresh biomass coupled with a shorter reaction time (~ 15 min), thus making real-time monitoring of FAMEs and fatty acid accumulation in microalgae culture feasible.
Similar content being viewed by others
Introduction
The key processes involved in the fatty acid profiling of microalgae are cultivation, biomass harvesting and drying, oil extraction and transesterification to produce fatty acid methyl esters (FAMEs). Among these processes, biomass drying and oil extraction are two costly and tedious processes. The conventional microalgae oil extraction method require several steps. The efficiency of the oil extraction depends on microalgae species, the cell wall disruption method and solvent system1, 2. To disrupt microalgae cell walls, various approaches such as grinding, ultra sonication1, microwave treatments3, enzymes digestion4 autoclaving, bead beating and high salt solution5 have been reported. The major problems faced by the conventional oil extraction-transesterification (OET) method are biomass drying and oil extraction processes. The drying of fresh/wet biomass prior to oil extraction requires high energy that is time-consuming. It is estimated that over 90% processed energy is used during the biomass drying and oil extraction steps6, 7. Furthermore, the solvent system cannot completely extract the fatty acids, especially the fatty acids which are attached to the membrane lipids such as phospholipids and glycolipids8. This leads to lower FAMEs recovery and compromises the fatty acid profiling6, 7. In addition, the oil extraction yield is adversely affected when wet biomass is used9.
Over the years, direct transesterification (DT) of biomass has received enormous attention due to its high efficiency in the reduction of energy and time10. The DT method enables simultaneous extraction of lipid and FAMEs conversion processes by alcohol in the presence of a catalyst. The alcohol acts as an extraction solvent and an acyl acceptor for transesterification11,12,13. The DT method makes it possible to produce FAMEs in a single step using microalgae fresh/wet biomass while also eliminating both biomass drying and oil extraction processes7, 14. There are plenty of recent research on DT of microalgae biomass along with relatively higher FAMEs yield reported. There are DT methods established using dry microalgal biomass15,16,17 or in combination with novel approaches such as microwave irradiation18, ultrasound treatment19 and vortex fluidic device (VFD)-based20, as well as the application of immobilized lipases19, 21, among others. One of the major drawback of the above-mentioned DT methods was the transesterification reaction time which was relatively longer and differs greatly depending on the approaches used that may range from less than one hour to several hours15,16,17,18,19,20,21,22,23,24. In addition, a large amount of dry/wet microalgal biomass was needed for the transesterification reaction15,16,17,18,19,20,21,22,23,24.
Nevertheless, there is a lack of research reported on small-scale DT using fresh/wet microalgal biomass directly harvested from microalgal cultures for real-time monitoring of oil accumulation and fatty acid composition. Therefore, this study compared the efficiency of the conventional OET and DT methods25 for total FAMEs recovery from small amount of microalgae biomass that was freshly and directly harvested from microalgae cultures. In this report, the DT method was optimized using different small sample volume sizes of freshly harvested microalgae biomass. The variation of fatty acid composition from the two methods was also compared.
Results and discussion
Cell growth, biomass production and oil content using conventional oil extraction-transesterification (OET) method
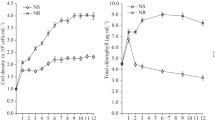
Figure 1 shows the growth curve of C. vulgaris and M. gracile. The cell density of the C. vulgaris culture increased exponentially in the first 5 days of the culture and attained early stationary growth phase at day 8. No significant change in cell density was observed until day 12 where the cell density of 2.26 × 107 cells mL−1 was recorded. On the other hand, the exponential growth of M. gracile lasted for 7 days and attained early stationary growth phase at day 8, where no significant change in cell density was observed until day 12 with cell density of 2.08 × 107 cells mL−1. Although an equal initial cell density (5 × 106 cells mL−1) was used for both species, the cell densities recorded everyday were different between the two species with C. vulgaris consistently recording higher cell density throughout the growth curve (Fig. 1). All cultures were harvested on day 12 where cell growth reached a plateau, which represents the stationary growth phase. The logistic growth pattern of microalgae involves lag phase, log phase, stationary phase and senescence phase26. In the lag phase, which was at the beginning of the culture, both species were adapting to a new culture environment. After full adaptation to the new culture condition, the log phase occurred in which the population growth was greatly increased until around day 7 (Fig. 1). When the cultures reached the stationary growth phase, the nutrients in the culture medium were expected to be depleted and cell growth turned into a regression straight line with no significant change in cell density at the end of the growth curve (Fig. 1).
