Abstract
Awake craniotomy enables mapping and monitoring of brain functions. For successful procedures, rapid awakening and the precise evaluation of consciousness are required. A prospective, observational study conducted to test whether intraoperative hand strength could be a sensitive indicator of consciousness during the awake phase of awake craniotomy. Twenty-three patients who underwent awake craniotomy were included. Subtle changes of the level of consciousness were assessed by the Japan Coma Scale (JCS). The associations of hand strength on the unaffected side with the predicted plasma concentration (Cp) of propofol, the bispectral index (BIS), and the JCS were analyzed. Hand strength relative to the preoperative maximum hand strength on the unaffected side showed significant correlations with the Cp of propofol (ρ = − 0.219, p = 0.007), the BIS (ρ = 0.259, p = 0.002), and the JCS (τ = − 0.508, p = 0.001). Receiver operating characteristic curve analysis for discriminating JCS 0–1 and JCS ≥ 2 demonstrated that the area under the curve was 0.76 for hand strength, 0.78 for Cp of propofol, and 0.66 for BIS. With a cutoff value of 75% for hand strength, the sensitivity was 0.76, and the specificity was 0.67. These data demonstrated that hand strength is a useful indicator for assessing the intraoperative level of consciousness during awake craniotomy.
Similar content being viewed by others
Introduction
The use of intraoperative neuromonitoring during awake craniotomy prevents serious neurological sequelae and allows more extensive tumor resection1,2. Rapid awakening and maintenance of full consciousness after the cessation of general anesthesia are important for accurate monitoring of motor and language functions, which have a significant impact on the patient's quality of life after craniotomy3. Thus, proper monitoring of the level of consciousness is particularly important for successful procedures.
Target-controlled infusion (TCI) of propofol is used to achieve an adequate speed and quality of recovery of neurological functions from anesthesia4,5. Propofol has a short half-life and is rapidly metabolized to lower blood levels, making it a widely used drug in awake craniotomy. Changes in a patient's level of consciousness can be monitored by the bispectral index (BIS)6,7, which correlates well with the propofol concentration8, and is thus routinely used to monitor depth of anesthesia. The anesthesiologist determines whether the patient can be extubated and moved to the awake phase after confirming that the patient's BIS is elevated.
During awake craniotomy, patients may be somnolent or unable to fully follow verbal instructions, even though they have been awakened from anesthesia and extubated9. It has been shown that the return of BIS to preinduction values was associated with patients’ ability to perform intraoperative language testing10. However, during intraoperative monitoring, the presence of electrical artifacts reduces the reliability of the BIS. In addition, effective measurement of the BIS may not be possible if the BIS sensor cannot be placed on the forehead (e.g., bilateral frontal craniotomy). When reliable data from the BIS are not available to assess the patient's level of consciousness (e.g., electrophysiological artifact), other monitoring modalities are required.
Hand strength is routinely monitored as one of the important neurological functions (motor function). Because motor evoked potential (MEP) amplitude and latency were shown to be closely correlated with depth of anesthesia11, we expected that hand strength may also reflect the depth of anesthesia and the level of consciousness. If this were the case, we could monitor both motor function and arousal by one examination modality. Hand strength on the unaffected side is expected to represent arousal, and that on the affected side represents motor function. However, hand strength is often measured qualitatively by the examiner, and the relationship with the level of consciousness has not been well studied. In the present investigation, hand strength was assessed quantitatively by a hand dynamometer, and it was standardized by calculating percent strength relative to the preoperative maximum hand strength. Then, the aim was to test the hypothesis that hand strength on the unaffected side reflects the depth of anesthesia and can be used to monitor the level of consciousness.
Globally, the Glasgow Coma Scale (GCS) is most widely used to assess the level of consciousness, with a total score of 3–15: 1–4 for eye opening (E), 1–5 for verbal response (V), and 1–6 for best motor response (M)12,13,14. A higher score indicates better consciousness. In Japan, the Japan Coma Scale (JCS) is widely used together with the GCS (Table 1)15. The 1-digit code in the JCS consists of the scores of 3 (able to keep eyes open and obey, but unable to respond with names or places), 2 (residual disorientation), and 1 (mostly conscious). Zero represents clear consciousness. JCS 0 and 1 correspond to GCS 15, and JCS 2 and 3 correspond to GCS 1416, meaning that the JCS can discriminate subtle changes in consciousness after the patient opens his/her eyes and obeys verbal commands. During brain mapping of motor/language function or extracting the eloquent area, it is important that the patient is awake at the level of JCS 0 or 1. In patients immediately after extubation with a JCS of 2 or more, it is difficult to validly assess motor and language functions because of their slow reaction time17. Therefore, the level of consciousness required for continuous neuromonitoring was considered to be JCS 0 or 1. In addition, patients can sometimes open their eyes and obey a command immediately after extubation, but they are unable to speak9,18,19. The patients are regarded as JCS 3, but it is difficult to tell whether they are not fully awake or they are awake but unable to speak due to a seizure or insufficient recovery of language function due to remaining effects of anesthesia. Other limitations of the evaluation of consciousness by the coma scale were described by Guay et al.20. It allows only intermittent assessments of arousal. It provides only a categorical measure and may also yield equivocal responses. Moreover, it offers low time resolution, since repetition of the same questions in a short time period reduces the reliability of the response. In this context, additional modalities that can support the evaluation of the level of arousal are warranted. Hand strength may provide alternative measures of arousal with continuous values at a high time resolution. To evaluate the usefulness of hand strength on the unaffected side as an indicator of the intraoperative level of consciousness during awake craniotomy, its ability to discriminate between JCS 0–1 and JCS ≥ 2 was evaluated and compared with that of the BIS and other modalities.
