Abstract
This study aimed to compare the effects of volatile anesthesia and total intravenous anesthesia (TIVA) on syndecan-1 shedding in patients with gastric cancer undergoing minimally invasive gastrectomy. Patients were randomly assigned to either the Volatile (n = 68) or the TIVA (n = 68) group. Anesthesia was maintained with sevoflurane/remifentanil or propofol/remifentanil in the Volatile and TIVA groups, respectively. Serum syndecan-1 was evaluated at pre-operation, end of operation, and postoperative day (POD) 1. Inflammatory markers including white blood cell (WBC) count, neutrophil-to-lymphocyte ratio (NLR), and C-reactive protein (CRP), were also measured at pre-operation, end of operation, and POD 1, 2, 3, and 5. The TIVA group showed significantly lower levels of syndecan-1 at the end of the operation compared to the Volatile group; however, no difference was seen between the groups at POD 1. The WBC count and NLR were significantly lower in the TIVA group at the end of the operation than the Volatile group, but there were no differences between the groups at POD 1, 2, 3, and 5. CRP levels were similar between the groups at all time points. In conclusion, despite TIVA being superior to volatile anesthesia in protecting endothelial glycocalyx during the operation, both did not prevent postoperative syndecan-1 shedding after gastrectomy.
Clinical trial registration number: NCT04183296 (ClinicalTrial.gov, 03/12/2019).
Similar content being viewed by others
Introduction
The endothelial glycocalyx (EG) is a gel-like layer that coats the luminal surface of the vascular endothelium, with thicknesses between 0.2 and 2 μm depending on the type of vasculature1,2,3. The EG is degraded under pathological conditions, such as ischemia–reperfusion (I/R) injury, oxidative stress, and inflammation1,2,3. Proteoglycans are the most important backbone molecule of the EG, and heparan sulfate proteoglycans represent about 50–90% of the proteoglycans1,2. Syndecans are a family of heparan sulfate proteoglycan, and syndecan-1 has been widely studied as a marker of EG breakdown in various conditions, including in surgical patients4,5,6,7,8,9,10,11,12,13.
Since no pharmacologic agents for the restoration of the EG are clinically available, strategies to prevent EG degradation in surgical patients are needed3. There have been experimental studies on the protective effect of sevoflurane against EG degradation from I/R injury14,15,16. Sevoflurane was superior to propofol in protecting the EG from I/R injury in a porcine model16. In contrast to experimental results, sevoflurane did not show a better protective effect on the EG than propofol in clinical studies of lung resection surgery and knee-ligament surgery9,10.
Minimally invasive laparoscopic surgery causes less surgical trauma compared to open surgery, which leads to less stress and attenuated inflammatory response17,18. In minimally invasive abdominal surgery, pneumoperitoneum is essential for adequate visualization and working space. However, abdomen insufflation causes a reduction in splanchnic blood flow, which causes organ ischemia followed by reperfusion injury upon deflation of the abdomen, resulting in oxidative stress19. Although I/R injury and oxidative stress are highly related to EG disruption, no study has evaluated the perioperative EG changes in minimally invasive abdominal surgery with pneumoperitoneum, nor the effect of different general anesthetics on these changes. Hence, this randomized controlled trial aimed to compare the effect of volatile anesthesia with sevoflurane/remifentanil and of total intravenous anesthesia (TIVA) with propofol/remifentanil on syndecan-1 shedding in patients with gastric cancer undergoing laparoscopic or robotic gastrectomy.
Results
Demographic and intraoperative characteristics
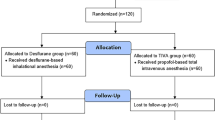
Of the 139 patients assessed for eligibility, 136 patients were randomly assigned into the two groups, and 132 of those patients completed the study (97%). Four patients were excluded from the final analysis (three patients from the Volatile group and one from the TIVA group) because of open and closure or conversion to open surgery (Fig. 1). The patients’ characteristics and intraoperative variables are shown in Table 1. The administered dose of remifentanil was significantly higher in the TIVA group (P < 0.001), whereas significantly higher doses of ephedrine and phenylephrine were administered to the Volatile group (both P < 0.001). No differences were observed between the two groups in other variables. Intraoperative MAP was significantly higher in the TIVA group at 30, 60, and 90 min after pneumoperitoneum compared to the Volatile group (Bonferroni corrected P = 0.012, 0.016, and 0.034, respectively; Fig. 2A). No statistical differences in heart rate were found between the two groups (Fig. 2B). Arterial partial pressures of oxygen and carbon dioxide at 90 min after pneumoperitoneum showed no significant differences between the Volatile and TIVA groups (202 ± 31 vs 206 ± 31 mmHg, P = 0.642 and 39 ± 4 vs 39 ± 3 mmHg, P = 0.977, respectively).
