Abstract
Malaria parasites develop as oocysts in the mosquito for several days before they are able to infect a human host. During this time, mosquitoes take bloodmeals to replenish their nutrient and energy reserves needed for flight and reproduction. We hypothesized that these bloodmeals are critical for oocyst growth and that experimental infection protocols, typically involving a single bloodmeal at the time of infection, cause nutritional stress to the developing oocysts. Therefore, enumerating oocysts disregarding their growth and differentiation state may lead to erroneous conclusions about the efficacy of transmission blocking interventions. Here, we examine this hypothesis in Anopheles coluzzii mosquitoes infected with the human and rodent parasites Plasmodium falciparum and Plasmodium berghei, respectively. We show that oocyst growth and maturation rates decrease at late developmental stages as infection intensities increase; an effect exacerbated at very high infection intensities but fully restored with post infection bloodmeals. High infection intensities and starvation conditions reduce RNA Polymerase III activity in oocysts unless supplemental bloodmeals are provided. Our results suggest that oocysts respond to crowding and nutritional stress with a dormancy-like strategy, which urges the development of alternative methods to assess the efficacy of transmission blocking interventions.
Similar content being viewed by others
Introduction
The lifecycle of the malaria parasite Plasmodium inside a mosquito vector begins with gametocytes ingested by a female mosquito during a bloodmeal. In the mosquito midgut lumen, gametes fuse to form zygotes that develop into motile ookinetes. Ookinetes traverse the midgut-epithelium and transform into oocysts. Multiple rounds of endomitotic replications inside an oocyst result in production of thousands of sporozoites, a process known as sporogony. Upon oocyst rupture, sporozoites migrate to the salivary gland for transmission to humans via a mosquito bite.
Current malaria control efforts focus on development of transmission blocking interventions including transmission blocking vaccines and drugs as well as genetically engineered mosquitoes expressing antimalarial effectors1,2. Experimental mosquito infections with Plasmodium parasites, and microscopic enumeration of oocysts is the gold standard for evaluation of transmission reduction, whereby mosquitoes are offered an infectious bloodmeal and maintained on sugar solution until oocysts are counted3. Infections often lead to high oocyst loads and consequently massive proliferation of sporozoites inside each oocyst, causing depletion of nutrients in the vector4,5,6,7. For example, ultra-high intensity infections of Anopheles stephensi mosquitoes with the rodent parasite P. berghei leads to reduced number of sporozoites reaching the salivary glands and high mosquito mortality compared with lower infection intensities8. High parasite infections have been linked to marked reduction in free amino acids in the mosquito6, which together with lipids are chiefly acquired from bloodmeals and are essential for most physiological processes and reproduction9. Free amino acids are also critical for parasite development; e.g. Isoleucine (Ile), a major component of P. falciparum proteins, is exclusively provided by the mosquito through digestion of blood serum10.
Under nutritionally limiting conditions, asexual P. falciparum is shown to enter a dormancy-like state, restricting protein synthesis to halt cell proliferation and inhibiting catabolism to maintain viability11,12. Plasmodium parasites lack the target of rapamycin (TOR), the key regulator of the canonical nutrient-sensing pathway in most eukaryotes, and use the mitochondrial association factor 1 (MAF1) to regulate this metabolic switch13. MAF1 acts as a repressor of RNA polymerase III (Pol III) that controls expression of highly abundant non-coding RNAs and is thus essential for cell maintenance, growth and proliferation14,15. Under nutritional stress, Plasmodium MAF1 binds to Pol III switching off tRNA transcription. When conditions become favourable, MAF1 is phosphorylated and reverse translocated to the cytoplasm, freeing Pol III13. MAF1 mutant P. falciparum exhibits increased tyrosine tRNA (tRNATyr) expression and fails to recover from dormancy after long periods of incubation in Ile-deficient medium.
To date, mostly indirect evidence exists for a Plasmodium oocyst strategy to cope with nutritional stress. P. berghei oocyst size and sporozoite output are markedly reduced in Anopheles gambiae mosquitoes silenced for Lipophorin (Lp), the main lipid transporter of insects4,16, and as mentioned previously very high P. berghei infections result in reduced number of sporozoites in A. stephensi salivary glands8. These reports and unpublished observations we and others have made over the years led us to hypothesize that experimental infections may cause a nutritional stress to the developing oocyst, affecting sporogonic development and the measurable infection outcome. Here we investigated and corroborated this hypothesis using P. berghei and P. falciparum infections of A. coluzzii (also known as M-form A. gambiae). Our results identify a dormancy-like phenomenon, where oocysts appear to slow down their metabolic state, hence their growth and differentiation, as a key strategy to cope with nutritional stress and underline the need for improved assays in studies of malaria transmission, especially those assessing the efficacy of transmission blocking interventions.
