Abstract
Dry reforming of methane (DRM) is a well-known process in which CH4 and CO2 catalytically react to produce syngas. Solid carbon is a well-known byproduct of the DRM but is undesirable as it leads to catalyst deactivation. However, converting CO2 and CH4 into solid carbon serves as a promising carbon capture and sequestration technique that has been demonstrated in this study by two patented processes. In the first process, known as CARGEN technology (CARbon GENerator), a novel concept of two reactors in series is developed that separately convert the greenhouse gases (GHGs) into multi-walled carbon nanotubes (MWCNTs) and syngas. CARGEN enables at least a 50% reduction in energy requirement with at least 65% CO2 conversion compared to the DRM process. The second process presents an alternative pathway for the regeneration/reactivation of the spent DRM/CARGEN catalyst using CO2. Provided herein is the first report on an experimental demonstration of a 'switching' technology in which CO2 is utilized in both the operation and the regeneration cycles and thus, finally contributing to the overall goal of CO2 fixation. The following studies support all the results in this work: physisorption, chemisorption, XRD, XPS, SEM, TEM, TGA, ICP, and Raman analysis.
Similar content being viewed by others
Introduction
Syngas production via DRM is being considered as an attractive CO2 conversion process. However, its industrial implementation is still subject to the development of more mature and advanced technology. The limitations of the DRM process can be attributed to the following: high endothermicity of the reaction; low syngas quality (i.e., H2: CO ≤ 1); and extremely high coking tendency that creates technological hurdles with prohibitive economic solutions. In contrast to the commercial technologies like: steam methane reforming (SRM), partial oxidation (POx), and auto-thermal reforming (ATR), the main benefit of DRM, however, is its ability to utilize CO2. The downstream plants like Fischer Tropsch (FTS) or methanol synthesis require an H2/CO ratio of 2:1, which cannot be fulfilled by the quality of the syngas produced from the DRM unit. To address this challenge, a study by Olah et al.1 reported a combination of SRM and DRM over NiO/MgO catalysts to produce an H2/CO ratio of 2:1, which is compatible with most of the downstream gas to liquid (GTL) facilities. However, a combination of DRM and SRM is less sustainable as both the processes are highly endothermic, resulting in significant indirect CO2 emissions. In this regard, life cycle assessment (LCA) presents a very promising technique in identifying and accounting for all the direct and indirect emissions in a particular process2. Regarding the second challenge of carbon formation, the accomplishments from the field of catalysis have resulted in the development of superior catalytic systems showcasing remarkable resistance towards carbon formation3,4,5,6,7,8,9,10,11. However, the third challenge of high endothermicity still remains, in addition to the fact that all the three challenges have never been addressed in a single process. Therefore, the research on the DRM process has always been active in the search for a radically new process targeting all the three challenges of DRM that would enable its commercial implementation.
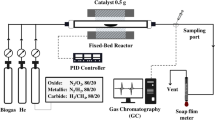
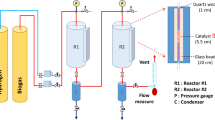
In this communication, reported is an experimental demonstration of a combination of two novel processes that enable DRM implementation12,13. The first process is a combination of two reactors in series, wherein the first reactor (also known as CARGEN or CARbon GENerator) converts CO2 and CH4 to multi-walled carbon nanotubes (MWCNTs). While the other reactor produces syngas with a desirable H2/CO ratio that meets downstream requirements. A systematic overview of the CARGEN process is provided in Fig. 1, and more details on its concept are provided in supplementary Section 1. The second process, on the other hand, utilizes CO2 during the catalyst regeneration cycle for the removal of surface carbon. A combination of CARGEN and the new CO2 regeneration process forms a switching technology that utilizes CO2 during both operations as well as the regeneration cycle.