Syndecan-1
The serum concentration of syndecan-1 was significantly increased at the end of the operation compared to preoperative values, and this increase was maintained until POD 1 in the Volatile group. However, in the TIVA group, syndecan-1 was significantly elevated at POD 1 but not at the end of the operation. Therefore, the TIVA group showed significantly lower levels of syndecan-1 at the end of the operation compared to the Volatile group (23.4 ± 11.7 vs 29.3 ± 14.7 ng/mL; Bonferroni corrected P = 0.021), but no difference existed between the groups at POD 1 (29.1 ± 13.1 ng/mL in the Volatile group vs 28.0 ± 12.9 ng/mL in the TIVA group; Fig. 3). Even after adjusting for confounding variables (MAP, dose of ephedrine, and dose of phenylephrine) which may contribute to organ ischemia, the syndecan-1 level was significantly lower in the TIVA group at the end of the operation compared to the Volatile group (Bonferroni corrected P = 0.042, data not shown), while there was no difference between the groups at POD1. When compared to preoperative values, 19 patients (44%) in the Volatile group and 24 patients (56%) in the TIVA group presented an elevation of syndecan-1 of more than 30% at POD 1 (P = 0.149, data not shown).
WBC count, NLR, and CRP
The WBC count and NLR were significantly lower in the TIVA group at the end of the operation than in the Volatile group (Bonferroni corrected P = 0.049 and 0.013, respectively), while no differences were found between the groups at POD 1, 2, 3, and 5 (Fig. 4A,B). In addition, there was no difference in the CRP levels between the groups at any time point (Fig. 4C).
Postoperative consumption of analgesics and hospital stay
The cumulative doses of fentanyl at postoperative 6, 12, and 24 h were similar in both groups (Fig. 5A). Likewise, no significant differences were observed in the number of patients who required rescue analgesics between the two groups (Fig. 5B). The postoperative hospital stay was of 6.5 ± 3.5 days in the Volatile group and 6.0 ± 2.2 days in the TIVA (P = 0.352).
Discussion
This randomized controlled study was the first to explore the perioperative changes of syndecan-1 and the effects of two commonly used general anesthetics on these changes in minimally invasive abdominal surgery with pneumoperitoneum. Our study showed that the serum concentration of syndecan-1 was significantly increased at POD 1 compared to preoperative levels, and that the increase was comparable between patients under volatile anesthesia with sevoflurane/remifentanil and those under TIVA with propofol/remifentanil. Our results further confirmed previous findings indicating that sevoflurane and propofol have similar effects regarding perioperative EG degradation9,10.
The EG layer is a fine structure that lines the endoluminal surface of the endothelium and acts as a regulator of endothelial function1,2,3. It is a labile structure and can be easily destroyed by a variety of enzymes and reactive oxygen species under inflammatory conditions1,2,3. Acute degradation of the EG has been demonstrated in patients after cardiac surgery, sepsis, and major trauma, and elevation of EG markers in blood is associated with poor outcomes in these patients3,6,8. Although previous studies have shown EG shedding in patients after major abdominal surgery5,7, literature on EG degradation after minimally invasive abdominal surgery was scarce. Minimally invasive abdominal surgery implies less inflammatory response compared to open surgical techniques17,18, from which it could be inferred that minimally invasive abdominal surgery would cause less EG damage. However, on the other hand, pneumoperitoneum applied in minimally invasive surgeries is known to induce I/R injury with reactive oxygen species formation19, which is another source of EG damage in addition to inflammatory response. Ultimately, our study revealed that there is a significant EG degradation in patients after minimally invasive laparoscopic or robotic gastrectomy.