Generally, microalgae grow slowly and attain low biomass production, but with higher lipid25,26,27 and carbohydrate28, 29 production when culture is cultivated under low nitrate concentration. Furthermore, stationary growth phase has been recognized as a period of active oil accumulation phase for microalgae species due to the conversion of carbohydrate to lipids30,31,32. Therefore, both C. vulgaris and M. gracile cultures were harvested at the stationary growth phase to ensure high oil accumulation for further analysis. As shown in Table 1, the total oil content of C. vulgaris and M. gracile at the stationary growth phase was 47.5% and 40.6% (percent of dry weight), respectively. Conversely, M. gracile recorded higher dry biomass production of 0.42 g L−1 against 0.37 g L−1 produced by C. vulgaris (Table 1). The higher biomass production was concomitant with lower cell density and total oil content recorded by M. gracile versus C. vulgaris. The larger cell size of M. gracile could compromise the efficiency of biological and physiological processes33, 34.
OET versus DT for determination of total FAMEs
The DT procedure was similar to the transesterification procedure in the OET method, except the oil sample, which was replaced with the fresh/wet microalgae biomass that was directly harvested from the stationary growth phase microalgal culture. Sample aliquots ranging from 5 to 30 mL were directly sampled from C. vulgaris cultures for DT to determine the total FAMEs. The good linearity (Supplementary Fig. S1) with correlation coefficient, r in the range of 0.9880–0.9913 was obtained from the calibration experiment and the linearity plots were then applied to quantitate the total FAMEs in the samples. Table 2 summarizes the total FAMEs obtained from different sample volumes of C. vulgaris cultures using the DT method. Generally, the results showed that the smaller sample volume used in DT produced higher total FAMEs. The 5-mL sample volume produced 68.3 mg (60.9% DW) of the total FAMEs, which was significantly higher (p < 0.05) than the total FAMEs obtained from the 20- and 30-mL sample volumes. Interestingly, the DT method using 5 mL sample volume managed to recover 36.4% more FAMEs when compared to the OET method, in which OET produced only 50.1 mg of total FAMEs (Table 2). In view of the higher total FAMEs yield from DT using 5 mL sample volume from C. vulgaris culture, therefore, the 5-mL sample volume was also chosen for the analysis of M. gracile. As shown in Table 2, the DT method using 5-mL sample volume managed to recover 76.3 mg (or 60.4% DW) total FAMEs from M. gracile, which was an increment of 53.0% (or 1.53-fold) compared to 49.9 mg (or 39.4% DW) obtained with the OET method (Table 2). The calculation methods for total FAMEs as outlined in this report was proven reliable and accurate when the total FAMEs calculated from OET method was expressed in percent of dry weight (% DW). For instance, the total FAMEs of 44.7% (% DW, Table 2) calculated from the OET method and the total oil content of 47.5% (% DW, Table 1) for C. vulgaris were not significantly different statistically. A similar result was observed for M. gracile (Tables 1 and 2).
Both C. vulgaris and M. gracile varied in cell sizes. The size of C. vulgaris cell was in the range of 2–10 μm in diameter with a spherical shape24 whereas the M. gracile was in the range of 13–16 μm in length and 1.7–3.5 μm in width with elongated, spindle-shaped, curved with pointed ends3, 34. The larger M. gracile cell including its organelles could consist of relatively greater double-layer membranes, which is embedded with phospholipids, glycolipids and other compositions8. In general, microalgal cell membranes vary in thickness depending on species and stages of growth33. When performing DT, a greater amount of fatty acids contained in the cell membrane of M. gracile were transesterified into FAMEs comparing to C. vulgaris. Furthermore, the total FAMEs obtained from M. gracile through DT method showed an increment of 53.0% against OET method (Table 2). On the other hand, the total FAMEs obtained from C. vulgaris through the DT method showed an increment of only 36.4% against the OET method (Table 2). These results revealed that the 5-mL sample volume withdrawn directly from the microalgal cultures for DT analysis was appropriate for real-time monitoring of FAMEs production in different microalgae species.
The result demonstrated that the DT method transesterified more fatty acid than the OET method from a single cell. According to Dong et al.35, the alkali catalyst might convert the lipid within the cell into cell wall-permeable form or it may make the cell wall permeable to lipids. Therefore, this would enable more or entire lipids to be transesterified into FAMEs from a single cell. Moreover, DT performed on the residual biomass, which had undergone solvent extraction revealed that the total fatty acid extracted and the fatty acid remaining in the residual biomass was similar to the total FAMEs obtained using the DT method11. This indicated the lipid extraction process did not extract all the fatty acids. A comparative study of several OET and DT methods by Cavonius et al.13 concluded that the total FAMEs recovered among the DT methods was observably similar or slightly higher than the OET methods for several microalgae species. On the other hand, Wahlen et al.12 reported FAMEs recovery between 39 and 82% of extractible lipid from several microalgae and cyanobacteria species. In contrast, the DT method used in this current research managed to recover far more FAMEs from the fresh microalgal biomass. The 36.4 and 53% increment of total FAMEs obtained from C. vulgaris and M. gracile, respectively, using the DT method indicated that there was far more other lipid classes composed in the cell structure (in particular the membrane of cells and organelles) which was not extractable using the OET method.