Results and discussion
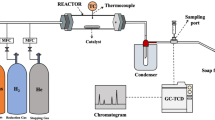
The thermodynamic equilibrium assessment of the CARGEN reactor shows that solid carbon is a favorable product in a temperature range of 420 °C to 600 °C as shown in Fig. 2a. The feed gas composition was CH4/CO2/O2 = 1/0.6/0.1, that closely resembles flue/landfill/biogas composition14 for ultimate FTS application15. However, this feed ratio may be changed depending upon available feed gas at site to get different results, and an optimization can also be done to achieve a desired quantity of carbon, or achieve a specific syngas ratio. Figure 2b shows the experimental conversion profile of the CARGEN reactor at a temperature of 550 °C, which closely matches with the theoretical results reported in Fig. 2a-ii. It should be noted that all the experimental studies in this work were conducted using commercially available 20% Ni/Al2O3 catalyst, as it is the most widely used reforming catalyst. However, other materials like Fe, Co, Mo, Ru, Pt, Rh etc. supported with SiO2, TiO2 etc. could be used, but will result in higher cost and sintering challenges. It can be seen in Fig. 2 that experimental results closely match thermodynamic results within a margin of 10–15%. It is important to note that the experimental conversions are lower compared to the estimated thermodynamic conversion due to kinetics and mass transfer limitations that arise during the catalytic reaction16. A closer examination of the actual spent catalyst from this experimental run using SEM and TEM (Fig. 2c) reveals the formation of MWCNTs.
(a) Thermodynamic profiles of the novel CARGEN reactor from 400 to 600 °C temperature, (i) Syngas ratio at CH4:CO2:O2 feed ratio of 1:/0.6/0.1. (ii) % Conversion of reactant gases. (iii) Energy requirement at reported temperature conditions, (iv) carbon formation tendency in moles at reported temperature conditions. (b) % Experimental conversions vs. time on stream (TOS) in a flow through reactor for a TOS of 500 min at 550 °C using 20 wt% Ni/Al2O3 catalyst (c-i, c-ii): SEM images at 10 μm and 1 μm respectively, (c-iii, c-iv): TEM images at 200 nm and 10 nm, respectively. Please note that the dotted lines in Fig. 2 a represent the temperature condition at which experiments were conducted that are reported in (b).
Raman analysis of a 30-min CH4 pyrolysis experiment on 20 wt% Ni/Al2O3 at 700, 600, 500, and 400 °C showed that the MWCNT formation regime is gradually diminished with a decrease in temperature, and only nano-carbon remains at 400 °C. These results demonstrate that MWCNT begins forming after between 400 and 500 °C. Figure 3 illustrates the corresponding ex-situ micro-Raman spectra, which indicates that the quality of the MWCNT gets better (D/G ratio decreases while G'/G ratio increases) with an increase in temperature. It should be noted that the operational window of CARGEN is in the temperature range of 400–600 °C, which is the most suitable condition for MWCNT formation per Raman results. High-value products coming from GHGs have attracted significant media attention17,18,19,20 as well as interest from global energy corporations due to its encouraging preliminary economic data21. The commercial value of high-quality MWCNTs can be in the range of 500–10,000 USD/kilogram22. Although a detailed techno-economic assessment is underway, the preliminary life cycle assessment (LCA) study revealed that both the CO2 footprint and the operational cost of the CARGEN process are approx. 40% that of conventional ATR processes21.
A thermo-gravimetric analysis (TGA) experiment coupled with material balance on residual gas analyzer (RGA) data enabled direct assessment of the carbon formation rate and feed conversions as a function of TOS. These results are provided in Fig. 4 and detailed calculation steps of material balance in supplementary Sect. 3. Around 20 mg carbon formation was observed in 138 min TOS at 550 °C on 20 mg of commercial 20 wt% Ni/γ-Al2O3 catalyst entailing a remarkable carbon formation rate of 0.00722 mgMWCNT/mgCat./min. It should be reiterated that the target for CARGEN reactor (the first reactor in CARGEN technology) is only to produce solid carbon. The second reactor on the other hand, which is a combined reformer offers a great flexibility in producing a desirable syngas ratio as discussed in detail in previous publications12,23.