The EG is composed of proteoglycans, glycoproteins, glycosaminoglycans, and associated plasma proteins, including albumin1,2. Proteoglycans consist of a core membrane-bound protein, such as those of the syndecan family, to which glycosaminoglycan side chains are attached1,2. Among glycosaminoglycans, heparan sulfate is the most prominent glycosaminoglycan1. Therefore, syndecan-1 (core protein) and heparan sulfate (glycosaminoglycan) have been the most commonly used markers of EG damage in medical and surgical patients4,5,6,7,8,9,10,11,12,13. The core protein syndecan-1 strongly binds to the cell membrane via a membrane-spanning domain, whereas heparan sulfate is a side chain attached to it1,2. Therefore, heparan sulfate is shed first and syndecan-1 is shed later, when severe EG damage occurs1,3. In cardiac surgery with cardiopulmonary bypass (CPB), syndecan-1 blood levels elevated following the elevation of heparan sulfate; syndecan-1 levels remained elevated until POD 3, whereas heparan sulfate levels were restored to baseline after weaning from CPB13. Therefore, syndecan-1 may be a better marker to detect severe injury and more stable than heparan sulfate.
There was approximately a 20% increase in serum syndecan-1 at POD 1 compared to preoperative values in our patients. This increase is lower than previously reported increases of syndecan-1 of 40–70% at 24 h following major abdominal surgery5,7. The discrepancy may arise from the difference in the extent of surgical trauma and the duration of the operation because (1) we studied only minimally invasive surgery, whereas previous studies included open surgery mostly, and (2) we included only gastrectomy, whereas previous studies included various types of abdominal surgery5,7. In fact, different types of surgery have been associated with perioperative variations in serum syndecan-1 levels; increases of 30–40% have been reported after lung resection surgery9,11, whereas up to 65-fold increases have been reported after major vascular surgery with CPB4.
After acute degradation of the EG, 5–7 days are required to endogenously restore the EG thickness in mice20. There are no clinically available pharmacologic agents for EG restoration; hence, efforts to minimize EG degradation may be important3. Strategies to minimize EG damage include avoiding hypervolemia, supplementing with albumin, and administering pharmacologic agents (e.g., glucocorticoids, antioxidants, and antithrombin III)2,3,12,21. Volatile anesthetics were also considered as one of the candidates for EG protection because of their protective effects against I/R injury14,15,16. Sevoflurane has been proven to reduce EG shedding caused by I/R injury through attenuation of the post-ischemic release of lysosomal protease cathepsin B14, and through the inhibition of post-ischemic leukocyte and platelet adhesion15. Moreover, sevoflurane significantly reduced heparan sulfate shedding after I/R injury compared to propofol by reducing the generation of unmeasured anions16. However, contradictory to the reported protective effects of sevoflurane on EG damage in animal models14,15,16, sevoflurane was not superior to propofol in reducing EG degradation in surgical patients9,10. In lung resection surgery with one lung ventilation, no difference was observed in heparan sulfate and syndecan-1 concentrations between sevoflurane and propofol groups during surgery9. Likewise, no difference existed in heparan sulfate and syndecan-1 concentrations between the two groups up to 90 min after tourniquet release in knee-ligament surgery10. These two studies presented design differences compared to our study: sevoflurane or propofol alone was used for general anesthesia maintenance without concomitant use of opioids9, and sevoflurane or propofol was used for hypnosis under spinal anesthesia10. On the contrary, we administered remifentanil in addition to sevoflurane or propofol because opioids are frequently used during general anesthesia with hypnotic agents. Hence, our study may reflect common clinical settings, and in these conditions we showed no superior effects of volatile anesthesia with sevoflurane/remifentanil over TIVA with propofol/remifentanil on perioperative EG damage.
Although there was no difference in syndecan-1 levels at POD 1 between the groups, the TIVA group showed significantly lower levels of syndecan-1 at the end of the operation compared to the Volatile group (Bonferroni corrected P = 0.021). This result is comparable to the significantly lower WBC count and NLR in the TIVA group at the end of the operation compared to the Volatile group (Bonferroni corrected P = 0.049 and 0.013, respectively). The NLR is the ratio of neutrophil count to lymphocyte count and is considered as a more sensitive marker of systemic inflammation than the measures of either cell type alone22. Therefore, lower levels of WBC count and NLR may reflect an attenuated inflammatory response in the TIVA group, which could have prevented EG degradation during the surgery. However, since sevoflurane also has anti-inflammatory properties23 and since CRP levels were similar between the groups, further studies are needed to draw definitive conclusions. Moreover, the protective effects of TIVA against EG damage were only limited to the surgery and were not maintained in the postoperative period. Hence, volatile anesthesia and TIVA can be considered comparable regarding EG protection in minimally invasive abdominal surgery.