Results
Oocyst intensity affects Plasmodium DNA content
We optimized a quantitative PCR (qPCR) assay that detects Plasmodium mitochondrial cytochrome-b (Cyt-b) gene in DNA extracted from as few as 50 merozoites spiked into A. coluzzii midgut homogenates (Fig. S1). The qPCR amplification-curves were distinct from background noise in uninfected midguts (Fig. S2). Using this assay, we found that PbCyt-b abundance (used as proxy for oocyst growth) in the midgut of mosquitoes infected 7 days earlier with P. berghei was positively and significantly (P < 0.0001) correlated with oocyst intensity recorded by microscopic enumeration prior to midgut homogenization (Fig. 1a). The results also showed that infection intensity does not influence PbCyt-b DNA abundance per capita oocyst (PCO; Fig. 1b,c). This pattern changes at later stages of development, as no correlation was detected between the PbCyt-b abundance and oocyst intensity at 14 days post infection (dpi; Fig. 1d). Interestingly, the oocyst intensity was negatively correlated (P = 0.001) with PbCyt-b abundance PCO (Fig. 1e). When midguts were grouped into two categories based on oocyst intensity, PbCyt-b abundance PCO was found to be markedly lower (P = 0.007) in those harbouring < 15 oocysts than those with ≥ 15 oocysts (Fig. 1f).
P. berghei Cyt-b DNA abundance is oocyst density dependent. (a) Correlation between oocyst density (log) and abundance of PbCyt-b mitochondrial DNA 7 dpi (r = 0.24; P < 0.0001). (b) Correlation between oocyst density (log) and PbCyt-b DNA abundance PCO 7 dpi. (c) PbCyt-b DNA abundance PCO in low and high oocyst density midguts 7 dpi. (d) Relationship between oocyst intensity (log) and PbCyt-b DNA abundance 14 dpi. (e) Correlation between oocyst density (log) and PbCyt-b DNA abundance PCO 14 dpi (r = -0.25; P = 0.001). (f) PbCyt-b DNA abundance PCO in low and high oocyst density midguts 14 dpi. In scatterplots, each dot represents a single midgut and lines show linear regression fit. Horizontal lines in boxplots indicate median. Data were generated from at least two independent experiments.
The impact of oocyst intensity on the abundance of parasite DNA PCO was further examined in mosquitoes where the immune factor LRIM1 was silenced with dsRNA injection 3 days prior to P. berghei infection. These mosquitoes harboured over 10 times more oocysts than control dsLacZ injected mosquitoes (Fig. S3). At 14 dpi, LRIM1-silenced mosquitoes yielded oocysts of significantly reduced (P < 0.0001) diameter compared to controls (Fig. 2a). A significantly decreased abundance of PbCyt-b PCO in LRIM1-silenced mosquitoes compared to controls was observed both pre-sporulation (7 dpi; P < 0.0001) and sporulation (14 dpi; P < 0.003) stages (Fig. 2b,c). These data suggested that high infection intensity negatively affects oocyst growth. Therefore, with standard post-infection mosquito husbandry, where mosquitoes obtain a single bloodmeal at the time of infection and are then maintained on sugar throughout the course of the infection, nutrient starvation may have detrimental impacts on the developing oocyst.
Effect of crowding on P. berghei oocyst growth. (a) Oocyst diameter (dia.) in LRIM1 and LacZ dsRNA injected mosquitoes. Data were generated from two independent biological replicates. (b, c) PbCyt-b DNA abundance in LRIM1 and LacZ dsRNA injected mosquitoes 7 (b) and 14 (c) dpi. Data were generated from three independent replicates. Horizontal lines in dot plots and boxplots indicate median.
Supplemental bloodmeals accelerate oocyst growth
To assess the impact of post-infection mosquito blood feeding on oocyst growth, parasite DNA abundance was compared between P. berghei oocysts in mosquitoes maintained under standard husbandry regime and mosquitoes provided a supplemental, uninfected bloodmeal 7 dpi. The results revealed that the supplemental bloodmeal significantly increased (P = 0.029) the oocyst size compared to control (Fig. 3a,b). In these mosquitoes, oocysts contained on average significantly more DNA (P = 0.003) than oocysts in control mosquitoes (Fig. 3c). The supplemental bloodmeal significantly increased (P < 0.0001) the correlation between P. berghei oocyst intensity and DNA abundance per midgut (Fig. 3d). It also abolished the negative correlation between oocyst intensity and abundance of parasite DNA PCO (Fig. 3e), which was observed in mosquitoes under standard husbandry regime (see Fig. 1e), revealing that oocyst growth ensues when mosquitoes receive a supplemental bloodmeal even when infection intensities are high.
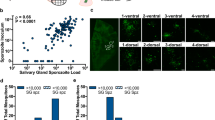
Effect of supplemental bloodmeals on oocyst growth. (a) P. berghei oocyst diameter (dia.) in mosquitoes provided no supplemental bloodmeal after the time of infection (non-blood-fed; NBF) or an additional supplemental bloodmeal 7 dpi (BF7) measured 14 dpi. (b) Representative microscopic pictures of P. berghei oocysts in NBF and BF7 mosquitoes taken 14 dpi. (c) PbCyt-b DNA abundance in NBF and BF7 mosquitoes 14 dpi. (d) Correlation between P. berghei oocyst density (log) and PbCyt-b DNA abundance in the midgut of NBF and BF7 mosquitoes (r = 0.35, P < 0.0001). (e) Correlation between P. berghei oocyst density (log) and PbCyt-b DNA abundance PCO. (f, g) Representative microscopic pictures and diameter of P. berghei oocysts on midguts of mosquitoes silenced for LRIM1 and LRIM1 + Lp and maintained under a standard husbandry regime (NBF) or provided a supplemental bloodmeal 7 dpi (BF7). For oocyst diameter, oocysts were randomly selected from 20 midguts from two independent infection experiments. (h) P. falciparum oocyst diameter (dia.) in mosquitoes provided no supplemental bloodmeal after the time of infection (NBF) or an additional supplemental bloodmeal 3 dpi (BF3) measured 9 dpi. (i) Representative microscopic pictures of P. falciparum oocysts in NBF and BF3. (j) PfCyt-b DNA abundance in NBF and BF3 mosquitoes. Horizontal lines in dot plots and boxplots indicate median. Data were generated from at least two independent experiments.
