Abstract
Large-scale studies have not been conducted to assess whether serum hypobicarbonatemia increases the risk for kidney function deterioration and mortality among East-Asians. We aimed to determine the association between serum total CO2 (TCO2) concentrations measured at the first outpatient visit and clinical outcomes. In this multicenter cohort study, a total of 42,231 adult nephrology outpatients from 2001 to 2016 were included. End-stage renal disease (ESRD) patients on dialysis within 3 months of the first visit were excluded. Instrumental variable (IV) was used to define regions based on the proportion of patients with serum TCO2 < 22 mEq/L. The crude mortality rate was 12.2% during a median 77.0-month follow-up period. The Cox-proportional hazard regression model adjusted for initial kidney function, alkali supplementation, and the use of diuretics demonstrated that low TCO2 concentration was not associated with progression to ESRD, but significantly increased the risk of death. The IV analysis also confirmed a significant association between initial TCO2 concentration and mortality (HR 0.56; 95% CI 0.49–0.64). This result was consistently significant regardless of the underlying renal function. In conclusion, low TCO2 levels are significantly associated with mortality but not with progression to ESRD in patients with ambulatory care.
Similar content being viewed by others
Introduction
Serum hypobicarbonatemia was observed in about 20% of patients with chronic kidney disease (CKD) stages 4–5 in the NHANES cohort1 and in 26.0% and 47.4% of patients with CKD stages 4 and 5, respectively, in South Korea2,3. Previous epidemiologic cohort studies showed that CKD patients with higher serum bicarbonate levels had lower risks of developing end-stage renal disease (ESRD) in various populations4,5,6. Several observational studies involving patients with CKD found that lower total serum CO2 (TCO2) levels, higher net endogenous acid production7, and high dietary acid loads8,9 were associated with higher risks of progressive renal function deterioration among various ethnic groups7,8,9,10,11. Reaven et al. recently conducted a well-designed observational study which included the medical records of > 50,000 community-based patients with advanced CKD (stages 3a-5) obtained from a diverse geographically distributed database12. The longitudinal study analyzed the10-year data of this large US community-based population to assess the effects of hypobicarbonatemia on mortality and kidney outcomes. The authors found that death, ESRD with ongoing dialysis, and eGFR reduction of ≥ 40% were significantly higher among patients with hypobicarbonatemia compared to those with normal serum bicarbonate levels (P < 0.001 for all results, regardless of age group)12. The prevalence of early CKD is increasing in South Korea, and data on the role of asymptomatic metabolic acidosis in CKD progression is limited13,14,15.
Notably, it is difficult to evaluate acid-base status in an outpatient setting because of the difficulty associated with performing arterial blood gas analysis in this setting. In this study, we investigated the long-term clinical impact of hypobicarbonatemia on the development of incident ESRD and overall mortality in East-Asians, using the level of TCO2 on venous sampling. We attempted to determine the effects of hypobicarbonatemia in a retrospective cohort from the patients with various regions of South Korea, using instrumental variable (IV) analysis.
Methods
Study subjects
This was a retrospective observational cohort study of patients tracked in the outpatient nephrology clinic of two tertiary hospitals in South Korea (Seoul National University Hospital and Seoul National University Boramae Hospital). Hospital administrative data were extracted, and the 10th International Code of Disease Classification (ICD-10) was used. Data were collected using electronic health records following the methods also described in previous studies16. In detail, the date of birth, sex, age, body mass index (kg/m2), and data related to other comorbidities (using the diagnostic code by ICD-10) and medication were recorded.
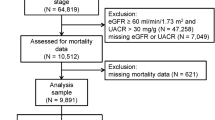
A total of 50,703 patients were examined from January 1, 2001, to December 31, 2016. Of the total 50,703 patients, we excluded 1,838 patients who had already been on dialysis with ESRD and 1,237 patients with acute kidney injury requiring renal replacement therapy who had been on dialysis within three months of hospital visit. Patients without record of serum creatinine levels or total CO2 (TCO2) (n = 5,298), patients with no address (n = 27), and those with extreme values of TCO2 (n = 72) were also excluded. The final analysis was performed including 42,231 patients (Fig. 1).