This study has some limitations. First, the sample size was not calculated beforehand due to the lack of previous reports. However, the number of patients in our study is larger than that of previous clinical studies comparing syndecan-1 between two treatment groups7,9,10,12,21. Second, we evaluated syndecan-1 only until POD 1 based on previous studies7,12. However, we evaluated WBC count, NLR, and CRP levels until POD 5 and no differences were found between the groups. Therefore, there may be no difference in syndecan-1 levels between the groups after POD 1 either. Moreover, syndecan-1 returned to baseline levels 48 h after abdominal surgery5; thus, evaluation after POD 1 may be meaningless.
In conclusion, EG damage was observed following minimally invasive laparoscopic or robotic gastrectomy with approximately 20% postoperative increase in serum syndecan-1. Although TIVA with propofol/remifentanil showed protective effects against EG damage during the surgery in contrast to volatile anesthesia with sevoflurane/remifentanil, both types of anesthetics could not prevent postoperative syndecan-1 shedding. These results argue against a favorable effect of volatile anesthetics over TIVA on perioperative EG damage in experimental studies, whereas support the previous clinical studies demonstrating comparable effects of these agents on EG damage in surgical patients.
Methods
Patients
This study was approved by the Institutional Review Board (IRB) and Hospital Research Ethics Committee (Yonsei University Health System, Seoul, Korea; IRB protocol No. 4-2019-0924) and was registered at http://clinicaltrials.gov (ClinicalTrial.gov, NCT04183296, 03/12/2019). This study was performed in accordance with relevant guidelines and regulations. After obtaining written informed consent from each patient, a total of 136 patients with gastric cancer who were waiting for a laparoscopic or robotic gastrectomy were enrolled between November 2019 and July 2020. Patients were excluded if they fulfilled any of the following criteria: emergency operation; severe hepatic dysfunction (alanine aminotransferase > 3 times of upper normal limit) or end-stage renal disease; allergy or hypersensitivity to sevoflurane or propofol; neurological or psychiatric impairment; history of thromboembolism; and treatment with oral contraceptives or anticoagulants.
Study design
Patients were randomly assigned to either the Volatile (n = 68) or the TIVA (n = 68) group with the permuted block size of four. In the Volatile group, a bolus of propofol (1.5–2 mg/kg) and target-controlled infusion (TCI) of remifentanil (effect-site concentration [Ce] of 4.0 ng/ml) were administered for anesthetic induction. In the TIVA group, anesthetic induction was achieved by TCI of propofol (Ce of 4.0–4.5 μg/mL) and remifentanil (Ce of 4.0 ng/ml). A commercial TIVA pump (Orchestra Base Primea, Fresenius-Vial, Sèvres, France) was used for TCI of remifentanil and propofol. For anesthesia maintenance, age-adjusted minimal alveolar concentration end-tidal sevoflurane of 0.8–1.0 and TCI of remifentanil were used in the Volatile group, while TCI of propofol and remifentanil were used in the TIVA group to target a Patient State Index (PSI) within the range of 25–50.
Anesthetic management
Upon arrival at the operating room, all required monitoring devices, including electrocardiography, non-invasive blood pressure, peripheral oxygen saturation, PSI using a SedLine electroencephalograph sensor (Masimo Corp., Irvine, CA), and peripheral nerve stimulator, were applied. After the administration 0.1 mg of intravenous glycopyrrolate as a premedication, anesthetic induction was performed as abovementioned. After loss of consciousness, 1.2 mg/kg of rocuronium was administered to facilitate tracheal intubation, and the degree of neuromuscular blockade was maintained, with 0–2 train-of-four as a target, by rocuronium infusion during pneumoperitoneum. Controlled ventilation was performed at a tidal volume of 7–8 mL/kg with 50% oxygen in air to maintain end-tidal carbon dioxide (CO2) at 35–40 mmHg, and a positive end-expiratory pressure of 5 cm H2O was applied. Radial artery cannulation was performed in all patients. Hypotension (mean arterial pressure [MAP] < 60 mmHg) was controlled with ephedrine in 4-mg increments or with phenylephrine in 20-μg increments. A forced-air warming system was applied to maintain body temperature at 36–37 °C. Pneumoperitoneum was induced by CO2 insufflation, and the intra-abdominal pressure was maintained at 12 mmHg. At the beginning of umbilical closure, 1 μg/kg of fentanyl and 0.3 mg of ramosetron were administered for the prevention of postoperative pain and nausea and vomiting, respectively. All patients were provided with an IV patient-controlled analgesia (PCA) device (Accumate 1100; WooYoung Medical, Seoul, Korea), which consisted of fentanyl infused basally at a rate of 8 µg/h with a bolus dose of 8 µg and a lockout time of 10 min. At the end of the operation, residual neuromuscular blockade was reversed with sugammadex 2 mg/kg intravenously.