We further investigated the effect of mosquito starvation on oocyst growth by augmenting oocyst numbers through silencing LRIM1 either alone or in combination with Lp, thus creating extreme crowding and starvation conditions4. The results revealed a remarkable variation of P. berghei oocyst size in midguts of both LRIM1 and LRIM1 + Lp silenced mosquitoes maintained under a standard husbandry regime, particularly in the latter mosquito cohort (Fig. 3f). This phenotype was reversed when mosquitoes were provided a supplemental bloodmeal 7 dpi, as shown by a significant increase of median oocyst size in both LRIM1 (P < 0.0001) and LRIM1 + Lp (P < 0.0001) silenced mosquitoes (Fig. 3f,g).
Analogous and indeed more pronounced differences were recorded for P. falciparum oocysts in mosquitoes that received a supplemental bloodmeal at day-3 pi compared to control mosquitoes that received only a single, infected bloodmeal (Fig. 3h–j). The supplemental bloodmeal was provided at day-3 pi due to the faster bloodmeal digestion and oocyst development in these mosquitoes which are maintained at 27 °C instead of 21 °C, while dissection was performed at day-9 pi.
Oocysts enter a dormancy-like state under starvation conditions
Female mosquitoes naturally lay their eggs before taking another bloodmeal to replenish energy reserves and embark on a new gonotrophic cycle. Follicular resorption, mediated by juvenile hormone, is employed to increase energy resources in case of nutrient limitation or when oviposition is not possible17, as is the case of most experimental infection protocols. This process is presumed to also provide nutrient supplies to the developing oocyst8,18. We reproduced the natural infection conditions by allowing mosquitoes infected with P. falciparum to lay their eggs at day 2 pi, before they were provided supplemental bloodmeals at either day 3 or day 6 pi. The results showed a dramatic difference reflected by significantly decreased oocyst sizes recorded at day 9 pi in mosquitoes deprived of either of supplemental bloodmeals (both P < 0.0001; Fig. 4a,b). This finding was consistent with lower parasite DNA abundance PCO in bloodmeal-deprived mosquitoes compared to those provided a supplemental bloodmeal at day 3 (P < 0.005) or 6 (P < 0.002) pi (Fig. 4c). No significant differences in oocyst size or parasite DNA abundance PCO were recorded between mosquitoes provided supplemental bloodmeals 3 or 6 dpi, suggesting that oocyst growth can quickly recover after a bloodmeal.
Effect of supplemental bloodmeal deprivation on oocyst development. (a) P. falciparum oocyst diameter (dia.) in mosquitoes provided no supplemental bloodmeal after the time of infection (non-blood-fed; NBF) or additional supplemental bloodmeals at 3 (BF3) or 6 (BF6) dpi measured 9 dpi. (b) Representative microscopic pictures of P. falciparum oocysts in NBF, BF3 and BF6 mosquitoes taken 9 dpi. (c) PfCyt-b DNA abundance in NBF, BF3 and BF6 mosquitoes 9 dpi. (d) P. falciparum oocyst diameter (dia.) in mosquitoes provided no supplemental bloodmeal after the time of infection (NBF) or additional supplemental bloodmeals 9 dpi (BF9) measured 13 dpi. (e) Representative microscopic pictures of oocysts in NBF and BF9 mosquitoes taken 13 dpi. (f) Oocyst size distribution fitting the Poisson distribution model in NBF and BF9 mosquitoes 13 dpi. Solid vertical lines show median and dotted vertical lines show mean. (g) Sporozoite load in NBF, BF3, BF6 and BF9 mosquitoes at 14 dpi. (h) Relative expression levels of P. falciparum pre-tRNATyr in NBF and BF3 mosquitoes measured 7 dpi. (i) Relative expression levels of P. berghei pre-tRNATyr in LRIM1 and LRIM1 + Lp silenced mosquitoes in NBF and BF7 mosquitoes at 14 dpi. Horizontal lines in dot plots and boxplots indicate median. Data were generated from at least two independent biological replicates.
Oocysts retained their propensity to grow even when a supplemental bloodmeal was provided as late as 9 dpi (P < 0.0001; Fig. 4d,e). In this experiment, midgut dissections and oocyst observations were carried out 13 dpi. Interestingly, the distribution of oocyst sizes in mosquitoes deprived of supplemental bloodmeal was positive-skewed (Fig. 4f), and several oocysts in these mosquitoes were as big as the biggest oocysts in mosquitoes provided a supplemental bloodmeal 9 dpi. This implies that, under limiting nutrient conditions, the oocyst population employs an effective resource management strategy whereby some oocysts continue to grow but most remain stunted. Furthermore, the distribution of oocyst sizes in mosquitoes receiving a supplemental bloodmeal 9 dpi was negative-skewed, and several oocysts were as small as the smallest oocysts in mosquitoes deprived of supplemental bloodmeals, suggesting that not all oocysts effectively exit this dormancy-like state following a long starvation period.