Data collection
The Low TCO2 group was defined as TCO2 < 22 mEq/L. Among the drugs used, medications potentially affecting the status of TCO2 level, data of patients who had a prescription of sodium bicarbonate, renin–angiotensin–aldosterone system (RAAS) blockades, including angiotensin-converting-enzyme inhibitors (ACE inhibitors, ACEi) and angiotensin II receptor blockers (ARBs), and diuretics were identified. Diuretics group included acetazolamide, spironolactone, amiloride, triamterene, furosemide, torasemide, thiazide, indapamide and metolazone.
We included only those patients who had undergone treatment for more than 30 days in the treatment group. The CKD grade was determined by calculating eGFR from creatinine level using Modification of Diet in Renal Disease (MDRD) GFR. The study was approved by the Seoul National University Boramae Medical Center Institutional Review Board (No. 10-2018-10/031), and requirement for consent was waived by virtue of the retrospective design. All clinical studies were conducted in accordance with the guidelines of the Declaration of Helsinki as amended in 2013.
Clinical outcomes
The primary outcome was to clarify the all-cause mortality according to the TCO2 level, and the secondary outcome was to find a new development of ESRD that required dialysis and progression to mortality from various causes. We obtained mortality information from the national statistics office17, and ESRD incidence was defined as follows: patients recently undergoing hemodialysis were extracted from the electronic medical records using the clinical data warehouse in our study centers. Patients who had started hemodialysis therapy were identified by the combination of operational definitions as follows: the occurrence of a new claim for the payment code of hemodialysis by screening ICD-10 codes, and vascular access operations; incident dialysis was also identified by the first date of when dialysis was suggested.
Statistical analysis
We used the chi-squared test for categorical variables and the Student’s t-test for continuous variables to compare the demographics. Categorical variables were reported as the percentage of all patients, and continuous variables as the mean ± standard deviation. The multivariate Cox proportional hazards model was used to calculate the hazard ratio (HR) with a 95% CI for all-cause mortality and renal outcomes. Kaplan–Meier curves and the multivariate Cox proportional hazard models were used to compare the outcomes between the Low TCO2 group and control groups. We included covariates, such as TCO2 values, age, sex, history of hypertension, diabetes mellitus, MDRD-GFR, and SB usage, in the final model using multivariate Cox regression analysis.
To minimize the bias of this retrospective observational study, we conducted an IV analysis18,19,20,21. The IV in this study was regional classification of proportion of patients with TCO2 < 22 mEq/L. We used the IV to independently associate the effects of low TCO2 level and clinical outcomes. All statistical analyses were performed using R version 3.5.022 (Comprehensive R Archive Network: http://cran.r-project.org) and SPSS version 22.0 (IBM Corp., Armonk, NY, USA). The maps in supplementary figures was generated that outlines of administrative districts were obtained from Statistical Geographic Information Service of Statistics Korea (URL, https://sgis.kostat.go.kr/jsp/english/index.jsp). Maps were produced with R in conjunction with the software packages ggmap, maptools, rgdal, abd ggplot223,24,25,26. In all analyses, P < 0.05 was considered statistically significant.
Results
Baseline characteristics and demographics
The baseline characteristics and demographics of the study population divided according to the CKD stage are shown in Table 1. The mean age of the study subjects was 54 years, and 48.9% were male. Hypertension and diabetes were found in 29.2% and 20.9% of patients, respectively. The mean eGFR was 70.5 mL/min/1.73 m2 and the TCO2 was 26.2 mEq/L. CKD stage 2 accounted for 44.8% of all patients, and about 10% of patients were in advanced stage with CKD stage 4–5. As CKD progressed, a decrease in TCO2 levels was observed, and the use of sodium bicarbonate, RAAS blockade, or diuretics was more frequent.
Meanwhile, the serum TCO2 level was < 22 mEq/L in 10.1% of all patients, and we graphically depicted the proportion of patients with a TCO2 < 22 mEq/L by the patient’s residential location (Supplementary Information Fig. S1). When we divided patients into four groups based on the proportion of serum TCO2 concentration < 22 mEq/L by residential area, we found that hypertension and smoking history were the highest in the 4th group (Supplementary Information Table S1). The first group comprised patients who were older, had a higher prevalence of diabetes mellitus, and had the best baseline renal function. The fourth group had the highest rate of low TCO2 level (P < 0.001).
