Abstract
Analysis of refractive outcomes, using biometry data collected with a new biometer (Pentacam-AXL, OCULUS, Germany) and a reference biometer (Lenstar LS 900, HAAG-STREIT AG, Switzerland), in order to assess differences in the predicted and actual refraction using different formulas. Prospective, institutional study, in which intraocular lens (IOL) calculation was performed using the Haigis, SRK/T and Hoffer Q formulas with the two systems in patients undergoing cataract surgery between November 2016 and August 2017. Four to 6 weeks after surgery, the spherical equivalent (SE) was derived from objective refraction. Mean prediction error (PE), mean absolute error (MAE) and the median absolute error (MedAE) were calculated. The percentage of eyes within ± 0.25, ± 0.50, ± 1.00, and ± 2.00 D of MAE was determined. 104 eyes from 76 patients, 35 males (46.1%), underwent uneventful phacoemulsification with IOL implantation. Mean SE after surgery was − 0.29 ± 0.46 D. Mean prediction error (PE) using the SRK/T, Haigis and Hoffer Q formulas with the Lenstar was significantly different (p > 0.0001) from PE calculated with the Pentacam in all three formulas. Percentage of eyes within ± 0.25 D MAE were larger with the Lenstar device, using all three formulas. The difference between the actual refractive error and the predicted refractive error is consistently lower when using Lenstar. The Pentacam-AXL user should be alert to the critical necessity of constant optimization in order to obtain optimal refractive results.
Similar content being viewed by others
Introduction
The Lenstar LS 900 (HAAG-STREIT AG, Switzerland) is a non-invasive biometer with OLCR (optical low coherence reflectometry), used in the calculation of intraocular lens (IOL) power through different formulas. It also provides readings on central corneal thickness (CCT), axial length (AL), anterior chamber depth (ACD), keratometry, pupillary diameter, retinal thickness measurements and white-to-white distance1.
The Pentacam (OCULUS, Germany) uses a rotating Scheimpflug camera which provides a three-dimensional scan of the anterior segment of the eye. The more recent Pentacam-AXL (OCULUS, Germany), by means of using partial coherence interferometry technology to obtain measurements of axial length (AL), has gained the ability to perform non-contact biometry. With this new feature, and in combination with the measurements obtained with the Scheimpflug rotating camera, this device can now perform the calculation of intraocular lens (IOL) power required in cataract and refractive surgery. Accurate and reliable measurements of ocular parameters and consequently of IOL power are essential to the success of surgery and patient satisfaction2,3,4,5.
We previously compared measurements of axial length (AL), anterior chamber depth (ACD) from the corneal epithelium (ACD ext) and endothelium (ACD int) to the anterior surface of the lens, central corneal thickness (CCT) and keratometry readings of the steepest and flattest corneal meridians (K1 and K2) obtained with both devices. Mean values of K1, K2 and CCT were different. Keratometry readings performed with the Lenstar were higher than with the Pentacam. CCT measurements retrieved from the Lenstar were also higher compared to the Pentacam. AL and ACD readings were not significantly different. We also performed IOL calculation with the Lenstar and Pentacam with SRK/T, Haigis and HofferQ formula. The comparison of these variables showed significant differences IOL power in all formulas between both devices, with the Pentacam showing higher IOL power values with all the three formulas. A possible reason for the differences found in IOL power calculation might be due to the different keratometric readings between both devices6.
Our goal now is to compare refractive outcomes after cataract surgery, evaluating the differences between real refraction and the predicted refraction obtained with both the Lenstar and the Pentacam.
Methods
This retrospective sub-analysis to the previous study6 included 136 eyes of 79 patients undergoing cataract surgery between November 2016 and August 2017 at Leiria Hospital Centre. Informed consent was obtained for the collection of data that is part of our standard practice and adhered to the tenets of the Declaration of Helsinki. The study protocol was approved by the institution’s review board.
Exclusion criteria included patients with previous anterior segment or vitreoretinal surgery, white mature or traumatic cataracts, patients with central corneal opacities or high corneal astigmatism and a difference in measurements of axial length bigger than 0.3 mm between both eyes. This latter exclusion criteria is due to the possibility of measurement error that is raised by the Pentacam´s software, suggesting the user performs a confirmatory AL measurement with another device or with ultrasound immersion biometry.
Biometry with the Lenstar LS900 (HAAG-STREIT AG, Switzerland) and Pentacam (Pentacam-AXL, OCULUS, Germany) was performed. Parameters retrieved were AL, IOL power chosen for each surgery and predicted refraction using Haigis, SRK/T and HofferQ with the selected IOL with both devices. IOL selection for surgery was performed with biometry data from our reference biometer, the Lenstar LS900, using the formula that yielded a value of diopter power closest to emmetropization.