We investigated the abundance of P. falciparum sporozoites in salivary glands 14 dpi by quantifying PfCyt-b in the mosquito thorax and head regions (Fig. 4g). The results revealed that deprivation of supplemental bloodmeals combined with oviposition at day 2 pi result in significantly lower levels of sporozoites compared to mosquitoes provided supplemental bloodmeals 3 (P = 0.009) or 6 (P = 0.019) dpi. Nevertheless, most of these mosquitoes (70%) did harbour detectable sporozoite loads in their salivary glands, like those provided supplemental bloodmeals (81% at 3 dpi and 86% at 6 dpi), indicating that some oocysts, presumably the fully-grown ones, were fully differentiated and produced sporozoites. Despite having much larger oocysts, mosquitoes provided a supplemental bloodmeal 9 dpi, showed equal sporozoite loads compared to those deprived of supplemental blood meals, suggesting that more time was needed for full oocyst differentiation.
Similar results were obtained by direct enumeration of sporozoites found in the salivary glands of mosquitoes provided with compared to those deprived of a supplemental bloodmeal 3 dpi (Fig. S4). The former group of mosquitoes showed significantly higher salivary gland sporozoite loads compared to the latter group (P = 0.0003). However, a large fraction of the supplemental bloodmeal-deprived mosquitoes did harbor sporozoites in their salivary glands, supporting our previous conclusion that some oocysts fully differentiate and produced sporozoite despite the nutrient scarcity.
The decelerated oocyst growth in nutrient limiting conditions suggested that oocysts might employ a dormancy-like strategy, i.e., slowing down of their metabolic activity, to overcome starvation. To examine this, parasite pre-tRNATyr abundance was quantified as a proxy for Pol III activity in oocysts. Measuring immature instead of mature tRNATyr served to overcome the long half-life of mature tRNA that might affect real-time quantification of gene expression13. Pre-tRNATyr expression was calibrated to parasite DNA abundance PCO. The results revealed that the levels of P. falciparum pre-tRNATyr expression levels in oocysts 7 dpi were significantly lower (P = 0.008) in mosquitoes that received a single bloodmeal at the time of infection compared to those that additionally received a supplemental bloodmeal 3 dpi (Fig. 4h). Similarly, P. berghei tRNATyr expression in LRIM1 (P < 0.0001) and LRIM1 + Lp silenced (P < 0.0001) conditions measured in oocysts 14 dpi were significantly lower in mosquitoes under standard husbandry regimes compared to those that received a supplemental bloodmeal 7 dpi (Fig. 4i). These data together suggest that oocysts enter a slowed transcriptional and metabolic state in the mosquito vector when faced with nutrient depletion caused by bloodmeal deprivation, high infection intensities or disruption of nutrient shuttling.
Discussion
Plasmodium spends a large part of its life as an oocyst within the Anopheles mosquito, where it faces a variety of challenges that can adversely affect its growth, differentiation and transmission potential19. A key challenge is the high nutritional demand to produce thousands of sporozoites. We demonstrate that under starvation conditions, when an oocyst-harbouring mosquito is deprived of supplemental bloodmeals, infection intensity plays a major role in oocyst growth, as measured by both DNA content and oocyst size. Mosquitoes harbouring many oocysts, a condition that can occur naturally in field infections20 and invariably in experimental infections and which can be further elevated by inactivating the mosquito immune response, exhibit substantial variability in oocyst growth. In such conditions of crowding and nutritional stress, expression of parasite pre-tRNATyr is significantly reduced, indicating that the oocyst enters a dormancy-like state of slowed metabolic activity. One of our most interesting findings is that, under such limiting nutrient conditions, the parasite population within the mosquito appears to employ a resource management strategy allowing a small number of oocysts grow by keeping the majority of them dormant. When faced with resource stress, Plasmodium is indeed thought to induce growth restriction mediated by extracellular vesicles, limiting growth to some parasite cells and ensuring transmission21.
Our findings, together with those from previous works, can bear important implications not only for understanding the mosquito-parasite interactions underpinning malaria transmission but also in evaluating the efficacy of transmission blocking interventions. The current gold standard in malaria transmission studies is microscopic enumeration of oocysts present in the mosquito midgut. It has been previously shown that the number of oocysts correlates well with the number of gametocytes ingested by the mosquito and can therefore be used as a measure of the efficacy of interventions acting on gametocytes, gametes and/or ookinetes22,23,24. However, this measurement does not consider any knock-on or additional impacts of the intervention on the oocyst. Although this additional impact is unlikely to reverse any detrimental effect on previous developmental stages, our data suggest that reduction in oocyst numbers may translate to a lesser or no impact on the sporozoite load, especially when the effect is small and oocyst competition for nutrients is reduced. Thus, a reduction in oocyst intensity alone is an insufficient measurement and may not accurately reflect the efficacy of a transmission blocking intervention. At the same time, interventions having a significant impact on high oocyst intensity in the laboratory setting may have insignificant effect in the field where the oocyst intensity is generally low.