OET versus DT for determination of fatty acid composition
Table 3 shows the fatty acid composition obtained from C. vulgaris using the DT method. The six major fatty acids, the palmitic (C16:0), stearic (C18:0), oleic (C18:1), linoleic (C18:2), γ-linolenic (C18:3n6) and α-linolenic (C18:3n3) acids, which together contributed over 98.0% of total fatty acids were analyzed. The analysis of the fatty acid composition revealed that DT using different sample volumes did not have any effect on the composition of C16:0, C18:1, C18:3n6 and C18:3n3 (Table 3). The C16:0 was the most abundant fatty acid with composition ranging from 42.2 to 46.7% (percent of total FAMEs), followed by C18:1 with composition ranging from 17.8 to 24.0%. In the meantime, C18:3n6 and C18:3n3 contributed relatively lower amounts of 4.3–4.5% and 3.3–4.4%, respectively (Table 3). On the other hand, DT using 5-mL sample volumes rendered higher (p < 0.05) C18:0 (11.9%) and lower (p < 0.05) C18:2 (13.0%) as compared to larger sample volumes (Table 3). A minimum sample volume was always preferred in DT analysis in order to keep the real-time monitoring of FAMEs production easy. However, a sufficient sample size was important for homogeneously representing the microalgal culture biomass, as the FAMEs recovered must exceed the instrument detection limits to minimize the systematic errors. The relatively consistent fatty acid composition coupled with far more total FAMEs recovery concluded that the 5-mL sample volume was selected for subsequent comparison with the OET method.
Figure 2 depicts the comparison of major fatty acids obtained from OET and DT using 5-mL sample volume for both microalgae species. The results clearly showed the consistency of the DT method in recovering specific fatty acids. The DT method obtained significantly higher (p < 0.05) C16:0 and C18:0 from both species and C18:3n6 from C. vulgaris. For C. vulgaris, the C16:0 increased from 32.8 to 46.7%, C18:0 increased from 6.8 to 11.9%, and C18:3n6 increased from 3.3 to 4.5% (Fig. 2a). As for M. gracile, the C16:0 increased from 35.7 to 42.5%, and C18:0 increased from 2.8 to 3.9% (Fig. 2b). On the other hand, the DT method produced consistently lower (p < 0.05) C18:1, C18:2 and C18:3n3 for both species. For C. vulgaris, the C18:1 decreased from 28.6 to 17.8%, C18:2 decreased from 21.2 to 13.0%, and C18:3n3 decreased from 5.4 to 3.3% (Fig. 2a). As for M. gracile, the C18:1 decreased from 49.5 to 43.5%, C18:2 decreased from 4.3 to 3.4%, and C18:3n3 decreased from 6.3 to 5.4% (Fig. 2b).
The fatty acid composition of (a) C. vulgaris UMT-M1 and (b) M. gracile SE-MC4 obtained from oil extraction-transesterification (OET) and direct-transesterification (DT) methods. The percent of fatty acid for OET and DT methods is of total oil content and total FAMEs, respectively. Asterisk (*) indicates significance higher among the same fatty acid according to t-test at p = 0.05. Data shown as the mean ± SD, n = 3.
There are several lipid classes contained within the microalgal cell and organelle membranes, which include neutral lipids, free fatty acids and polar lipids such as glycolipids and phospholipids33, 36, 37. The neutral lipids or mainly triacylglycerols (TAGs) of microalgae plays their role as storage lipids as a means for carbon and energy reserves38. According to Goold et al.39, the lipid droplet of nitrogen-starved Chlamydomonas reinhardtii cells that contained 80–90% TAGs and about 5–10% of polar lipids, which form a monolayer surrounding the neutral lipid core of lipid droplets. The fatty acid composition of microalgal TAGs differs greatly depending on species and culture conditions. In general, it contains fatty acids with chain-length ranging from 12 to 22 carbons with different degree of unsaturation40. While the polar lipids mainly consist of phospholipids and glycolipids that are important constituents of the cells and organelle membranes8, 39, 40. In this current study, the OET method uses hexane to extract the neutral lipids (mainly the storage TAGs) from microalgal dry biomass and subsequently the extracted lipid was transesterified into FAMEs for GC-FID analysis. Hexane (C6H14) is an alkane of six carbon atoms and serves as a non-polar solvent. It has been widely used in industries and laboratories to extract vegetable oils from crops, due to the solubility of the neutral lipids in this non-polar solvent8. On the other hand, the DT method used was similar to the transesterification step for OET method, but the oil sample was replaced with microalgae fresh biomass. Firstly, the fresh biomass underwent saponification when sodium hydroxide in methanol was added and the solution was boiled to saponify the oil into free fatty acid salts (soaps). These fatty acids were then transesterified to FAME with the aid of catalyst boron triflouride in methanol. The transesterification process took place where the free fatty acids, TAGs and other lipid classes (e.g. glycolipids and phospholipids) from the whole cells were converted to FAMEs for GC-FID analysis.