Subsequent to the production of milli-grams of carbon in TGA, a larger batch of solid carbon was produced in a chemical vapor deposition (CVD) setup to produce multi-grams of carbon. One of the sample produced from this experiment was at ~ 80 wt% carbon purity (and remaining catalyst weight). In order to deduce the quality of the as-produced samples, a TGA air oxidation experiment was then conducted. The protocol for this test involved air combustion of carbon at 50 mL/min in the TGA on ca. 10 mg of the sample under a temperature ramp of 10 °C/min from room temperature to 400 °C, and at 5 °C/min from 400 °C to 800 °C. Figure 5a,b shows that only < 1% of the sample was oxidized between 200 and 500 °C, which is the literature reported range of amorphous carbon24,25,26. As the combustion occured in the range of 480 to 700 °C temperature indicates that crystalline form of carbon was primarily present, of which MWCNTs are predominant category24,25,26. Consolidating the TGA information to the SEM and TEM micrographs infers that most of the carbon belongs to MWCNT category with diameters in the range of 50 to 100 nm and length in the range of 10 to 30 µm as shown in Fig. 5. Moreover, the HR-TEM images at 10 nm scale bar shows that a tip-growth mechanism of CNT production would have taken place during the CARGEN process.
(a) Differential Thermogravimetric profiles of the MWCNTs produced from CARGEN and from commercial source, (b) Weight loss profiles of the commercial MWCNTs and the CARGEN produced MWCNTs, (c) SEM micrograph showing the length and diameter of the MWCNTs produced from CARGEN, (d) TEM images of the MWCNTs showing tip-growth mechanism and the multi-walled hollow characteristic of the as-produced MWCNTs from CARGEN.
One of the primary challenges in any industrial process that involves carbon formation like DRM, or catalytic cracker units is catalyst deactivation. Due to this, the entire plant has to be taken into maintenance, and the bulk of carbon has to be removed by either scraping or by using other carbon removal techniques like etching, sonication, etc. However, even after the removal of bulk carbon, the catalyst nano sites are still covered with surface carbon27, which needs to be removed to reactivate the catalyst. In this paper, a novel approach of a single-step catalyst regeneration procedure, applicable for any process that suffers from catalyst deactivation via coke formation17 is presented. The said process utilizes CO2 as a soft oxidant28 for the removal of surface carbon that deactivated the catalyst. This presents a great improvement over conventional procedure, as the conventional procedure requires two steps- first, oxidation with O2 for carbon removal as CO2, followed by the reduction of nickel oxide with H2 to produce active nickel. The new procedure is precisely similar to temperature-programmed oxidation (TPO) using O2 but utilizes CO2 in its place. The reaction is proposed to happen via reverse-boudouard route as follows:
This reaction was first tested using thermodynamic equilibrium assessment at temperatures in the range of 650 to 800 °C, followed by laboratory proof of concept study in a flow-through reactor. Under a temperature ramp from 650 to 800 °C, when the CO2 gas was passed through the spent DRM catalyst bed, it reacted with surface carbon and produced CO (which could be used as a precursor for several industrial chemicals). A subsequent DRM immediately after the regeneration showed activity without the need for a second reduction step by H2. Figure 6 shows the results of this test for three cycles that ensures repeatability. A combination of the operation and regeneration cycle as in Fig. 6 demonstrates an interesting switching process wherein uninterrupted CO2 utilization happens during both the cycles.
Three cycles of repeatability of the new regeneration technique demonstrating that there is no need for reduction after every surface carbon removal step using CO2. Inset figure: Concentration plot from RGA showing the release of CO during CO2 TPO. Conditions: 200 mg 20% Ni/γ-Al2O3, flow: 94 mL/min, T: 550 °C.
In order to evaluate the CO2 oxidation capability, pre-reduced 20 wt% Ni/Al2O3 catalyst was heated continuously from room temperature to 700 °C in CH4, then CH4 supply was cut off and one CO2 pulse was injected for 5 s and for 40 s respectively in two independent experiments. Figure 7 shows the SEM and Raman results of this study. It was observed that the quality of the CO2-treated MWCNTs becomes worse (D/G ratio increases while G'/G ratio decreases). It seems that the more defective carbon structures are preferentially removed from the Ni surface while the robust ones remain on the surface with more defects.
Results of XPS analysis of a coked commercial catalyst showed that the surface carbon atom percentage was about 75%. On the other hand, the surface carbon coverage of post-CO2 TPO treated catalyst was about 5%, while for post O2 TPO was 4.5%. This shows the equivalency of both the regeneration techniques in removing surface carbon. It should be emphasized that even though pure CO2 is required for CO2 TPO, it has a greater importance for DRM since its use will enable overall CO2 fixation.