Data collection
Hemodynamic variables (MAP and heart rate) were measured at the following operation time points: before induction, at 30, 60, and 90 min of CO2 pneumoperitoneum, and at the end of the operation and arterial blood gas analysis was performed at 90 min of CO2 pneumoperitoneum. The primary outcome was serum syndecan-1, which was evaluated at 3 time points: pre-operation (baseline), at the end of the operation, and at postoperative day (POD) 1. Extracted blood samples were centrifuged at 5000 rpm for 5 min at 4 °C to obtain serum and were stored in a -80 °C freezer until analyzed. The analysis was performed by using a specific immunoassay kit (Abnova,Cat. No. KA3851,Taiwan) and all samples were tested in duplicate. In addition, the inflammatory markers, white blood cell (WBC) count, neutrophil-to-lymphocyte ratio (NLR), and C-reactive protein (CRP) were measured at pre-operation, at the end of the operation, and at POD 1, 2, 3, and 5. Postoperatively administered doses of fentanyl at postoperative 6, 12, and 24 h were registered using the PCA device, which automatically recorded the delivered quantities of drug every 30 min. The number of patients who required rescue analgesics (25 mg of intravenous pethidine) was also registered up to postoperative 24 h.
Statistical analysis
The primary outcome was perioperative syndecan-1, which was compared between volatile anesthesia and TIVA groups. Previous studies on perioperative changes of syndecan-1 concentration after abdominal surgery with pneumoperitoneum were not found; thus, this study was designed with 68 patients per group. This number is higher than that of previous studies on the effect of sevoflurane and propofol on perioperative syndecan-1 shedding9,10.
Continuous variables are shown as mean ± SD and categorical variables are expressed as number of patients (percentage). Group differences for continuous variables were analyzed by the Student’s t test, which is valid according to the central limit theorem because of the large number of patients (65 and 67). Differences in categorical variables were checked by the Chi-square test or by the Fisher’s exact test: the former was employed if the portion of cells with an expected cell frequency of less than 5 was less than 20% of all the cells, and the latter was employed otherwise. For repeated-measure variables, a linear mixed model analysis was employed to determine group and time effects and a compound symmetry covariance structure was used for the within-subject effect. The Bonferroni correction was used to adjust for multiple comparison in post-hoc analyses. A P value of less than 0.05 was considered to be significant. All analyses were conducted using SAS version 9.4 (Cary, NC).
References
Reitsma, S., Slaaf, D. W., Vink, H., van Zandvoort, M. A. M. J. & oude Egbrink, M. G. A. The endothelial glycocalyx: Composition, functions, and visualization. Pflugers Arch. 454, 345–359 (2007).
Bashandy, G. M. Implications of recent accumulating knowledge about endothelial glycocalyx on anesthetic management. J. Anesth. 29, 269–278 (2015).
Song, J. W. & Goligorsky, M. S. Perioperative implication of the endothelial glycocalyx. Korean J. Anesthesiol. 71, 92–102 (2018).
Rehm, M. et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 116, 1896–1906 (2007).
Steppan, J. et al. Sepsis and major abdominal surgery lead to flaking of the endothelial glycocalix. J. Surg. Res. 165, 136–141 (2011).
Gonzalez Rodriguez, E. et al. Syndecan-1: A quantitative marker for the endotheliopathy of trauma. J. Am. Coll. Surg. 225, 419–427 (2017).
Pustetto, M. et al. Intravenous lidocaine to prevent endothelial dysfunction after major abdominal surgery: A randomized controlled pilot trial. BMC Anesthesiol. 20, 155 (2020).