Therefore, the differences in fatty acid composition obtained from the OET and DT methods could serve as a tool to study fatty acid deposition dynamic between the TAGs and the polar lipids in the cell and organelle membranes. As shown in Fig. 2, DT method produced significantly higher amounts of C16:0 and C18:0 from both C. vulgaris and M. gracile which may imply the important role fatty acids have under nitrogen deficiency (BBM supplemented with only 25% of its original sodium nitrate concentration) culture condition. A previous study showed that the polar lipids of C. reinhardtii, C. vulgaris UTEX 265 and Nannochloropsis sp. constituted a larger amount (21.7–25.8%) of C16:041. In addition, the phosphatidylcholine (PC) and phosphatidylethanolamine (PE) of C. vulgaris was also found to constitute C16:0 of between 34.5 and 45.5% under high- and low-CO2 culture conditions42. Therefore, the DT method not only transesterifies the storage lipids (TAGs), but also transesterifies the polar lipids inside the cells membrane and organelles membrane into FAMEs.
Furthermore, the higher recovery of C16:0 and C18:0 from both C. vulgaris and M. gracile (Fig. 2) with DT method could be an added value for biodiesel application as higher saturated fatty acids content would contribute to higher cetane number (CN)43. The CN is an important measurement to determine the engine ignition delay time and combustion performance43. The biodiesel produced from oil producing crops such as palm and coconut44, as well as several microalgae from the Chlorophyta strains such as C. vulgaris, Chlamydomonas sp. and Scenedesmus obliquus44 is rich in these saturated compounds, thus rendering a high CN value in the range of 61–6544. These CN values are well above the minimum requirement under the European specification (UNE-EN 14214) of 5145. However, the DT method recovered fewer C18:1 from both microalgae species under study which could be a drawback for biodiesel application. The C18:1 has been suggested for biodiesel enrichment to improve the fuel quality due to its superior attributes such as low melting point (at − 20 °C), greater oxidative stability than that of PUFAs and CN that exceeds the minimum requirement given in major biodiesel standards45. Nevertheless, the fatty acid composition of microalgae can be altered through the manipulation of various culture conditions to increase the C18:1 content. For examples, nitrogen limitation25 and phytohormones (e.g. jasmonic acid and gibberellin) supplementation46, 47 in C. vulgaris culture, while phosphate limitation48 in M. gracile culture were found to increase the C18:1 content. However, it is elusive to find a feedstock that possesses the ‘perfect’ fatty acid composition for biodiesel conversion thus the binary blending of oils25 from potential feedstock could be further explored.
Conclusions
This study demonstrated the superiority of the DT method against the OET method in recovering the total FAMEs and for fatty acid composition analysis of microalgal culture. Microalgal fresh biomass directly harvested from as little as 5 mL of the microalgal culture managed to render a 36.4 and 53.0% increase in total FAMEs production from C. vulgaris and M. gracile, respectively. Generally, both methods have their advantages, respectively, in the multidisciplinary applications. In edible oil industries such as for human consumption, pharmaceutical and nutraceutical applications, OET method is preferred as it involves the extraction of oil from microalgal dry biomass. While the DT method is favoured in biodiesel production as it eliminates the needs for dewatering, biomass drying and oil extraction processes that could further reduces the energy input, time and cost of biodiesel production. On the other hand, the small-scale and rapid DT method used in this study enables faster screening for potential lipid producing microalgae strains, as well as for real-time monitoring of FAMEs production and fatty acid profile changes during microalgae cultivation.
Materials and methods
Microalgae cultivation
C. vulgaris strain UMT-M1 and M. gracile (previously known as Ankistrodesmus gracilis) strain SE-MC449 were obtained from a collection of purified microalgae stock culture at Universiti Malaysia Terengganu, Malaysia. The inoculum was initiated from a single colony taken from the stock collection and cultured into 300 mL of Bold’s Basal Medium (BBM). The inoculum was then supplied with constant filtered (0.22 μm filter membrane) aeration and maintained at 24 ± 1 °C under 24 h light illustrations (~ 80 μmol m−2 S−1). The cell number was determined after one week of cultivation, and harvested for the next experiment.
