Abstract
The purpose of this retrospective interventional case series is to compare the functional and anatomical outcomes in eyes with diabetic macular edema (DME) and proliferative diabetic retinopathy (PDR) treated intravitreally with aflibercept or ranibizumab under the Taiwan National Insurance Bureau reimbursement policy. 84 eyes were collected and all eyes were imaged with spectral-domain optical coherence tomography (SD-OCT), color fundus photographs (CFPs), and fluorescein angiography (FA). At 24 months after therapy initiation, the logMAR BCVA improved from 0.58 ± 0.33 to 0.47 ± 0.38 (p < 0.01), the CRT decreased from 423.92 ± 135.84 to 316.36 ± 90.02 (p < 0.01), and the number of microaneurysms decreased from 142.14 ± 57.23 to 75.32 ± 43.86 (p < 0.01). The mean injection count was 11.74 ± 5.44. There was no intergroup difference in logMAR BCVA (p = 0.96), CRT (p = 0.69), or injection count (p = 0.81). However, the mean number of microaneurysms was marginally reduced (p = 0.06) in eyes treated with aflibercept at the end of the follow-up, and the incidence rates of supplementary panretinal photocoagulation (PRP) (p = 0.04) and subthreshold micropulse laser (SMPL) therapy sessions (p = 0.01) were also reduced. Multivariate analysis revealed that only initial logMAR BCVA influenced the final VA improvements (odds ratio (OR) 0.49, 95% confidence interval (CI) 0.21 ~ 0.93, p < 0.01); in contrast, age (OR − 0.38, 95% CI − 6.97 ~ − 1.85, p < 0.01) and initial CRT (OR 0.56, 95% CI 0.34 ~ 0.84, p < 0.01) both influenced the final CRT reduction at 24 months. To sum up, both aflibercept and ranibizumab are effective in managing DME with PDR in terms of VA, CRT and MA count. Eyes receiving aflibercept required less supplementary PRP and SMPL treatment than those receiving ranibizumab. The initial VA influenced the final VA improvements at 24 months, while age and initial CRT were prognostic predictors of 24-month CRT reduction.
Similar content being viewed by others
Introduction
Anti-vascular endothelial growth factor (anti-VEGF) agents have been accepted as the first-line treatment for diabetic macular edema (DME). Recently, protocol S also showed the good efficacy of ranibizumab in regressing preretinal and prepapillary neovascularization and lowering the stage of diabetic retinopathy (DR) as part of the management of proliferative DR (PDR)1. In addition, eyes receiving ranibizumab had a reduced chance of developing peripheral visual field loss and center-involved macular edema. Similar study results were shown at the 1-year follow-up in the CLARITY randomized clinical trial using aflibercept2.
Anti-VEGF agents, including both ranibizumab and aflibercept, have been reimbursed by the National Taiwan Health Insurance Bureau for DME but not PDR since 2014. A lifetime maximum of 8 injections per eye are eligible for reimbursement, and patients are not allowed to shift from one anti-VEGF agent to another. In this study, we aimed to observe the clinical outcomes of anti-VEGF treatment in eyes with DME and PDR under the reimbursement policy of the National Taiwan Health Insurance Bureau and compare the clinical outcomes of ranibizumab and aflibercept treatment in eyes with DME and coexistent PDR.
Results
Eighty-four eyes of 72 patients (M:F = 41:31, mean age: 58.57 ± 10.76) were included in the study. Forty-one eyes that received aflibercept were categorized into group 1, and 43 eyes that received ranibizumab were categorized into group 2. The mean logMAR best-corrected visual acuity (BCVA) at baseline was 0.58 ± 0.33. There was no significance intergroup difference in the patients’ basic profiles, initial logMAR BCVA, CRT, or retinal morphology; these data are summarized in Table 1.
At 24 months after the initiation of the treatment, the mean number of injections was 11.74 ± 5.44 (p = 0.91), the logMAR BCVA improved from 0.58 ± 0.33 to 0.47 ± 0.38 (p < 0.01), the central retinal thickness (CRT) decreased from 423.92 ± 135.84 to 316.36 ± 90.02 (p < 0.01), and the counts of microaneurysms (MAs) decreased from 142.14 ± 57.23 to 75.32 ± 43.86 (p < 0.01). There was no difference in the injection count, logMAR BCVA or CRT between the two groups (Table 2).