Anand, D., Ray, S., Srivastava, L. M. & Bhargava, S. Evolution of serum hyaluronan and syndecan levels in prognosis of sepsis patients. Clin. Biochem. 49, 768–776 (2016).
Kim, H. J. et al. Sevoflurane did not show better protective effect on endothelial glycocalyx layer compared to propofol during lung resection surgery with one lung ventilation. J. Thorac. Dis. 10, 1468–1475 (2018).
Maldonado, F. et al. Effect of sevoflurane and propofol on tourniquet-induced endothelial damage: A pilot randomized controlled trial for knee-ligament surgery. BMC Anesthesiol. 20, 121 (2020).
Wang, J., Wu, A. & Wu, Y. Endothelial glycocalyx layer: A possible therapeutic target for acute lung injury during lung resection. Biomed. Res. Int. 2017, 5969657 (2017).
Lindberg-Larsen, V. et al. The effect of pre-operative methylprednisolone on early endothelial damage after total knee arthroplasty: A randomised, double-blind, placebo-controlled trial. Anaesthesia 72, 1217–1224 (2017).
Dekker, N. A. M. et al. Postoperative microcirculatory perfusion and endothelial glycocalyx shedding following cardiac surgery with cardiopulmonary bypass. Anaesthesia 74, 609–618 (2019).
Annecke, T. et al. Sevoflurane preserves the endothelial glycocalyx against ischaemia–reperfusion injury. Br. J. Anaesth. 104, 414–421 (2010).
Chappell, D. et al. Sevoflurane reduces leukocyte and platelet adhesion after ischemia–reperfusion by protecting the endothelial glycocalyx. Anesthesiology 115, 483–491 (2011).
Annecke, T. et al. Ischemia–reperfusion-induced unmeasured anion generation and glycocalyx shedding: Sevoflurane versus propofol anesthesia. J. Invest. Surg. 25, 162–168 (2012).
Sista, F. et al. Systemic inflammation and immune response after laparotomy vs laparoscopy in patients with acute cholecystitis, complicated by peritonitis. World J. Gastrointest. Surg. 5, 73–82 (2013).
Okholm, C., Goetze, J. P., Svendsen, L. B. & Achiam, M. P. Inflammatory response in laparoscopic vs. open surgery for gastric cancer. Scand. J. Gastroenterol. 49, 1027–1034 (2014).
Sammour, T. et al. Systematic review of oxidative stress associated with pneumoperitoneum. Br. J. Surg. 96, 836–850 (2009).
Potter, D. R., Jiang, J. & Damiano, E. R. The recovery time course of the endothelial cell glycocalyx in vivo and its implications in vitro. Circ. Res. 104, 1318–1325 (2009).
Dostalova, V. et al. The effect of fluid loading and hypertonic saline solution on cortical cerebral microcirculation and glycocalyx integrity. J. Neurosurg. Anesthesiol. 31, 434–443 (2019).
Zahorec, R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy 102, 5–14 (2001).
Cruz, F. F., Rocco, P. R. M. & Pelosi, P. Anti-inflammatory properties of anesthetic agents. Crit. Care 21, 67 (2017).
Funding
This study was funded by a National Research Foundation of Korea grant from the Korean government (MSIP) (NRF-2017R1C1B5017365).
Author information
Authors and Affiliations
Contributions
Conceptualization: N.Y.K., K.J.K. K.-Y.L. and S.Y.K.; Data curation: N.Y.K., J.C., D.J.N., and S.Y.K.; Project administration: N.Y.K., K.J.K., K.-Y.L. and S.Y.K.; Methodology: N.Y.K., H.J.S., and S.Y.K.; Investigation and formal analysis: N.Y.K., H.J.S., J.C., D.J.N., and S.Y.K.; Writing-original draft: N.Y.K., and S.Y.K.; Writing-Review & Editing: N.Y.K., K.J.K., K.-Y.L., H.J.S., J.C., D.J.N., and S.Y.K.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, N.Y., Kim, K.J., Lee, KY. et al. Effect of volatile and total intravenous anesthesia on syndecan-1 shedding after minimally invasive gastrectomy: a randomized trial. Sci Rep 11, 1511 (2021). https://doi.org/10.1038/s41598-021-81012-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-81012-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.