Uncomplicated phacoemulsification with IOL implantation in the capsular bag was performed on 104 eyes by the same surgeon. IOL type was Acrysoft IQ monofocal SN60WF in all surgeries. Main incisions were done on the steepest corneal axis with a 2.4 mm keratome in all cases.
Post-operatively, eyes with IOL not in-the bag or with a secondary procedure occurring at the same time as phacoemulsification were excluded from analysis. Four to six weeks after surgery, the spherical equivalent (SE) was derived from the objective refraction obtained with auto refractometer (NIDEK ARK-1a). Mean prediction error (PE) or the difference between the actual refractive error and the predicted refractive error was calculated, based on the predicted spherical equivalent derived from both the Lenstar and the Pentacam separately, for the IOL power selected for the surgery, with the SRK/T, Haigis and HofferQ formulas. Mean absolute error (MAE) and the median absolute error (MedAE) were also calculated for better characterization and comparison purposes. The percentage of eyes within ± 0.25, ± 0.50, ± 1.00, and ± 2.00 D of MAE was also determined in order to compare refractive predictability.
Statistical analysis
The data was analyzed with SPSS version 22 software (IBM Inc., Chicago, Illinois, USA). Normality was guaranteed by the Kolmogorov–Smirnov test and graphical analysis. Statistical analysis was performed using descriptive statistics and AL was compared using a paired Student t-test. The Friedman test was used to test the difference among instruments for PE, MAE and MedAE. The tests were considered significant when p < 0.05 with 95% confidence intervals.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent for anonymous biometric data collection was obtained from all individual participants included in the study, as part of the departments standard of care practices.
Results
Our study sample included 136 eyes of 79 patients, 53.2% were female. Mean age was 76.2 ± 6.8 years. From the initial sample, 104 eyes from 76 patients, 35 males (46.1%) and 42 females (55.3%), underwent uncomplicated phacoemulsification with IOL implantation in the capsular bag.
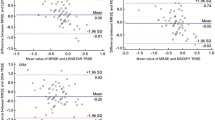
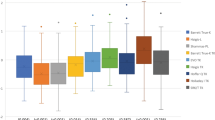
Mean AL measured with the Lenstar was 23.01 ± 0.77 mm [20.89; 24.86] and 22.97 ± 0.77 [20.84; 24.78] mm with the Pentacam. There were no significant differences between both variables (p < 0.0001). Mean SE 4–6 weeks after surgery was − 0.29 ± 0.46 D. Evaluating the difference between predicted refraction and actual refraction using the SRK/T formula, it was found that the final refractive outcome was 0.07 diopters (D) more hypermetropic than the predicted refraction when using the Lenstar and 0.16 D more myopic when using the Pentacam (p = 0.001). In a similar way, using the Haigis formula, the final refractive outcome was 0.09 D more hypermetropic than predicted refraction using Lenstar and 0.23 D more myopic when using the Pentacam (p < 0.0001). When using the HofferQ formula, it was observed that final refractive outcome was 0.04 D more hypermetropic using the Lenstar and 0.25 more myopic using the Pentacam (p < 0.0001). The MAE did not differ significantly between devices (p = 0.2 SRK/T; p = 0.21 Haigis; p = 0.31 HofferQ). PE, MAE and MedAE are further described in Table 1.
Percentage of eyes within ± 0.25, ± 0.50, ± 1.00 and ± 2.00 D MAE using SRK/T with the Lenstar device was 45.2%; 26.0%; 19.2% and 5.8% respectively. Using the same formula in the Pentacam, the percentage of eyes within ± 0.25, ± 0.50, ± 1.00, and ± 2.00 would be 40.4%, 31.7%, 23.1% and 4.8%. If the refractive outcome was considered as the proportion of those who differed from the predicted refraction then 74% were within 0.5 D and 94% were within 1.0 D when the Lenstar was used, compared with 72% within 0.5 D and 95.2% when using the Pentacam with the SRK/T formula.
Proportion of eyes whose postoperative refraction differed from preoperative predicted refraction with the SRK/T, Haigis and Hoffer Q formulas using both devices, is illustrated in Fig. 1.
Discussion
The precise acquisition of ocular parameters is the first step to correctly determine the IOL power required to achieve target refraction as predictability of the refractive error after cataract surgery in biometry is key to meet high standards of refractive outcomes.
In our previous study6, AL and ACD readings showed excellent correlation and agreement but keratometry readings performed with the Lenstar were significantly higher than with the Pentacam. Moreover, IOL power closest to emmetropization calculated with the Pentacam showed higher values for the same eye.
Muzyka-Woźniak et al.7 found flatter corneal curvature measurements in the Pentacam-AXL when comparing it with the IOL Master 500. ACD measurements were not statistically significant and differences in AL were clinically insignificant. They showed the proneness of the Pentacam-AXL to provide higher calculated IOL power. They suggested it could be explained by its flatter keratometric measurements and proposed optimization of constant used in IOL power calculation formulas. These results are in line with our previous work6. Sel et al.8 have reached similar conclusions when comparing IOL Master to Pentacam-AXL. In contrast, Shajari and colleagues9 found no significant differences in keratometric parameters.