On the other hand, for cases that had more than 8 injections, macular edema was the main reason (93.86%), other reasons including vitreous hemorrhage (3.22%), new retinal neovascularization (0.58%), neovascularization of iris (0.58%), and preparing for pars plana vitrectomy (PPV) to eliminate possible hemorrhage during operation (1.17%). There was no difference between cases received less or equal to 8 and cases had additional injections regarding final BCVA, CRT, and MAs.
New-onset vitreous hemorrhage (VH) was noted in 27.38% of eyes. The incidence of new VH during the follow-up period was higher in the ranibizumab group than in the aflibercept group, but the difference was not statistically significant (p = 0.33). PPV was performed in 7.14% of eyes for recurrent or non-clearing VH. Although the rate of PPV was higher in eyes receiving ranibizumab, there was no significant difference (4.88% in the aflibercept group and 9.30% in the ranibizumab group, p = 0.68).
Supplementary pan-retinal photocoagulation (PRP) was needed in 33.33% of eyes. The supplementary PRP were prescribed in cases whom had new onset VH (71.42%), neovascular glaucoma (14.29%), faint PRP scars based on the physician’s experience (10.71%), and neovascularization of iris (3.57%). More cases in the ranibizumab group than in the aflibercept group required supplementary PRP (p = 0.04), and more sessions of supplementary PRP were needed (p = 0.02). A comparison was made between PRP naïve and PRP-treated at baseline, but no difference was noticed regarding the supplementary sessions (aflibercept group p = 0.46, ranibizumab group p = 0.08), and the rate (aflibercept group p = 0.68, ranibizumab group p = 0.52). Subthreshold macular pulse laser (SMPL) for persistent DME was performed in 15.48% of eyes, with a significantly higher number in the ranibizumab group (p = 0.01). In contrast, the rate of focal retinal photocoagulation (FRP) in all eyes was 8.33%, with no significant difference between the two groups (p = 0.43). For neovascular glaucoma (NVG) and neovascularization of the iris (NVI), the incidence was 7.14% and 1.19%, respectively, for all eyes, and no differences were noted between these 2 groups. The area of retinal neovascularization within the seven field areas also markedly decreased for all eyes (from 1.30 ± 1.90 to 0.10 ± 0.52, p < 0.01), and no difference was noted between the 2 groups (p = 0.47). A decreased area of disorganization of Retinal Inner Layers (DRIL) (from 324.19 ± 306.46 to 299.55 ± 272.79, p = 0.5) was also noticed; however, the difference was not statistically significant.
Regarding procedure-related complications, 1 eye in group 1 had rhegmatogenous retinal detachment; 1 eye in group 2 had endophthalmitis; and 1 eye in group 2 had tractional retinal detachment, which required PPV.
The multivariate analysis revealed that only initial logMAR BCVA influenced the final VA improvements (OR 0.49, 95% CI 0.21 ~ 0.93, p < 0.01); on the other hand, age (OR − 0.38, 95% CI − 6.97 ~ − 1.85, p < 0.01) and initial CRT (OR 0.56, 95% CI 0.34 ~ 0.84, p < 0.01) both influenced the final CRT reduction at 24 months (Table 3).
Discussion
Recently, there has been increasing interest in the use of anti-VEGF agents to treat PDR1. Both ranibizumab and aflibercept have been shown to prevent DR progression and improve scores on the Diabetic Retinopathy Severity Scale (DRSS)1,2,3,4,5.
In this retrospective study, patients with DME and PDR had significant visual improvement, CRT reduction, and decreased MA counts after 24 months of anti-VEGF treatment. MA counts have been well characterized in a previous study as a biomarker for grading DR severity6. MA counts have recently been shown to improve after intravitreal injection of anti-VEGF7,8,9, which suggests an improvement in DR. Although the total number of injections is limited by the reimbursement policy of the Taiwan Insurance Bureau, good anatomical and functional outcomes can be achieved.