At high intra-population densities, endoparasites are known to evoke competition for nutrients when these are of limited supply25. This phenomenon is ubiquitous in nature and often leads to negative density‐dependent processes with significant effects on parasite fitness such as proliferation and ability to avoid host immune defences26. The density-dependent effect on Plasmodium sporogony reported here is not related to mosquito immune responses known to reduce the number of oocysts but to the rate of oocyst growth through endoreplication. The effect is indeed exacerbated upon silencing the mosquito complement-like system due to the significant increase of the number of ookinetes that successfully develop to oocysts.
The negative density‐dependent effect on the oocyst is exacerbated in standard laboratory infections where infected mosquitoes are often not provided supplemental bloodmeals, leading to acute depletion of nutrient reserves4,5,6,7,27. In nature, a female mosquito could acquire a bloodmeal every 2–3 days28,29, which helps it meet the high energy and nutrient demand for flight and reproduction. These additional bloodmeals are thought to also be important for the proliferating oocyst18. Indeed, early studies have revealed intense competition for nutrients between the reproductive mosquito and the developing parasite by demonstrating that oocyst infection can lead to significant reduction in mosquito fecundity30. However, the relationship between parasite infection and mosquito fecundity is not as clear cut as it may seem with other studies suggesting a positive relationship between these two parameters31.
Plasmodium is auxotrophic for most amino acids; of these, Isoleucine exclusively derives from bloodmeal digestion as neither the parasite nor the mosquito can synthesize it de novo10,32,33. Bloodmeal-derived fatty acids are also critical for mosquito fitness and reproduction, chiefly derived from haemoglobin metabolism in the gut cells and distributed across mosquito tissues by Lp. Silencing the Lp gene abolishes egg development and compromises growth of both P. berghei and P. falciparum oocysts4,16; albeit studies showing that mature late-stage P. falciparum oocysts synthesize lipids de novo via the type two fatty acid biosynthesis pathway (FAS-II), in contrast to P. berghei and asexual stages that scavenge fatty acids exclusively from the host34. We show that silencing Lp together with the complement-like immune pathway exacerbates the negative effects of crowding on oocyst growth but cannot further reduce pre-tRNATyr expression, indicating that the slowed metabolic state is likely to be regulated by amino acid sensing12.
Previous studies have shown that oocyst intensity is negatively correlated with salivary gland sporozoite load and that a supplemental bloodmeal both synchronizes sporozoite maturation and increases the salivary gland sporozoite load, suggesting that supplemental bloodmeals serve to counteract the inter-oocyst competition for nutrients within the mosquito28,35,36. A recent work using the rodent parasite Plasmodium yoelii has revealed a clear yet non-linear correlation between transmission success and salivary gland sporozoite load37, suggesting that the increase of sporozoite load upon supplemental bloodmeal can significantly enhance transmission.
Thus, better understanding of the oocyst response to crowding and nutritional stress could assist development of novel interventions to limit malaria transmission. For example, a temporally tuned targeting of pathways involved in mosquito amino acid or lipid metabolism using genetic engineering could prolong completion of the parasite extrinsic incubation period (EIP), delaying oocyst growth and differentiation and sporozoite release. In nature, only a small proportion of mosquitoes can survive through the P. falciparum EIP38,39; hence, even a small delay in oocyst growth and differentiation could have a drastic negative impact on malaria transmission. Indeed, a recent study has revealed accelerated salivary gland invasion by P. falciparum sporozoites by over two days in mosquitoes that received a supplemental bloodmeal 3 dpi40. This suggests that the infective age of mosquitoes in nature may be smaller than previously predicted and that depriving infected mosquitoes from supplemental bloodmeals through various interventions including genetic engineering could have huge implications on malaria transmission. Although the frequency with which infected A. gambiae mosquitoes take bloodmeals in the field vary with the ambient temperature, volume of preceding bloodmeal and other factors, one could hypothesize that multiple supplemental bloodmeals compared to the one examined here may have an even greater impact on the parasite EIP.
Importantly, our findings in conjunction with those of earlier studies highlight a major drawback of typical laboratory infection protocols, which can lead to inaccurate conclusions when evaluating the transmission-blocking efficacy of an intervention in the laboratory setting. First, an intervention that inhibits or delays oocyst growth and/or differentiation without or by weakly affecting oocyst counts may be rejected owing to a decision merely based on reduction of oocyst numbers. This pertains to interventions targeting both mosquito or parasite molecules and pathways, e.g. the mosquito Lp as described earlier16,41 and the parasite misfit gene of which the knockout produces oocysts arrested at early mitotic stages but continuing to grow in size42. Second, inducing a dormancy-like state, a universally resilient life stage, may make oocysts resistant to transmission blocking interventions. Third, the current standard protocol may lead to false positive conclusions in favour of an intervention reducing oocyst numbers whilst it has no real impact on malaria transmission owing to the density-dependent effects described here. In other words, oocyst enumeration may conceal intrinsic or extrinsic variations in individual oocysts or oocyst populations to produce and release sporozoites, and hence cannot be used as a direct proxy for transmission43. We conclude that the provision of supplemental bloodmeals to mitigate any parasite density-dependent effects on oocyst growth and the assessment of the actual parasite load in the oocyst that escape the intervention using a molecular diagnostic method are profoundly important. Nonetheless, a decision on whether an intervention can be adopted must be based on assays with epidemiological endpoints applied at the population level such as the multicycle transmission assay developed for interventions targeting rodent malaria transmission44.