In this study, analyzing the actual refractive outcomes, we found that the difference between the actual refractive error and the predicted refractive error is consistently lower when using Lenstar. This is represented by a lower value of mean error and mean absolute error when using the values obtained with Lenstar. Percentage of eyes within ± 0.25 D MAE were also larger with the Lenstar device, using all three formulas. When using the SRK/T formula the difference in MAE between both machines was inferior thus implying greater inter-device proximity when using this formula.
Refractive outcomes using optic biometry and other biometry techniques such as applanation and immersion were compared by different authors10,11,12,13,14.
Naicker et al.10 found no significant differences in the predicted post-operative refractive outcome between the Lenstar LS900 and the Immersion A-scan ultrasound devices. Trivedi et al.11 showed Immersion A-scan biometry would be more desirable for IOL power calculation on a pediatric population. Landers12 and colleagues compared the IOL Master to immersion ultrasound and revealed a more predictable refractive outcome with the IOL Master, with patients’ SE closer to target refraction. Lam et al.13 found the Lenstar superior to the immersion biometry in refractive outcomes. Nemeth et al.14 showed slightly better outcomes with optical biometry comparing to immersion biometry with no optimized formulas and significantly better results with optimization of IOL-constants.
Refractive outcomes comparison between an optical biometer and an instrument with Dual Scheimpflug/Placido’s discs technology and low coherence interferometry biometry (Galilei G6 ZIEMER OPHTHALMIC SYSTEMS AG) have been performed15,16. IOL powers determined by the IOLMaster (CARL ZEISS MEDITEC), were compared to the refractive outcome that would have been achieved using IOL powers determined with the Galilei G6 Lens Professional, a Dual-Scheimpflug Corneal Topographer/Keratometer (ZIEMER OPHTHALMIC SYSTEMS)15. No significant differences were found between them. Jung et al.16 comparing the IOL Master and the Galilei G6 found that the proportion of eyes with an MAE within 0.5 D was 85.0% for the IOLMaster and was 80.0% for the Galilei G6 based on the SRK/T formula.
The Pentacam-AXL accounts for astigmatism of both anterior and posterior corneal surfaces. It estimates changes in the corneal shape suffered by previous refractive surgery (PRK or LASIK). These functions come as highly desirable in patients with previous refractive surgery. Patients with these characteristics represent a challenge in IOL calculation, because, among other reasons, the keratometric index of 1.3375 used to convert the anterior corneal curvature into diopters is no longer valid after LASIK or PRK17. For these reasons, this device represents a resource of great value in achieving the best refractive results in populations with characteristics as diverse as those we find today.
The Acrysoft IQ monofocal SN60WF constants for the Pentacam-AXL were the same as for the Lenstar in our study. In the same way as Muzyka-Woźniak et al.7, in the comparison of the Pentacam with the IOL Master, we believe these constants are not the ideal for the Pentacam-AXL and are the main reason for IOL calculation and mean error dissimilarity with the Lenstar. However, some studies with constant optimization have shown diverging results in the incidence of prediction errors in IOL calculation with the Pentacam18,19.
The main limitations to this study are the inclusion of both eyes, the lack of constant optimization to the formulas and the execution of biometry exams by several technicians. Regardless, we believe that this comparison is relevant to the daily clinical practice as it alerts the Pentacam-AXL user to the critical necessity of constant optimization in order to obtain optimal refractive results.
References
HAAG-STREIT AG. Lenstar LS900. Accessed November 28, 2019 from https://www.haag-streit.com/haag-streit-usa/products/haag-streitdiagnostics/lenstar-biometry/lenstar-ls-900/ (n.d.)
OCULUS. Pentacam-AXL. Accessed November 28, 2019 from https://www.pentacam.com/int/ophthalmologist-surgeon-without-pentacam/technology/ (n.d).
Khoramnia, R., Rabsilber, T. M. & Auffarth, G. U. Central and peripheral pachymetry measurements according to age using the Pentacam rotating Scheimpflug camera. J. Cataract. Refract. Surg. 33, 830–836 (2007).
Swartz, T., Marten, L. & Wang, M. Measuring the cornea: the latest developments in corneal topography. Curr. Opin. Ophthalmol. 18, 325–333 (2007).
Belin, M. W., Khachikian, S. S. & Ambrósio, R. J. Introduction and Overview. In Elevation Based Corneal Tomography 2nd edn 1–14 (Jaypee Highlights Medical Publishers, Panama City, 2012).