On the other hand, the length of DRIL did not change in the present study (p = 0.51). Nicholson et al. reported the association between DRIL and capillary nonperfusion on FA10. A recent study further demonstrated the presence of multilayer ischemia in DRIL by OCT angiography11. The stationary length of DRIL may imply that the ischemic process of DR remains undeteriorated during the treatment period, which echoes the finding of another study that the area of nonperfusion did not change despite 3 monthly injections of anti-VEGF8.
PPV for the complications of PDR was carried out in 6/84 patients (7.14%) in our series. Compared to the previous study of protocol S, which compared the efficacy of PRP and intravitreal injection (IVI) of ranibizumab in controlling PDR1, the incidence of PPV in our study was lower than that in the PRP group but higher than that in the IVI group in protocol S. However, since the number of injections per patient was far lower in our study than in protocol S, it is not surprising that there was a higher incidence of PPV.
In this study, although there was no intergroup difference in BCVA or CRT at 24 months, MAs were marginally higher in the ranibizumab group. In addition, supplementary PRP was performed significantly more in the ranibizumab group than in the aflibercept group (p = 0.04). These results may implicate that aflibercept improved the grade of DR more effectively than ranibizumab, as shown in previous reports12,13. Since the placental growth factor (PGF) correlates with the severity of PDR14, the different molecular mechanisms between aflibercept and ranibizumab might be one of the causative reasons15. In addition, there was a significantly higher chance of eyes in the ranibizumab group receiving SMPL (p = 0.01) to further control the status of macular edema.
However, in the multivariate analysis, when we considered each patient’s basic profile, the type of anti-VEGF the patient received, the number of injections, the initial VA, the CRT, the MA count, and the use of laser therapy, only the initial VA predicted the VA improvements after 24 months of treatment (OR 0.49, p < 0.01): the worse the initial VA, the more it would improve by 24 months. Similar results were noticed for the CRT reduction: the greater the initial CRT, the more it would decrease. In addition, age was another factor influencing CRT reduction. These results were similar to previous reports that younger patients with greater initial CRT were likely to have greater CRT reduction12,16.
There was no detected difference in ocular safety concerns between aflibercept and ranibizumab during the follow-up period. The rates of endophthalmitis and other injection-related adverse events were low, as in previous studies [1 ~ 5, 12]. The rates of NVI, NVG, and tractional retinal detachment were also low and showed no difference between the two groups.
Previous studies have tried to reflect the clinical practice in DME treated with anti-VEGF in Taiwan, but the follow-up period was relatively shorter17,18,19. In addition, no study has presenting the relationship between anti-VEGF and cases coexist PDR yet. To our best knowledge, this is the first real-world data demonstrating such cases treated with anti-VEGF in Taiwan. However, this study had several limitations. The data were collected retrospectively, and in place of strict protocols, doctors and patients decided at their own discretion whether procedures would be performed. Moreover, in Taiwan, only a limited number of anti-VEGF injections can be reimbursed, which may affect patients’ willingness to have additional IVIs beyond the reimbursed injections. Future prospective studies with larger sample sizes and long-term follow-up are necessary to clarify the benefit of anti-VEGF agents and the difference between different anti-VEGF agents in the treatment of PDR and DME.
In conclusion, anti-VEGF agents including ranibizumab and aflibercept were effective in improving visual acuity and reducing CRT in eyes with DME and PDR under the reimbursement policy of the Taiwan National Insurance Bureau. Although there was no difference in BCVA and CRT outcomes between ranibizumab and aflibercept at 2 years after treatment, the numbers of MAs, supplementary PRP sessions, and SMPL sessions were higher in the ranibizumab group than in the aflibercept group. However, only the initial VA influenced the VA improvements at 2 years.
Methods
The study was a retrospective, interventional, comparative study of eyes with DME and PDR that were reimbursed for either ranibizumab or aflibercept from the National Insurance Bureau for DME treatment at Changhua Christian Medical Center, a tertiary center in Taiwan, from November 2014 to March 2020. The study was approved by the Institutional Review Board of Changhua Christian Hospital and was conducted according to the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients.
All the eyes included in this study fulfilled the criteria for reimbursement for DME by the National Taiwan Insurance Bureau, which included the following: 1. presence of DME, 2. CRT greater than 300 microns as measured by spectral domain optical coherence tomography (SD-OCT), 3. Best-corrected decimal visual acuity ranging from 0.05 to 0.5, and 4. HbA1c below 10%.