The initial cell number for each experimental replicate was standardized at 5 × 106 cells mL−1 in 300 mL of BBM (supplemented with only 25% of its original sodium nitrate concentration) prepared in a 0.5 L Erlenmeyer flask. Our previous research had shown that both C. vulgaris UMT-M150 and M. gracile SE-MC451 produced high amount of total oil content when the culture medium was supplemented with 25% of its original nitrate concentration. In this current study, the cultivation of both microalgae under 25% nitrate supplementation was aimed to produce reasonable high amount of total oil content for the DT and OET experiments as outlined below. This allowed sample standardization and accurate measurement and comparison of the total FAMEs produced from both methods.
The cultures were maintained at 24 ± 1 °C under 24 h light illustrations (~ 80 μmol m−2 S−1) and supplied with constant filtered (0.22 μm filter membrane) air flow. The culture growth was monitored daily until stationary growth phase was attained when cell growth reached a plateau. The cell number was determined by a Neubauer haemocytometer. The optical density was measured at a wavelength of 750 nm by spectrophotometer (Shimadzu UV-1800). The cell density was determined using the calibration curve of optical density at wavelength 750 nm (OD750) versus cell number. The calibration curve of optical density against cell number was drawn and the slope of the curve was used to determine the cell density of the cultures. Three microalgal culture replicates were harvested, and used for DT or conventional OET methods.
Conventional oil extraction-transesterification (OET) procedure
At the stationary growth phase, cells in 300 mL cultures were harvested by centrifugation at 8000 rpm for 5 min. The liquid was removed while the cell pellet was washed with distilled water to remove the excess nutrients. The cell pellets were then dried at 60 °C in an oven for 24 h until consistent weight was achieved. The oil extraction procedure used was previously described by Cha et al.25 with modifications as outlined below. Dry biomass (X) of 0.1 g was immersed in 5 mL of concentrated HCl in a test tube and vortexes for 2 min to thoroughly wet the dry biomass. The stopper was removed and the test tube was placed in a beaker containing boiling water and boiled for 30 min, while the test tube was swirled at a 5 min interval. Subsequently, the test tube was removed from the beaker and cooled to room temperature. Hexane (12.5 mL) was added into the mixture and the test tube was stoppered and shaken vigorously for 1 min. Then, the stopper was removed and the hexane (upper layer) was transferred to a pre-dry pre-weigh 250 mL flat bottom flask (X1) using a pasteur pipette. The extraction was repeated twice with 7.5 mL of hexane. The combined hexane extracts were evaporated in a rotary evaporator. The flask was then dried at 60 °C until consistent weight was obtained. Finally, the flask was cooled in desiccator prior to weighing (X2). The total oil content was calculated using formula (1) as shown below. Fatty acid methyl esters (FAMEs) was prepared using 50 mg of the extracted oil as previously described25. The fatty acid composition of the FAMEs was determined using gas chromatography-flame ionization detection (GC-FID) as described in section below.
Direct transesterification (DT) of microalgal fresh/wet biomass procedure
Four different small aliquots (5, 10, 20, and 30 mL) of C. vulgaris fresh cells were sampled at the stationary growth phase and the cells were pelleted by centrifugation at 8000 rpm for 5 min. The supernatant was removed while the cell pellet was washed once with distilled water to remove residual nutrients.
The DT method was modified from Cha et al.25 The freshly harvested cell pellet was transferred, by flushing it with 5 mL of 0.5 N NaOH (in methanol), into a 250 mL flat bottom flask containing a few pieces of boiling chips. Then, the flask was attached to a Liebig Condenser and the mixture was boiled with a heating mantle for 10 min. Next, 5 mL of boron-triflouride (in 20% methanol) was added and the solution was boiled for 2 min. Subsequently, 2 mL of n-heptane was added and the mixture was further boiled for another 1 min. Finally, the flask was removed from the heating mantle and vapor was allowed to condense before removing the condenser. The resulting solution was cooled at room temperature prior to being transferred into a test tube, followed by the addition of 15 mL saturated NaCl. The test tube was stoppered and shook vigorously for 15 s for phase separation. The upper n-heptane layer was then withdrawn, filtered with a nylon syringe filter and transferred into a 1.5 mL vial prior to FAMEs analysis using GC-FID as described in “Chromatographic conditions and calculation of total FAME”.
The DT method applied on M. gracile culture was based-on the optimized sample volume of C. vulgaris culture that rendered the highest total FAMEs as described above.