Pereira, J. M. M. et al. Lenstar LS900 vs Pentacam-AXL: comparative study of ocular biometric measurements and intraocular lens power calculation. Eur. J. Ophthalmol. https://doi.org/10.1177/1120672118771844 (2018).
Muzyka-Woźniak, M. & Oleszko, A. Comparison of anterior segment parameters and axial length measurements performed on a Scheimpflug device with biometry function and a reference optical biometer. Int. Ophthalmol. https://doi.org/10.1007/s10792-018-0927-x (2018).
Sel, S., Stange, J., Kaiser, D. & Kiraly, L. Repeatability and agreement of Scheimpflug-based and swept-source optical biometry measurements. Contact Lens Anterior Eye 40(5), 318–322. https://doi.org/10.1016/j.clae.2017.03.007 (2017).
Shajari, M. et al. Comparison of axial length, corneal curvature, and anterior chamber depth measurements of 2 recently introduced devices to a known biometer. Am. J. Ophthalmol. 178, 58–64. https://doi.org/10.1016/j.ajo.2017.02.027 (2017).
Naicker, P. et al. Refractive outcomes comparison between the Lenstar LS 900 optical biometry and immersion A-scan ultrasound. Int. Ophthalmol. 35(4), 459–466. https://doi.org/10.1007/s10792-014-9970-4 (2015).
Trivedi, R. H. & Wilson, M. E. Prediction error after pediatric cataract surgery with intraocular lens implantation: contact versus immersion A-scan biometry. J. Cataract. Refract. Surg. 37(3), 501–505. https://doi.org/10.1016/j.jcrs.2010.09.023 (2011).
Landers, J. & Goggin, M. Comparison of refractive outcomes using immersion ultrasound biometry and IOLMaster biometry. Clin. Exp. Ophthalmol. 37(6), 566–569. https://doi.org/10.1111/j.1442-9071.2009.02091.x (2009).
Lam, S. Comparing optical low coherence reflectometry and immersion ultrasound in refractive outcome after cataract surgery. J. Cataract. Refract. Surg. 39(2), 297–298. https://doi.org/10.1016/j.jcrs.2012.12.003 (2013).
Nemeth, G., Nagy, A., Berta, A. & Modis, L. Jr. Comparison of intraocular lens power prediction using immersion ultrasound and optical biometry with and without formula optimization. Graefes Arch. Clin. Exp. Ophthalmol. 250(9), 1321–1325. https://doi.org/10.1007/s00417-012-2013-9 (2012).
Pajic, B., Mueller, M., Allemann, R. & Vastardis, I. Galilei G6 Lens Professional vs. IOL Master and Lenstar LS900—A Comparison study (Ziemer Opthalmic Systems AG, Port, 2014).
Jung, S., Chin, H. S., Kim, N. R., Lee, K. W. & Jung, J. W. Comparison of repeatability and agreement between swept-source optical biometry and dual-Scheimpflug topography. J. Ophthalmol. 2017, 5. https://doi.org/10.1155/2017/1516395 (2017).
Savini, G. & Hoffer, K. J. Intraocular lens power calculation in eyes with previous corneal refractive surgery. Eye Vis. (Lond.) 5, 18. https://doi.org/10.1186/s40662-018-0110-5 (2018).
Savini, G., Barboni, P., Carbonelli, M. & Hoffer, K. J. Accuracy of Scheimpflug corneal power measurements for intraocular lens power calculation. J. Cataract. Refract. Surg. 35(7), 1193–1197 (2009).
Saad, E., Shammas, M. C. & Shammas, H. J. Scheimpflug corneal power measurements for intraocular lens power calculation in cataract surgery. Am. J. Ophthalmol. 156(3), 460-467.e2. https://doi.org/10.1016/j.ajo.2013.04.035 (2013).
Acknowledgements
We would like to thank the team of orthoptists from the Ophthalmology Department in Leiria Hospital Center (Carla Silva, Nélia Ferreira, Susana Feliciano, Mara Gonçalves, Ana Rita Baptista and Ana Cunha) for performing the biometry exams in all patients. All the authors have read and approved the final manuscript. The authors have declared that no competing interests exist.
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arruda, H.A., Pereira, J.M., Neves, A. et al. Lenstar LS 900 versus Pentacam-AXL: analysis of refractive outcomes and predicted refraction. Sci Rep 11, 1449 (2021). https://doi.org/10.1038/s41598-021-81146-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-81146-2
This article is cited by
-
Comparison of anterior corneal aberrations measured by Scheimpflug and Placido Disc System for myopes
BMC Ophthalmology (2022)
-
The effect of implantable collamer Lens V4c on ocular biometric measurements and intraocular lens power calculation based on Pentacam-AXL and IOLMaster 500
BMC Ophthalmology (2022)
-
Agreement analysis of Lenstar with other four techniques of biometry before cataract surgery
International Ophthalmology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.