In addition to the reimbursement criteria, we applied additional inclusion criteria as follows: 1. age 18 to 80 years, 2. available standard 7-field color fundus photography (CFP), 3. PDR according to fluorescein angiography (FA), 4. no VH obscuring the posterior pole at baseline so that that macular morphological status in the central 6 mm could be obtained by SD-OCT (Spectralis, Heidelberg Engineering, Heidelberg, Germany), 4. treatment with either aflibercept or ranibizumab, without switching to other agents, and 5. more than 2 years of follow-up.
Each patient’s age, sex, and HbA1c level were recorded. BCVA, slit-lamp biomicroscopy findings, indirect ophthalmoscopy results and SD-OCT data from each visit were documented. The procedures and surgeries received during each clinic visit were documented as well.
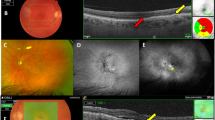
CFP and FA images were assessed for MAs, retinal neovascularization area, and nonperfusion within the standard seven fields. MAs were quantified in FA and CFP by two independent physicians (Hsieh MC and Cheng CY) using Image J. In the event of disagreement, a third investigator (Chen SN) was consulted for final determination. The MAs were defined as small circular hyperfluorescence objects with surrounding choroidal background in early phase FA20.
The CRT was measured by SD-OCT at a diameter of 100 microns centered at the fovea. DRIL was defined as any unidentifiable boundaries between the ganglion cell-inner plexiform layer complex, inner nuclear layer, and outer plexiform layer21,22. The length of DRIL was measured at a 6 mm scan centered on the fovea.
Outcomes
Outcome measures included the final BCVA, mean changes in BCVA from baseline to 2 years, CRT, the regression rate of neovascularization, numbers of injections, sessions of supplementary PRP, FRP, SMPL, presence of VH, NVI, NVG, new-onset epiretinal membrane, and the numbers of MAs within zone 1 and the seven fields. The number of cases that underwent vitrectomy because of tractional retinal detachment and persistent or frequent recurrence of VH was also documented. Other related condition including endophthalmitis, cataract surgery, and retinal detachment were also recorded.
Statistical analysis
Decimal visual acuity was converted to logMAR units for statistical analysis. All analyses were performed using SPSS 23.0 software (SPSS, Chicago, IL, USA). The Mann–Whitney U test and the Wilcoxon signed-rank test were used for continuous variables. The chi-square test or Fisher’s exact test was used for categorical variables. Factors influencing the outcome were analyzed by logistic regression. The odds ratio was calculated, and the 95th percentile confidence intervals (95% CI) were determined. A p value less than 0.05 was considered statistically significant.
References
Diabetic retinopathy clinical research network. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy. A randomized clinical trial. JAMA Ophthalmol. 314(20), 2137–2146 (2015).
Sivaprasad, S. et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): A multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet 389(10085), 2193–2203 (2017).
Ip, M. S., Domalpally, A., Hopkins, J. J., Wong, P. & Ehrlich, J. S. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch. Ophthalmol. 130(9), 1145–1152 (2012).
Ip, M. S., Domalpally, A., Sun, J. K. & Ehrlich, J. S. Long-term effects of therapy with ranibizumab on diabetic retinopathy severity and baseline risk factors for worsening retinopathy. Ophthalmology 122(2), 367–374 (2015).
Brown, D. M. et al. Evaluation of intravitreal aflibercept for the treatment of severe nonproliferative diabetic retinopathy. Results from the PANORAMA randomized clinical trial. JAMA Ophthalmol. 139(9), 946–955 (2021).
Ehlers, J. P. et al. Quantitative ultra-widefield angiography and diabetic retinopathy severity. An assessment of panretinal leakage index, ischemic index and microaneurysm count. Ophthalmology 126(11), 1527–1532 (2019).
Babiuch, A. et al. Longitudinal panretinal microaneurysm dynamics on ultra-widefield fluorescein angiography in eyes treated with intravitreal aflibercept for proliferative diabetic retinopathy in the recovery study. Br. J. Ophthalmol. 2, 316952 (2020).