Methods
Ethics statement
Animal procedures were reviewed and approved by the Imperial College Animal Welfare and Ethical Review Body and carried out in accordance with the Animal Scientifics Procedures Act 1986 under the United Kingdom Home Office license PPL70/8788. Human red blood cells provided by the National Blood Service of the United Kingdom National Health Service were obtained from healthy donors upon taking a written informed consent.
Mosquito infections
A. coluzzii of the N’gousso strain were reared following standard protocols and infected with P. berghei ANKA507m6cl1 that constitutively expresses GFP45 by direct feeding on infected mice or with P. falciparum gametocytes from a laboratory culture (NF54 strain) using standard membrane feeding. Engorged mosquitoes were provided 10% sucrose and maintained at 21 °C for P. berghei infections and 27 °C for P. falciparum infections until midgut dissection. Supplemental bloodmeals on human blood was provided via membrane feeding.
Midgut preparations and oocyst enumeration
Gut dissections were performed in ice-chilled PBS. For P. berghei infection, guts were mounted on glass slides and oocysts were counted with fluorescence microscopy. For P. falciparum infection, guts were stained with 0.5% Mercurochrome for 10 min on ice and washed twice in ice chilled PBS for 30 min, and oocysts were enumerated using light microscopy. After oocyst enumeration, the foregut and hindgut were removed, and midguts were individually transferred to 1.5 ml screw-top tubes with 350 µl RLT Plus buffer from Qiagen AllPrep DNA/RNA Mini Kit and kept at -20 °C until DNA and RNA extraction.
Sporozoite enumeration
Salivary glands were dissected from P. falciparum infected mosquitoes 14 dpi on ice-cold PBS. The glands were transferred to Eppendorf tubes with 200 µl PBS and loaded to the fast-read 102® system (Biosigma SPA, Italy) after homogenising. Sporozoites in four different squares were counted under the microscope (40x) and total sporozoite loads for each mosquito was calculated. Three biological replicates were considered.
Genomic DNA and RNA extraction
Midguts were homogenized using PRECELLYS® 24 (Bertin Technologies) before simultaneous extraction of genomic DNA and total RNA using the AllPrep DNA/RNA Mini Kit. DNA and RNA concentrations were determined using Nanodrop (Thermo Scientific), and samples were kept at 20 °C until cDNA synthesis and qPCR analysis. To measure the abundance of P. falciparum sporozoites in salivary glands, mosquitoes were killed with 75% ethanol and placed on dry ice and their abdomens were removed. Genomic DNA was extracted from the remaining thorax and head regions of each mosquito using Qiagen DNeasy 96 Blood and Tissue kit.
Quantitative PCR analysis
SYBR Green based qPCR was used to quantify Plasmodium Cyt-b mitochondrial DNA. Assay sensitivity and specificity were determined with serial dilutions of P. falciparum merozoites from a synchronized asexual culture after heat inactivation, quantified and collected using a BD FACSAria III cell sorter. QPCR reactions were performed in 20 µl volumes according to the manufacturer’s instructions and previously described primers for P. berghei46 and P. falciparum47. Pre-tRNATyr expression levels were determined with the Qiagen Quantinova SYBR Green PCR kit using the stem-loop qPCR technique and qPCR primers described previously13.
DNA and RNA abundances were measured using the standard curve-based qPCR method. Curves for each target gene and the reference gene (A. gambiae S7 ribosomal protein gene48) were constructed after serial dilution of nucleic acid templates. Ct-values were standardized using their respective standard curves, and the target gene Ct value was normalized to that of the reference gene. Pre-tRNATyr PCO was further normalized to Cyt-b abundance.
Data analysis
GraphPad Prism v.8.4.0 software package was used for data analysis and presentation. Spearman’s rank-order correlation analysis was used to measure the relationship between oocyst intensity (log10 transformed) and relative DNA abundance per single midgut or oocyst. All other statistical analyses were performed using the Kolmogorov–Smirnov test.
References
Gantz, V. M. et al. Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc. Natl. Acad. Sci. USA 112, E6736–E6743. https://doi.org/10.1073/pnas.1521077112 (2015).
Sinden, R. E. Developing transmission-blocking strategies for malaria control. PLoS Pathog 13, e1006336–e1006336. https://doi.org/10.1371/journal.ppat.1006336 (2017).
Churcher, T. S., Blagborough, A. M. & Sinden, R. E. Measuring the blockade of malaria transmission: analyzing the results of mosquito feeding assays. Malaria J. 11, P18 (2012).
Costa, G. et al. Non-competitive resource exploitation within mosquito shapes within-host malaria infectivity and virulence. Nat. Commun. 9, 3474. https://doi.org/10.1038/s41467-018-05893-z (2018).
Ferguson, H. M. & Read, A. F. Genetic and environmental determinants of malaria parasite virulence in mosquitoes. Proc. Biol. Sci. 269, 1217–1224. https://doi.org/10.1098/rspb.2002.2023 (2002).
Maier, W. A., Becker-Feldman, H. & Seitz, H. M. Pathology of malaria-infected mosquitoes. Parasitol. Today 3, 216–218. https://doi.org/10.1016/0169-4758(87)90063-9 (1987).
Takken, W. et al. Larval nutrition differentially affects adult fitness and Plasmodium development in the malaria vectors Anopheles gambiae and Anopheles stephensi. Parasites Vectors 6, 345 (2013).