Chromatographic conditions and calculation of total FAME
FAMEs measurement was performed using gas chromatography coupled with a flame ionization detector (GC-2010 Plus, Shimadzu, Japan). The chromatographic separation of FAMEs was carried out on HP-88 capillary column (0.25 mm × 100 m, 0.2 µm) (Agilent). The injector volume and injector temperature was 1 μL and 250 °C, respectively. The split ratio for the reference standard and sample was fixed at ratio 1:50. Helium was used as the carrier gas at constant flow rate of 1.9 mL min−1. The flow rate for hydrogen gas and air was 40 mL min−1 and 450 mL min−1, respectively. The initial oven temperature applied was 175 °C for 10 min and then increased to 220 °C for 15 min at a rate of 3 °C min−1. Identification of FAMEs was done by comparing the retention time with a reference standard, the Supelco 37 Component FAMEs Mix (Sigma-Aldrich).
A five-point calibration was performed using 37 Component FAMEs Mix diluted with n-heptane. The calibration standard solutions were vortexed to ensure the homogeneity prior to GC-FID analysis. The linearity plots obtained from the calibration experiment was then applied to quantitate the total FAMEs derived from OET and DT methods. The total FAMEs obtained from the 300 mL cultures was expressed in milligrams (mg) and percentage of the total dry biomass (% DW).
Statistical analysis
All experiments were performed in triplicates. The difference in major fatty acid composition and the total FAMEs yield of the C. vulgaris under volume optimization were analyzed statistically by one-way analysis of variance (ANOVA) followed by Tukey test at 5% significant level. A comparison between the OET and the optimized DT methods was carried out using t-test using statistical software, SPSS where statistical significance was assigned at p < 0.05.
References
Phong, W. N. et al. Improving cell disruption efficiency to facilitate protein release from microalgae using chemical and mechanical integrated method. Biochem. Eng. J. 135, 83–90 (2018).
Anto, S. et al. Algae as green energy reserve: Technological outlook on biofuel production. Chemosphere 242, 125079 (2020).
Cheng, J., Yu, T., Li, T., Zhou, J. & Cen, K. Using wet microalgae for direct biodiesel production via microwave irradiation. Bioresour. Technol. 131, 531–535 (2013).
Al-Ameri, M. & Al-Zuhair, S. Using switchable solvents for enhanced, simultaneous microalgae oil extraction-reaction for biodiesel production. Biochem. Eng. J. 141, 217–224 (2019).
Tran, D. T., Yeh, K. L., Chen, C. L. & Chang, J. S. Enzymatic transesterification of microalgal oil from Chlorella vulgaris ESP-31 for biodiesel synthesis using immobilized Burkholderia lipase. Bioresour. Technol. 108, 119–127 (2012).
Lardon, L., Sialve, B., Steyer, J. & Bernard, O. Life-cycle assessment of biodiesel production from microalgae. Environ. Sci. Technol. 43, 6475–6481 (2009).
Kim, T. H. et al. Development of direct conversion method for microalgal biodiesel production using wet biomass of Nannochloropsis salina. Bioresour. Technol. 191, 438–444 (2015).
Salam, K. A., Velasquez-Orta, S. B. & Harvey, A. P. A sustainable integrated in situ transesterification of microalgae for biodiesel production and associated co-product: A review. Renew. Sust. Energ. Rev. 65, 1179–1198 (2016).
Hidalgo, P., Toro, C., Ciudad, G. & Navia, R. Advances in direct transesterification of microalgal biomass for biodiesel production. Rev. Environ. Sci. Biotechnol. 12, 179–199 (2013).
Sati, H., Mitra, M., Mishra, S. & Baredar, P. Microalgal lipid extraction strategies for biodiesel production: A review. Algal Res. 38, 101413 (2019).
Griffiths, M. J., Hille, R. V. & Harrison, S. T. L. Selection of direct transesterification as the preferred method for assay of fatty acid content of microalgae. Lipids 45, 1053–1060 (2010).
Wahlen, B. D., Willis, R. M. & Seefeldt, L. C. Biodiesel production by simultaneous extraction and conversion of total lipids from microalgae, cyanobacteria, and wild mixed cultures. Bioresour. Technol. 102, 2724–2730 (2011).
Cavonius, L. R., Carlsson, N. G. & Undeland, I. Quantification of total fatty acids in microalgae: Comparison of extraction and transesterification methods. Anal. Bioanal. Chem. 406, 7313–7322 (2014).
Park, J. Y., Park, M. S., Lee, Y. C. & Yang, J. W. Advances in direct transesterification of algal oils from wet biomass. Bioresour. Technol. 184, 267–275 (2015).