Association between low TCO2 and patient mortality
During the median 77.0-month follow-up period, the crude mortality rate was 12.2%. According to the Kaplan–Meier curve (Fig. 2), patients with TCO2 < 22 mEq/L had a higher risk of mortality (HR 0.899; 95% CI 0.893–0.905; P < 0.001) than those in the other groups. The Cox-proportional hazard regression model adjusted for sex, age, hypertension, diabetes mellitus, and alkali supplementation showed that low TCO2 concentration increased the risk for mortality (model 3, Table 2). After adding of initial eGFR levels for adjustment, the association between low TCO2 concentration and mortality remained consistently significant (model 5). These results were consistently significant even when the use of RAAS blockades and diuretics were additionally adjusted (model 6). In the IV analysis, low TCO2 concentration also increased the mortality risk. When adjusted for baseline renal function, alkali supplementation, RAAS blockade, and diuretics, the mortality risk reduced by approximately 44% with each 1 mEq/L increase in serum TCO2 concentration (model 6; 95% CI 0.485–0.644; P < 0.001).
Next, the TCO2 level was set as a continuous variable and the correlation with the risk of death was visually shown (Fig. 3). As a result, it was confirmed that the lower the TCO2 level, the higher the risk of death (Fig. 3A). Even after adjusting for several variables including age, sex, hypertension, diabetes, baseline kidney function, usage of sodium bicarbonate, RAAS blockades and diuretics, a consistent pattern was shown (Fig. 3B).
Non-linear association between the TCO2 levels and the risk of patient death. (A) Univariate analysis (B) Multivariate analysis. These graphs were adjusted for TCO2 level (as continuous variable), age, sex, history of hypertension and diabetes, baseline kidney function (as continuous variable using MDRD-eGFR), usage of sodium bicarbonate, RAAS blockades and diuretics.
It was also verified that these results were consistent in Table 3, which was expressed as a categorical variable. Based on the serum TCO2 concentration, patients were divided into three groups as follows: low, < 22 mEq/L; normal, 22–29 mEq/L; high, ≥ 30 mEq/L. Compared to the normal group, mortality risk was significantly higher in patients with serum TCO2 concentration below 22 mEq/L; however, the risk decreased when TCO2 concentrations were above 30 mEq/L. These results were significant even after adjusting for other variables.
In the subgroup analysis by initial eGFR, mortality risk increased with lower serum TCO2 concentrations, regardless of initial GFR values (Table 4). These results were verified both in the Cox-proportional hazard regression model and on IV analysis. This also showed significant results when adjusting for underlying renal function, underlying disease, and medications taken, and the effect was more pronounced in patients with relatively good renal function with a baseline GFR of 60 mL/min/1.73 m2 or higher.
Association between low TCO2 and the risk of progression to ESRD
The risk of progression to ESRD steadily increased over time and occurred in 8.5% of patients during the follow-up period. Patients with TCO2 < 22 mEq/L showed higher risks of progression to ESRD (HR 0.844; 95% CI 0.838–0.850; P < 0.001) than the other groups in univariate analysis (Supplementary Information Fig. S2, Table S2). However, the protective effect was the lowest in final model 6, which was adjusted for baseline renal function and medications. These results are consistent with the IV analysis. After adjusting for baseline renal function and medications taken, there was no association between serum TCO2 concentration and progression to ESRD (model 6; HR 1.057, 95% CI 0.892–1.251, P = 0.5234).
The subgroup analysis of the relationship between the TCO2 level and ESRD progression risk by TCO2 level groups is also presented in Supplementary Information Fig. S3 and Table S3. In subgroup IV analysis by initial eGFR, serum TCO2 concentration did not affect ESRD progression, regardless of eGFR (Supplementary Information Table S4).