Pollitt, L. C. et al. Costs of crowding for the transmission of malaria parasites. Evolut. Appl. 6, 617–629. https://doi.org/10.1111/eva.12048 (2013).
Rivera-Pérez, C., Clifton, M. E. & Noriega, F. G. How micronutrients influence the physiology of mosquitoes. Curr. Opin. Insect. Sci. 23, 112–117. https://doi.org/10.1016/j.cois.2017.07.002 (2017).
Divo, A. A., Geary, T. G., Davis, N. L. & Jensen, J. B. Nutritional requirements of Plasmodium falciparum in culture: I—exogenously supplied dialyzable components necessary for continuous growth. J. Protozool. 32, 59–64. https://doi.org/10.1111/j.1550-7408.1985.tb03013.x (1985).
Babbitt, S. E. et al. Plasmodium falciparum responds to amino acid starvation by entering into a hibernatory state. Proc. Natl. Acad. Sci. USA 109, E3278–E3287 (2012).
Mancio-Silva, L. et al. Nutrient sensing modulates malaria parasite virulence. Nature 547, 213. https://doi.org/10.1038/nature23009 (2017).
McLean, K. J. & Jacobs-Lorena, M. Plasmodium falciparum Maf1 confers survival upon amino acid starvation. MBio 8, e02317–e2316 (2017).
Boguta, M. Maf1, a general negative regulator of RNA polymerase III in yeast. J. Biochim. Biophys. Acta Gene Regul. Mech. 1829, 376–384 (2013).
Moir, R. D. & Willis, I. Regulation of pol III transcription by nutrient and stress signaling pathways. J. Biochim. Biophys. Acta Gene Regul. Mech. 1829, 361–375 (2013).
Mendes, A. M. et al. Conserved mosquito/parasite interactions affect development of Plasmodium falciparum in Africa. PLoS Pathog 4, e1000069. https://doi.org/10.1371/journal.ppat.1000069 (2008).
Clifton, M. E. & Noriega, F. G. Nutrient limitation results in juvenile hormone-mediated resorption of previtellogenic ovarian follicles in mosquitoes. J. Insect. Physiol. 57, 1274–1281. https://doi.org/10.1016/j.jinsphys.2011.06.002 (2011).
Hurd, H., Hogg, J. C. & Renshaw, M. Interactions between bloodfeeding, fecundity and infection in mosquitoes. Parasitol. Today 11, 411–416 (1995).
Gamage-Mendis, A. C. et al. Infectivity of Plasmodium vivax and P. falciparum to Anopheles tessellatus; relationship between oocyst and sporozoite development. Trans. R. Soc. Trop. Med. Hygiene 87, 3–6. https://doi.org/10.1016/0035-9203(93)90396-8 (1993).
Bompard, A. et al. High Plasmodium infection intensity in naturally infected malaria vectors in Africa. Int. J. Parasitol. 50, 985–996. https://doi.org/10.1016/j.ijpara.2020.05.012 (2020).
Mantel, P. Y. & Marti, M. The role of extracellular vesicles in Plasmodium and other protozoan parasites. Cell Microbiol. 16, 344–354. https://doi.org/10.1111/cmi.12259 (2014).
Churcher, T. S. et al. Measuring the blockade of malaria transmission—an analysis of the Standard Membrane Feeding Assay. Int. J. Parasitol. 42, 1037–1044. https://doi.org/10.1016/j.ijpara.2012.09.002 (2012).
Da, D. F. et al. Experimental study of the relationship between Plasmodium gametocyte density and infection success in mosquitoes; implications for the evaluation of malaria transmission-reducing interventions. Exp. Parasitol. 149, 74–83. https://doi.org/10.1016/j.exppara.2014.12.010 (2015).
Stone, W. J. R. et al. The relevance and applicability of oocyst prevalence as a read-out for mosquito feeding assays. Sci. Rep. 3, 3418. https://doi.org/10.1038/srep03418 (2013).
Anderson, R. The regulation of host population growth by parasitic species. Parasitology 76, 119–157 (1978).
Churcher, T. S., Filipe, J. A. & Basanez, M. G. Density dependence and the control of helminth parasites. J. Anim. Ecol. 75, 1313–1320 (2006).
Nyasembe, V. O. et al. Plasmodium falciparum infection increases Anopheles gambiae attraction to nectar sources and sugar uptake. Curr. Biol. 24, 217–221 (2014).
Ponnudurai, T. et al. Sporozoite load of mosquitoes infected with Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hygiene 83, 67–70 (1989).
Beier, J. C. Malaria parasite development in mosquitoes. Annu. Rev. Entomol. 43, 519–543 (1998).
Hogg, J. C. & Hurd, H. The effects of natural Plasmodium falciparum infection on the fecundity and mortality of Anopheles gambiae s. l. in north east Tanzania. Parasitology 114 ( Pt 4), 325–331. https://doi.org/10.1017/s0031182096008542 (1997).
Werling, K. et al. Steroid hormone function controls non-competitive plasmodium development in anopheles. Cell 177(315), e314–e325. https://doi.org/10.1016/j.cell.2019.02.036 (2019).
Liu, J., Istvan, E. S., Gluzman, I. Y., Gross, J. & Goldberg, D. Plasmodium falciparum ensures its amino acid supply with multiple acquisition pathways and redundant proteolytic enzyme systems. Proc. Natl. Acad. Sci. 103, 8840–8845 (2006).