Velasquez-Orta, S. B., Lee, J. G. M. & Harvey, A. P. Evaluation of FAME production from wet marine and freshwater microalgae by in situ transesterification. Biochem. Eng. J. 76, 83–89 (2013).
Zhang, Y., Li, Y., Zhang, X. & Tan, T. Biodiesel production by direct transesterification of microalgal biomass with co-solvent. Bioresour. Technol. 196, 712–715 (2015).
Sung, M. & Han, J.I. Alkaline in situ transesterification of Aurantiochytrium sp. KRS 101 using potassium carbonate. Bioresour. Technol. 205, 250–253 (2016).
Patil, P. D. et al. Power dissipation in microwave-enhanced in situ transesterification of algal biomass to biodiesel. Green Chem. 14, 809–818 (2012).
Sivaramakrishnan, R. & Incharoensakdi, A. Direct transesterification of Botryococcus sp. catalysed by immobilized lipase: Ultrasound treatment can reduce reaction time with high yield of methyl ester. Fuel 191, 363–370 (2017).
Sitepu, E. K. et al. Vortex fluidic mediated direct transesterification of wet microalgae biomass to biodiesel. Bioresour. Technol. 266, 488–497 (2018).
Marcon, N. S. et al. Production of ethyl esters by direct transesterification of microalga biomass using propane as pressurized fluid. Appl. Biochem. Biotechnol. 187, 1288–1299 (2019).
Haas, M. J. & Wagner, K. Simplifying biodiesel production: the direct or in situ transesterification of algal biomass. Eur. J. Lipid Sci. Technol. 113, 1219–1229 (2011).
Mathimani, T., Uma, L. & Prabaharan, D. Homogeneous acid catalysed transesterification of marine microalga Chllorella sp. BDUG 91771 lipid: An efficient biodiesel yield and its characterization. Renew. Energ. 81, 523–533 (2015).
Mathimani, T., Uma, L. & Prabaharan, D. Optimization of direct solvent lipid extraction kinetics on marine trebouxiophycean alga by central composite design: Bioenergy perspective. Energy Convers. Manag. 142, 334–346 (2017).
Cha, T. S., Chen, J. W., Goh, E. G., Aziz, A. & Loh, S. H. Differential regulation of fatty acid biosynthesis in two chlorella species in response to nitrate treatments and the potential of binary blending microalgae oils for biodiesel application. Bioresour. Technol. 102, 10633–10640 (2011).
Adamczyk, M., Lasek, J. & Skawińska, A. CO2 biofixation and growth kinetics of Chlorella vulgaris and Nannochloropsis gaditana. Appl. Biochem. Biotechnol. 179, 1248–1261 (2016).
Illman, A. M., Scragg, A. H. & Shales, S. W. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb. Technol. 27, 613–635 (2000).
Ho, S. H. et al. Characterization and optimization of carbohydrate production from an indigenous microalga Chlorella vulgaris FSP-E. Bioresour. Technol. 135, 157–165 (2013).
Ho, S. H., Chen, C. Y. & Chang, J. S. Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour. Technol. 113, 244–254 (2012).
Feng, Y., Li, C. & Zhang, D. Lipid production of Chlorella vulgaris cultured in artificial wastewater medium. Bioresour. Technol. 102, 101–105 (2011).
Ruangsomboon, S. Effect of light, nutrient, cultivation time and salinity on lipid production of newly isolated strain of the green microalga, Botryococcus braunii KMITL 2. Bioresour. Technol. 109, 261–265 (2012).
Wang, H., Gao, L., Shao, H., Zhou, W. & Liu, T. Lipid accumulation and metabolic analysis based on transcriptome sequencing of filamentous oleaginous microalgae Tribonema minus at different growth phases. Bioprocess Biosyst. Eng. 40, 1327–1335 (2017).
Safi, C., Zebib, B., Merah, O., Pontalier, P. Y. & Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sust. Energ. Rev. 15, 265–278 (2014).
Yee, W. Microalgae from the Selenastraceae as emerging candidates for biodiesel production: A mini review. World J. Microbiol. Biotechnol. 32, 64 (2016).
Dong, T. et al. Direct quantification of fatty acids in wet microalgal and yeast biomass via a rapid in situ fatty acid methyl ester derivatization approach. Appl. Microbiol. Biotechnol. 99, 10237–10247 (2015).
Berge, J. P., Gouygou, J. P., Dubacq, J. P. & Durand, P. Reassessment of lipid composition of the diatom Skeletonema costatum. Phytochemistry 39, 1017–1021 (1995).
Lage, S. & Gentili, F. G. Quantification and characterisation of fatty acid methyl esters in microalgae: Comparison of pretreatment and purification methods. Bioresour. Technol. 257, 121–128 (2018).