Discussion
This study is the first population-scale study to show that low TCO2 level affected clinical outcomes in an East-Asian population. The main objective of this study was to determine whether the single measurement of TCO2 level affected the clinical outcomes in patients with ambulatory care in out-patients clinic, whose diet patterns were thought to be less acidic than that of their Western counterparts. According to the Korea National Health and Nutrition Examination Survey data27,28, regional differences were noted in meat intake between urban and rural areas. These differences in animal-based acidic diet pattern were likely related to the rate of dietary acid loading, causing eubicarbonatic H+ retention without overt metabolic acidosis13,14,15. We used the fraction of low TCO2 levels (< 22 mEq/L) by the residence area of participants as the IV to determine the independent relationship between TCO2 level and clinical outcomes. To be selected as an IV, several important assumptions must be met19,20. First, the exposure values should have definite predictive associations with the causal inference for IV. This IV should not be directly related to the outcome to be predicted. The four groups in Supplementary Information Table S1 did not differ significantly in terms of renal function by eGFR or other baseline characteristics, but only in terms of the proportion of patients with low TCO2 level.
Final Cox regression models and IV analysis including covariates of initial eGFR and information on the use of medications such as SB, RAAS blockade, and diuretics, showed that higher TCO2 concentrations were protective in patients at risk of all-cause mortality, but not for preventing progression to ESRD (Tables 2 and Supplementary Information Table S2). Serum bicarbonate concentrations were not very low in most of the patients in this study; only 10% of the cohort (4,242/42,331) had a serum bicarbonate level of < 22 mEq/L. Our results are consistent with those of previous studies which showed that long-term prognosis improved in patients with preserved renal function when serum bicarbonate levels were at the upper limit of the normal range6. Notably, in this study, the authors noted that hypobicarbonatemia (identified by low TCO2 levels) may not indicate metabolic acidosis, and this is a possible limitation of the study. The identification of acid–base status based on TCO2 as bicarbonate level may have led to misclassification because low TCO2 might be due to chronic respiratory or mixed acid–base disorders. These misclassifications could be seen in patients with ESRD receiving hemodialysis29 and in patients with acute renal failure requiring continuous renal replacement therapy30. However, in patients with preserved renal function in an outpatient setting, TCO2 is considered effective for predicting serum bicarbonate level31. Thus, we believe that the low TCO2 group in this study mainly reflects hypobicarbonatemia, irrespective of pH status. A recent study by Hirai et al. showed that serum TCO2 correlated with bicarbonate concentration in Japanese patients with pre-dialysis CKD (sensitivity 91.7%, specificity 73.4%)32, supporting our results.
In terms of ESRD development, there was significance in model 3 without eGFR as a covariate; however, the protective effect of high TCO2 disappeared when baseline eGFR was considered (Supplementary Information Table S2, model 4 to 6). Recent studies on early CKD (CKD2-3a) patients reveal that H+ retention is first seen, even if low TCO2 is not present yet as eubicarbonatemia. This H+ retention itself affects CKD progression, which can be diagnosed by hypocitrauria13 and urine ammonium15. In addition, low TCO2 itself is a highly independent risk factor, which is more pronounced in early CKD and above (Table S3), and in advanced CKD (GFR 30 mL/min/1.73 m2 and below), the influence on the ESRD of various factors other than TCO2 level itself is much greater (e.g., vascular calcification, cardiovascular disease, and commodities). In the results of our study, the final Cox model and IV analysis were inconclusive in terms of ESRD incidence as there were limitations due to the retrospective study design, and heterogeneity of the operational definition of ESRD was also limitation. Another possible rationale for hypobicarbonatemia being associated only with mortality but not with ESRD incidence in our study involves systemic compensation capacity. A recent study in patients with CKD found that hypobicarbonatemia was involved in CKD progression only when pH reached acidemia (pH < 7.32)33. The acid–base status (using gas analysis of blood pH) on the clinical outcome of hypobicarbonatemia was not considered in existing studies4,6,11. According to researchers at the University of Osaka, in a study of 1,058 patients with CKD (eGFR < 60 mL/min), TCO2 level and pH were measured simultaneously through venous blood gas analysis (VBGA). This study showed that the strength of this association is significantly changed by acidemia. Hypobicarbonatemia is associated with an increased risk of ESRD only when pH is low (pH < 7.32) but not when pH is normal. This association suggests that the compensatory reservoir might be important in terms of CKD prognosis15. In our study, patients with CKD 1,2 (eGFR > 60 mL/min) were 67% of the total. These patients are likely to have a normal pH. Results from the Osaka researchers also found that 40% of the group with bicarbonate levels under 21.5 mEq/L did not present acidemia as a result of systemic compensation33. In addition, in a prospective cohort study of an elderly population without CKD, similar to our results, 60% of the group with low bicarbonate levels did not have acidemia34. This study showed a correlation between low bicarbonatemia levels and high mortality, regardless of pH, and the modification of the effects by pH did not affect this association, which supports our findings. These findings suggest that combining information on pH with bicarbonate levels, especially to improve renal outcome, helps identify patients at high risk of ESRD. In addition, needless alkali treatments could be reduced in non-acidic hypobicarbonatemic patients15, 33. Further studies may identify the need for clinicians to further improve non-acidicidc hypobicarbonatemic or eubicarbonatic H+ retension in early CKD patients for slowing CKD progression. Treatment and consequent improvement of acidosis in CKD patients are expected to improve the clinical outcomes; however, the optimal interventional methods and population remain inconclusive, and The cautions is needen for the replacement of alkali using SB which is associated with complications, such as deterioration of sodium retention, edema, and heart failure aggravation.