Bonnin, S. et al. Anti-vascular endothelial growth factor therapy can improve diabetic retinopathy score without change in retinal perfusion. Retina 39(3), 426–434 (2019).
Sugimoto, M. et al. Multiple effects of intravitreal aflibercept on microvascular regression in eyes with diabetic macular edema. Ophthalmol. Retina. 3(12), 1067–1075 (2019).
Nicholson, L. et al. Diagnostic accuracy of disorganization of the retinal inner layers in detecting macular capillary non-perfusion in diabetic retinopathy. Clin. Exp. Ophthalmol. 43(8), 735–741 (2015).
Onishi, A. C., Ashraf, M., Soetikno, B. T. & Fawzi, A. A. Multilevel ischemia in disorganization of the retinal inner layers on projection-resolved optical coherence tomography. Retina 39(8), 1588–1594 (2019).
Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N. Engl. J. Med. 372(13), 1193–1203 (2015).
Bressler, S. B. et al. Change in diabetic retinopathy through 2 years. Secondary analysis of a randomized clinical trial comparing Aflibercept, Bevacizumab, and Ranibizumab. JAMA Ophthalmol. 135(6), 558–568 (2017).
Kahtani, E. A. et al. Vitreous levels of placental growth factor correlate with activity of proliferative diabetic retinopathy and are not influenced by bevacizumab treatment. Eye (Lond.). 31(4), 529–536 (2017).
Stewart, M. W., Grippon, S. & Kirkpatrick, P. Aflibercept. Nat. Rev. Drug Discov. 11(4), 269–270 (2012).
Chen, Y. P., Wu, A. L., Chuang, C. C. & Chen, S. N. Factors influencing clinical outcomes in patients with diabetic macular edema treated with intravitreal ranibizumab: Comparison between responder and non-responder cases. Sci. Rep. 9, 10952 (2019).
Lai, T. T., Yang, C. M., Yang, C. H., Ho, T. C. & Hsieh, Y. T. Treatment outcomes and predicting factors for diabetic macular edema treated with ranibizumab—one-year real-life results in Taiwan. J. Formos. Med. Assoc. 118(1 Pt 1), 194–202 (2019).
Lin, H. W., Guo, C. Y. & Chou, K. P. Characteristics and treatment patterns of anti-VEGF use among patients with diabetic macular edema in Taiwan: Real-world data from population-based health claims. Value Health Retional Issues. 2, 2 (2020).
Tsai, M. J. & Cheng, C. K. Intravitreal aflibercept versus ranibizumab for diabetic macular edema in a Taiwanese health service setting. Semin. Ophthalmol. 36(3), 132–138 (2021).
Ehlers, J. P., Wang, K., Vasanij, A., Hu, M. & Srivastava, S. K. Automated quantitative characterization of retinal vascular leakage and microaneurysms in ultrawidefield fluorescein angiography. Br. J. Ophthalmol. 101(6), 696–699 (2017).
Sun, J. K. et al. Disorganization of the retinal inner layers as a predictor of visual acuity in eyes with center-involved diabetic macular edema. JAMA Ophthalmol. 132(11), 1309–1316 (2014).
Sun, J. K. et al. Neural retinal disorganization as a robust marker of visual acuity in current and resolved diabetic macular edema. Diabetes 64, 2560–2570 (2015).
Acknowledgements
We thank Yi-Ling Chen [Research assistant, Surgery Clinical Research Center, Changhua Christian Hospital for giving help in data collection and statistical analysis.
Author information
Authors and Affiliations
Contributions
S.N.C. and M.C.H. designed the project; C.Y.C., K.H.L., C.C.C., J.S.W., S.T.L., W.Y.L., S.L.C., Y.L.L., and S.N.C. participated in managing patients; M.C.H collected the data; S.N.C., and M.C.H. analyzed and interpreted the results; M.C.H. wrote the manuscript; S.N.C. contributed to the improvement of project and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hsieh, MC., Cheng, CY., Li, KH. et al. Diabetic macular edema and proliferative diabetic retinopathy treated with anti-vascular endothelial growth factor under the reimbursement policy in Taiwan. Sci Rep 12, 711 (2022). https://doi.org/10.1038/s41598-021-04593-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-04593-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.