Klok, A. J., Lamers, P. P., Martens, D. E., Draaisma, R. B. & Wijffels, R. H. Edible oils from microalgae: Insights in TAG accumulation. Trends Biotechnol. 32, 521–528 (2014).
Goold, H., Beisson, F., Peltier, G. & Li-Beisson, Y. Microalgal lipid droplets: Composition, diversity, biogenesis and functions. Plant Cell Rep. 34, 545–555 (2015).
Rivera, E. C. et al. Mechanical cell disruption of Parachlorella kessleri microalgae: Impact on lipid fraction composition. Bioresour. Technol. 256, 77–85 (2018).
Yao, L., Gerde, J. A., Lee, S. L., Wang, T. & Harrata, K. A. Microalgae lipid characterization. J. Agric. Food Chem. 63, 1773–1787 (2015).
Tsuzuki, M., Ohnuma, E., Sato, N., Takaku, T. & Kawaguchi, A. Effects of CO2 concentration during growth on fatty acid composition in microalgae. Plant Physiol. 93, 851–856 (1990).
Ramos, M. J., Fernández, C. M., Casas, A., Rodríguez, L. & Pérez, A. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 100, 261–268 (2009).
Nascimento, I. A. et al. Microalgae versus land crops as feedstock for biodiesel: Productivity, quality, and standard compliance. BioEnergy Res. 7, 1002–1013 (2014).
Knothe, G. “Designer” biodiesel: Optimizing fatty ester composition to improve fuel properties. Energy Fuels 22, 1358–1364 (2008).
Jusoh, M., Loh, S. H., Chuah, T. S., Aziz, A. & Cha, T. S. Elucidating the role of jasmonic acid in oil accumulation, fatty acid composition and gene expression in Chlorella vulgaris (Trebouxiophyceae) during early stationary growth phase. Algal Res. 9, 14–20 (2015).
Jusoh, M., Loh, S. H., Aziz, A. & Cha, T. S. Gibberellin promotes cell growth and induces changes in fatty acid biosynthesis and upregulates fatty acid biosynthetic genes in Chlorella vulgaris UMT-M1. Appl. Biochem. Biotechnol. 188, 450–459 (2019).
Anne-Marie, K., Yee, W., Loh, S. H., Aziz, A. & Cha, T. S. Effects of excess and limited phosphate on biomass, lipid and fatty acid contents and the expression of four fatty acid desaturase genes in the tropical Selenastraceaen Messastrum gracile SE-MC4. Appl. Biochem. Biotechnol. 190, 1438–1456 (2020).
Teh, K. Y. et al. De novo whole genome sequencing data of two mangrove-isolated microalgae from Terengganu coastal waters. Data Brief 27, 104680 (2019).
Norashikin, M. N., Loh, S. H., Aziz, A. & Cha, T. S. Metabolic engineering of fatty acid biosynthesis in Chlorella vulgaris using an endogenous omega-3 fatty acid desaturase gene with its promoter. Algal Res. 31, 262–275 (2018).
Anne-Marie, K., Yee, W., Loh, S. H., Aziz, A. & Cha, T. S. Influence of nitrogen availability on biomass, lipid production, fatty acid profile, and the expression of fatty acid desaturase genes in Messastrum gracile SE-MC4. World J. Microbiol. Biotechnol. 36, 17 (2020).
Acknowledgements
This research was supported by Japan Science and Technology Agency (JST)/Japan International Cooperation Agency (JICA), Science and Technology Research Partnership for Sustainable Development (SATREPS) through the project for Continuous Operation System for Microalgae Production Optimized for Sustainable Tropical Aquaculture (COSMOS), and the SATREPS-COSMOS Matching Fund from the Ministry of Education Malaysia (MOE) (The project VOT53222). Equipment used in this study was obtained with financial support from the Japan Science and Technology Agency (JST)/Japan International Cooperation Agency (JICA), Science and Technology Research Partnership for Sustainable Development (SATREPS) through the project for Continuous Operation System for Microalgae Production Optimized for Sus.
Author information
Authors and Affiliations
Contributions
S.H.L, T.S.C and A.A conceived and designed the research; M.K.C and N.S.F conducted experiments. S.H.L, T.S.C, A.A, and M.K.C analyzed and interpreted data. S.H.L, T.S.C and A.A wrote the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Loh, S.H., Chen, M.K., Fauzi, N.S. et al. Enhanced fatty acid methyl esters recovery through a simple and rapid direct transesterification of freshly harvested biomass of Chlorella vulgaris and Messastrum gracile. Sci Rep 11, 2720 (2021). https://doi.org/10.1038/s41598-021-81609-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-81609-6
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.