In conclusion, low TCO2 levels were significantly associated with mortality, but not with progression to ESRD in IV analysis using regional classification by proportion of low TCO2 as an IV. Further studies, for a prospective interventional study on a nation-wide scale for the effects of alkali therapy on CKD progression in patients with eubicarbonatemia with early CKD and explanations for possible biologic mechanisms, in Korean CKD patients are needed.
Data availability
All data associated with this study are available in the main text or the supplementary materials following reasonable request.
References
Eustace, J. A., Astor, B., Muntner, P. M., Ikizler, T. A. & Coresh, J. Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney Int. 65, 1031–1040 (2004).
Kim, H. J. et al. Metabolic acidosis is associated with pulse wave velocity in chronic kidney disease: results from the KNOW-CKD study. Sci. Rep. 9, 16139 (2019).
Oh, K. H. et al. The KNOW-CKD study: what we have learned about chronic kidney diseases. Kidney Res. Clin. Pract. 39, 121–135 (2020).
Shah, S. N., Abramowitz, M., Hostetter, T. H. & Melamed, M. L. Serum bicarbonate levels and the progression of kidney disease: a cohort study. Am. J. Kidney Dis. 54, 270–277 (2009).
Dobre, M., Rahman, M. & Hostetter, T. H. Current status of bicarbonate in CKD. J. Am. Soc. Nephrol. 26, 515–523 (2015).
Raphael, K. L., Wei, G., Baird, B. C., Greene, T. & Beddhu, S. Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney Int. 79, 356–362 (2011).
Scialla, J. J. et al. Net endogenous acid production is associated with a faster decline in GFR in African Americans. Kidney Int. 82, 106–112 (2012).
Rebholz, C. M. et al. Dietary acid load and incident chronic kidney disease: results from the ARIC study. Am. J. Nephrol. 42, 427–435 (2015).
Kanda, E., Ai, M., Kuriyama, R., Yoshida, M. & Shiigai, T. Dietary acid intake and kidney disease progression in the elderly. Am. J. Nephrol. 39, 145–152 (2014).
Banerjee, T. et al. High dietary acid load predicts esrd among adults with CKD. J. Am. Soc. Nephrol. 26, 1693–1700 (2015).
Menon, V. et al. Serum bicarbonate and long-term outcomes in CKD. Am. J. Kidney. Dis. 56, 907–914 (2010).
Reaven, N. L., Funk, S., Tangri, N. & Mathur, V. Adverse clinical outcomes in patients with chronic kidney disease and metabolic acidosis: a longitudinal analysis from electronic medical records of >50,000 patients. Am. J. Kidney Dis. 73, 722 (2019).
Goraya, N., Simoni, J., Sager, L. N., Madias, N. E. & Wesson, D. E. Urine citrate excretion as a marker of acid retention in patients with chronic kidney disease without overt metabolic acidosis. Kidney Int. 95, 1190–1196 (2019).
Goraya, N. et al. Urine citrate excretion identifies changes in acid retention as eGFR declines in patients with chronic kidney disease. Am. J. Physiol. Renal Physiol. 317, F502–F511 (2019).
Madias, N. E. Metabolic acidosis and CKD progression. Clin. J. Am. Soc. Nephrol. https://doi.org/10.2215/CJN.07990520 (2020).
Kang, M. W. et al. An independent validation of the kidney failure risk equation in an Asian population. Sci. Rep. 10, 12920 (2020).