Mack, S. R., Samuels, S. & Vanderberg, J. P. Hemolymph of Anopheles stephensi from uninfected and Plasmodium berghei-infected mosquitoes. 2. Free amino acids. J. Parasitol. 65, 130–136 (1979).
van Schaijk, B. C. et al. Type II fatty acid biosynthesis is essential for Plasmodium falciparum sporozoite development in the midgut of Anopheles mosquitoes. Eukaryot Cell 13, 550–559. https://doi.org/10.1128/EC.00264-13 (2014).
Sinden, R. E. et al. Progression of Plasmodium berghei through Anopheles stephensi is density-dependent. PLoS Pathog 3, e195. https://doi.org/10.1371/journal.ppat.0030195 (2007).
Bell, A. S. & Ranford-Cartwright, L. C. A real-time PCR assay for quantifying Plasmodium falciparum infections in the mosquito vector. Int. J. Parasitol. 34, 795–802. https://doi.org/10.1016/j.ijpara.2004.03.008 (2004).
Aleshnick, M., Ganusov, V. V., Nasir, G., Yenokyan, G. & Sinnis, P. Experimental determination of the force of malaria infection reveals a non-linear relationship to mosquito sporozoite loads. PLoS Pathog 16, e1008181. https://doi.org/10.1371/journal.ppat.1008181 (2020).
Charlwood, J. et al. Survival and infection probabilities of anthropophagic anophelines from an area of high prevalence of Plasmodium falciparum in humans. Bull. Entomol. Res. 87, 445–453 (1997).
Ryan, S. J., Ben-Horin, T. & Johnson, L. R. Malaria control and senescence: the importance of accounting for the pace and shape of aging in wild mosquitoes. Ecosphere 6, 1–13 (2015).
Shaw, W. R. et al. Multiple blood feeding in mosquitoes shortens the Plasmodium falciparum incubation period and increases malaria transmission potential. bioRxiv. https://doi.org/10.1101/2020.03.24.991356 (2020).
Rono, M. K., Whitten, M. M., Oulad-Abdelghani, M., Levashina, E. A. & Marois, E. The major yolk protein vitellogenin interferes with the anti-plasmodium response in the malaria mosquito Anopheles gambiae. PLoS Biol 8, e1000434. https://doi.org/10.1371/journal.pbio.1000434 (2010).
Bushell, E. S. et al. Paternal effect of the nuclear formin-like protein MISFIT on Plasmodium development in the mosquito vector. PLoS Pathog 5, e1000539. https://doi.org/10.1371/journal.ppat.1000539 (2009).
Medley, G. F. et al. Heterogeneity in patterns of malarial oocyst infections in the mosquito vector. Parasitology 106, 441–449. https://doi.org/10.1017/s0031182000076721 (1993).
Blagborough, A. M. et al. Transmission-blocking interventions eliminate malaria from laboratory populations. Nat. Commun. 4, 1812. https://doi.org/10.1038/ncomms2840 (2013).
Janse, C. J. et al. High efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol. Biochem. Parasitol. 145, 60–70. https://doi.org/10.1016/j.molbiopara.2005.09.007 (2006).
Shimizu, S., Osada, Y., Kanazawa, T., Tanaka, Y. & Arai, M. J. M. j. Suppressive effect of azithromycin on Plasmodium berghei mosquito stage development and apicoplast replication. Malaria J. 9, 73 (2010).
Farrugia, C. et al. Cytochrome b gene quantitative PCR for diagnosing Plasmodium falciparum infection in travelers. J. Clin. Microbiol. 49, 2191–2195 (2011).
Meister, S. et al. Immune signaling pathways regulating bacterial and malaria parasite infection of the mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. USA 102, 11420–11425. https://doi.org/10.1073/pnas.0504950102 (2005).
Acknowledgements
We thank J. Srivastava and J.E. Rowley for assisting with parasite cell sorting and M.G. Inghilterra for gametocyte culturing. We are also grateful to R.E. Sinden for critically reviewing our manuscript. The work was funded by the Bill and Melinda Gates Foundation grant OPP1158151 to N.W. and G.K.C and the Wellcome Trust Investigator Award 107983/Z/15/Z to G.K.C.
Author information
Authors and Affiliations
Contributions
T.H. and G.K.C. contributed conceptualization; T.H., A.A.S., C.A.S.W. and E.K.G.M. contributed methodology; T.H. and A.A.S. conducted investigation; T.H. and G.K.C. performed formal analysis; T.H. and A.A.S. wrote original draft; T.H. and G.K.C. wrote the paper; T.H., N.W. and G.K.C. provided supervision; G.K.C. conducted project administration; N.W. and G.K.C. acquired funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Habtewold, T., Sharma, A.A., Wyer, C.A.S. et al. Plasmodium oocysts respond with dormancy to crowding and nutritional stress. Sci Rep 11, 3090 (2021). https://doi.org/10.1038/s41598-021-81574-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-81574-0
This article is cited by
-
Sugar and blood: the nutritional priorities of the dengue vector, Aedes aegypti
Parasites & Vectors (2024)
-
Plasmodium berghei oocysts possess fatty acid synthesis and scavenging routes
Scientific Reports (2023)
-
A non-destructive sugar-feeding assay for parasite detection and estimating the extrinsic incubation period of Plasmodium falciparum in individual mosquito vectors
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.