In terms of sustainability benefits, it was observed that, the new CO2 TPO process is capable of converting at least 3 kg of CO2 per kg of surface carbon removed, while O2 TPO leads to about 0.5 kg of CO2 emission per kg of surface carbon. Detailed calculation steps are provided in Sect. 5 of the Supplementary Information. It should be emphasized that the use of CO2 regeneration technique also saves the active catalyst (Ni) from undergoing cycles of oxidation (forming inactive NiO) and reduction (forming Ni) as in the case of conventional regeneration technique.
Conclusion
In conclusion, the reported work experimentally demonstrates the formation of MWCNTs in the novel CARGEN process. Additionally, a unique single step procedure to regenerate coked spent DRM/ CARGEN catalyst using CO2 as an activation media is also reported. A combination of the two processes (DRM/CARGEN + CO2 regeneration) provides a novel switching technique that utilizes CO2 during both operations as well regeneration cycles, proving to be a strong candidate for the commercialization of the DRM/CARGEN process that enables overall CO2 fixation.
References
Olah, G. A., Goeppert, A., Czaun, M. & Prakash, G. K. S. Bi-reforming of methane from any source with steam and carbon dioxide exclusively to metgas (CO-2H2) for methanol and hydrocarbon synthesis. J. Am. Chem. Soc. 135(2), 648–650 (2013).
Afzal, S., Sengupta, D., Sarkar, A., El-Halwagi, M. & Elbashir, N. Optimization approach to the reduction of CO2 emissions for syngas production involving dry reforming. ACS Sustain. Chem. Eng. 6(6), 7532–7544 (2018).
Shi, L.-Y. et al. Fabrication of highly dispersed nickel in nanoconfined spaces of as-made SBA-15 for dry reforming of methane with carbon dioxide. Chem. Eng. J. 390, 124491 (2020).
Gavrilova, N. N., Sapunov, V. N. & Skudin, V. V. Intensification of dry reforming of methane on membrane catalyst. Chem. Eng. J. 374, 983–991 (2019).
Durán, P., Sanz-Martínez, A., Soler, J., Menéndez, M. & Herguido, J. Pure hydrogen from biogas: intensified methane dry reforming in a two-zone fluidized bed reactor using permselective membranes. Chem. Eng. J. 370, 772–781 (2019).
Benguerba, Y., Virginie, M., Dumas, C. & Ernst, B. Computational fluid dynamics study of the dry reforming of methane over Ni/Al2O3 catalyst in a membrane reactor. Coke deposition. Kinet. Catal. 58(3), 328–338 (2017).
Song, Y. et al. Dry reforming of methane by stable Ni–Mo nanocatalysts on single-crystalline MgO. Science 367(6479), 777–781 (2020).
Kim, S. M. et al. Cooperativity and dynamics increase the performance of NiFe dry reforming catalysts. J. Am. Chem. Soc. 139(5), 1937–1949 (2017).
Wang, Y. et al. Low-temperature catalytic CO2 dry reforming of methane on Ni-Si/ZrO2 Catalyst. ACS Catal. 8(7), 6495–6506 (2018).
Akri, M. et al. Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat. Commun. 10(1), 1–10 (2019).
Foppa, L. et al. Contrasting the role of Ni/Al2O3 interfaces in water-gas shift and dry reforming of methane. J. Am. Chem. Soc. 139(47), 17128–17139 (2017).
Elbashir, N. O., Challiwala, M. S., Sengupta, D. & El-Halwagi, M. M. System and method for carbon and syngas production, PCT/US2018/025696 (2018).
Challiwala, M., Choudhury, H. A. & Elbashir, N. O. Regeneration & activation of dry reforming of methane catalysts using CO2, PCT/QA2019/050005 (2019).
Usman, M., Wandaud, W. M. A. & Abbas, H. F. Dry reforming of methane: influence of process parameters—a review. Renew. Sustain. Energy Rev. 45, 710–744 (2015).
Buragohain, B., Mahanta, P. & Moholkar, V. S. Thermodynamic optimization of biomass gasification for decentralized power generation and Fischer-Tropsch synthesis. Energy 35(6), 2557–2579 (2010).
Xu, J. & Froment, G. F. Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics. AIChE J. 35(1), 88–96 (1989).