Oh, H. J. et al. Exploring mortality rates for major causes of death in Korea. Open Public Health J. 12, 16–25 (2019).
Fu, R., Kim, S. J., de Oliveira, C. & Coyte, P. C. An instrumental variable approach confirms that the duration of pretransplant dialysis has a negative impact on the survival of kidney transplant recipients and quantifies the risk. Kidney Int. 96, 450–459 (2019).
Stel, V. S., Dekker, F. W., Zoccali, C. & Jager, K. J. Instrumental variable analysis. Nephrol. Dial. Transplant. 28, 1694–1699 (2013).
Baiocchi, M., Chengb, J. & Smallc, D. S. Tutorial in biostatistics: instrumental variable methods for causal inference. Stat. Med. 33, 2297–2340 (2014).
Butala, N. M., Reese, P. P., Doshi, M. D. & Parikh, C. R. Is delayed graft function causally associated with long-term outcomes after kidney transplantation? Instrumental variable analysis. Transplantation 95, 1008–1014 (2013).
Team, R.C. R version 3.5. 0. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2018).
Bivand, R. & Lewin-Koh, N. maptools: Tools for reading and handling spatial objects, 2014. R package version 0.8-29 (2015).
Bivand, R., Keitt, T., Rowlingson, B. & Pebesma, E. rgdal: Bindings for the geospatial data abstraction library. R package version 1(2016).
Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, Berlin, 2016).
Kahle, D. & Wickham, H. ggmap: spatial visualization with ggplot2. R J. 5, 144–161 (2013).
Kim, J.-M., Kim, H. S. & Kim, K. N. A study on nutritional intakes in middle income adults based on data from the 5th Korean National Health and Nutrition Examination Survey. J. Nutr. Health 48, 364 (2015).
Kim, Y., Seo, S., Kwon, O. & Cho, M. S. Comparisons of dietary behavior, food intake, and satisfaction with food-related life between the elderly living in urban and rural areas. Korean J. Nutr. 45, 252 (2012).
Woodell, T. B., Rifkin, D. E., Ellison, D. H., Negoianu, D. & Weiss, J. W. Serum bicarbonate as a surrogate for pH in hemodialysis: a pilot study. Kidney Med. 2, 42–48 (2019).
Woodell, T. B., Webster, L., Mehta, R., Macedo, E. & Rifkin, D. E. Total carbon dioxide versus pH for determining acid-base status in patients on continuous kidney replacement therapy: a cohort study. Am. J. Kidney Dis. https://doi.org/10.1053/j.ajkd.2020.06.015 (2020).
Nasir, N. M., Sthaneshwar, P., Yunus, P. J. & Yap, S. F. Comparing measured total carbon dioxide and calculated bicarbonate. Malaysian J. Pathol. 32, 21–26 (2010).
Hirai, K. et al. Approximation of bicarbonate concentration using serum total carbon dioxide concentration in patients with non-dialysis chronic kidney disease. Kidney Res. Clin. Pract. 38, 326–335 (2019).
Kajimoto, S., Sakaguchi, Y., Asahina, Y., Kaimori, J.-Y. & Isaka, Y. Modulation of the association of hypobicarbonatemia and incident kidney failure with replacement therapy by venous pH: a cohort study. Am. J. Kidney Dis. 77, 35–43 (2020).
Raphael, K. L. et al. Bicarbonate concentration, acid-base status, and mortality in the health, aging, and body composition study. Clin. J. Am. Soc. Nephrol. 11, 308–316 (2016).
Author information
Authors and Affiliations
Contributions
K.D.Y., J.N.A., S.O., and J.P.L. designed the study. J.N.A., Y.C.K., J.L., K.J., and Y.S.K. collected the data. K.D.Y., J.N.A., C.S.L., and S.O. analyzed the data. K.D.Y., J.N.A., Y.K.O., and S.O. created the figures. K.D.Y., J.N.A., and J.P.L. drafted and revised the paper. All authors approved the final version of manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoo, K.D., An, J.N., Kim, Y.C. et al. Low serum total CO2 and its association with mortality in patients being followed up in the nephrology outpatients clinic. Sci Rep 11, 1711 (2021). https://doi.org/10.1038/s41598-021-81332-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-81332-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.