Qatar Researchers Develop Natural Gas Processing Technology That Could Reduce Qatar’s Carbon Footprint - Texas A&M Today. [Online]. https://today.tamu.edu/2019/10/24/qatar-researchers-develop-natural-gas-processing-technology-that-could-reduce-qatars-carbon-footprint/. Accessed: 16 Feb 2020.
Qatar Researchers Develop Natural Gas Processing Technology That Could Reduce Qatar’s Carbon Footprint. [Online]. https://www.pollutiononline.com/doc/qatar-researchers-develop-natural-gas-processing-carbon-footprint-0001. Accessed 16 Feb 2020.
Researchers Develop Technology to Reduce Qatar’s Carbon Footprint | Qatar-America Institute. [Online]. https://qataramerica.org/researchers-develop-technology-to-reduce-qatars-carbon-footprint/. Accessed 16 Feb 2020.
Challiwala, M. S., Choudhury, H. A., Sengupta, D., El-Halwagi, M. M. & Elbashir, N. O. Turning CO2 into carbon nanotubes, Chemical Engineering Progress, New York, pp. 9–10 (2020).
Challiwala, M. S., Afzal, S., Choudhury, H. A., Sengupta, D., El-halwagi, M. & Elbashir, N. O. Alternative pathways for CO2 utilization for enhanced methane dry reforming technology. In Advances in Carbon Management Technologies, Chap. 12 (eds Sikdar, Subhas K. and Princiotta, Frank) 253–272 (2019).
Cheaptubes.com, Multi Walled Carbon Nanotubes. [Online]. https://www.cheaptubes.com/product-category/multi-walled-carbon-nanotubes/.
Noureldin, M. M., Elbashir, N. O., Gabriel, K. J. & El-Halwagi, M. M. (2015). A process integration approach to the assessment of CO2 fixation through dry reforming. ACS Sustain. Chem. Eng. 3(4), 625–636.
Mansfield, E., Kar, A. & Hooker, S. A. Applications of TGA in quality control of SWCNTs. Anal. Bioanal. Chem. 396(3), 1071–1077 (2010).
Trigueiro, J. P. C. et al. Purity evaluation of carbon nanotube materials by thermogravimetric, TEM, and SEM methods. J. Nanosci. Nanotechnol. 7(10), 3477–3486 (2007).
Murakami, Y., Miyauchi, Y., Chiashi, S. & Maruyama, S. Characterization of single-walled carbon nanotubes catalytically synthesized from alcohol. Chem. Phys. Lett. 374(1–2), 53–58 (2003).
Gili, A. et al. Surface carbon as a reactive intermediate in dry reforming of methane to syngas on a 5% Ni/MnO catalyst. ACS Catal. 8(9), 8739–8750 (2018).
Mahamulkar, S. et al. Formation and oxidation/gasification of carbonaceous deposits: a review. Ind. Eng. Chem. Res. 55(37), 9760–9818 (2016).
Acknowledgements
This work was supported by the Qatar National Research Fund (QNRF), a member of Qatar Foundation (Grant NPRP X-100- 2-024). The statements made herein are solely the responsibility of the authors. We would like to acknowledge help from Dr. Zafar Khan Ghouri at Texas A&M University at Qatar (TAMUQ) on XPS data. We would also like to acknowledege help from Dr. Said Mansoor at Qatar Environment and Energy Research Institute (QEERI) for his most valuable inputs on HR-TEM, STEM and XPS studies.
Funding
Open access funding provided by the Qatar National Library..
Author information
Authors and Affiliations
Contributions
M.S.C. conducted theoretical concept development, experimental work, and manuscript writing. H.A.C. was involved in experimental apparatus development, conducting various experimental activities, and in manuscript writing. D.W. contributed to Raman analysis and characterization studies. M.M. E.-H. supervised contribution to theoretical and conceptual activities during the CARGEN technology development stage. E.W. contributed to supervising Raman analysis, characterization studies, manuscript drafting, and reviewing. N.O.E. supervised and contributed to theoretical concept development, experimental activities, manuscript drafting, and reviewing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Challiwala, M.S., Choudhury, H.A., Wang, D. et al. A novel CO2 utilization technology for the synergistic co-production of multi-walled carbon nanotubes and syngas. Sci Rep 11, 1417 (2021). https://doi.org/10.1038/s41598-021-80986-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-80986-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.