Results
Characteristics of the study population
Twenty-three patients were included in the analysis; their characteristics are shown in Table 2. The mean age of the patients was 49.6 ± 17.4 years, and 19 (82.6%) were male. The lesions were in the left hemisphere in 17 patients and in the right hemisphere in six patients. The mean hand strength of the unaffected side measured preoperatively was 34.2 ± 9.1 kg, and that of the affected side was 31.4 ± 9.4 kg. During the awake craniotomy, the mean time from cessation of anesthetic administration to extubation was 19.4 ± 7.9 min.
Chronological changes in consciousness after extubation
The chronological changes in the patients' consciousness after extubation were evaluated using the JCS. Table 3 shows the mean (± standard deviation (SD)) minutes to reach each JCS level after extubation. The mean predicted plasma concentration (Cp) of propofol and the BIS score for each JCS are also shown. The number of patients at each JCS level was different because some did not become fully conscious, and others rapidly gained consciousness by skipping the level of JCS 10. Immediately after extubation, 18 patients were at JCS 10; 14.9 min were needed to reach JCS 3, 16.6 min to reach JCS 2, 26.4 min to reach JCS 1, and 30.0 min to reach JCS 0. The changes in consciousness level and hand strength for each patient are shown in Fig. 1. Percent hand strength relative to the preoperative maximum hand strength used as intraoperative hand strength in the analysis. The intraoperative hand strengths increased according to the improvement in the JCS in most cases, on both the unaffected and affected hands (Fig. 1).
The representative values of hand strength tended to increase with an increase in the Japan Coma Scale (JCS). The line graphs show the change in hand strength at each JCS for each patient (A: unaffected hand, B: affected hand). Several plots show a patient that had no change in consciousness from the beginning to the end of the measurement of hand strength during the awake state.
Correlations of hand strength, the Cp of propofol and the BIS with the JCS
The relationships between the JCS and the Cp of propofol, the BIS, and hand strength on both the affected and unaffected sides were evaluated using Kendall’s rank correlation coefficient. The Cp of propofol decreased from 0.82 ± 0.20 to 0.46 ± 0.06 µg/ml when the JCS decreased from 10 to 0, and this relationship showed a significant correlation (τ = 0.48, p < 0.01) (Fig. 2A). The BIS increased from 78.7 ± 10.8 to 88.7 ± 5.5 as the JCS decreased from 10 to 0, demonstrating a significant correlation (τ = − 0.27, p < 0.01) (Fig. 2B). As the JCS decreased from 10 to 0, hand strength on the unaffected side increased from 55.0 ± 24.7% to 84.4 ± 12.2%, showing a significant correlation (τ = − 0.51, p = 0.001) (Fig. 2C). Hand strength on the affected side also increased from 37.9 ± 34.2% to 89.4 ± 19.5%, and a significant correlation was observed (τ = − 0.35, p = 0.002) (Fig. 2D). Deviation in the hand strength was greater on the affected side than the unaffected side. The coefficients of variation at JCS 10, 3, 2, 1, and 0 were 0.45, 0.49, 0.24, 0.14, and 0.14 for the unaffected hand, and 0.90, 0.61, 0.40, 0.23, and 0.22 for the affected hand, respectively.
Plots showing the representative values averaged over the values for each Japan Coma Scale (JCS) period. The black bar shows the mean, and the values below the bars indicate the mean (standard deviation). Kendall’s rank correlation coefficient (τ) was used for analyses. (A) The predicted plasma concentration (Cp) of propofol decreases with the JCS (τ = 0.484, p < .0001). (B) The BIS increases with the JCS (τ = − 0.269, p = 0.003). (C) Hand strength on the unaffected side increases with the JCS (τ = − 0.508, p = 0.001). (D) Hand strength on the affected side increases with the JCS (τ = − 0.351, p = 0.002).
Hand strength was correlated with the Cp of propofol, reflecting depth of anesthesia
As shown above, the Cp of propofol, the BIS, and hand strength were significantly correlated with the JCS, indicating that they were good indicators of consciousness during awake craniotomy. Similarly, Spearman’s rank correlation coefficient analysis was conducted to determine the interactive effects of these parameters. A significant correlation was found between the Cp of propofol and hand strength on the unaffected side (Table 4; ρ = − 0.219, p = 0.007), but not with hand strength on the affected side (ρ = − 0.098, p = 0.151). This finding suggests that hand strength is also a good indicator of the depth of anesthesia. A significant correlation was also found between the BIS and hand strength on both sides (unaffected hand ρ = 0.259, p = 0.002; affected hand ρ = 0.277, p < 0.0001). A significant correlation was also found between the BIS and the Cp of propofol (ρ = − 0.165, p < 0.001).
Hand strength as an indicator of consciousness on Receiver operating characteristic (ROC) curve analysis
To further evaluate the clinical significance of hand strength for assessment of the level of consciousness, ROC analysis was conducted (Fig. 3). The area under the curve (AUC) to discriminate whether the patient is in JCS level 0–1 or ≥ 2 was 0.75 for hand strength (p < 0.01). The sensitivity was 0.76, and the specificity was 0.67 at the cut-off value of 75% strength compared with preoperative maximum hand strength. The AUC of the affected hand was 0.73, and the sensitivity and specificity were 0.95 and 0.54, respectively, with a cut-off value of 56%. The AUC for the Cp of propofol was 0.78, the sensitivity was 0.86, and the specificity was 0.65 when the Cp of propofol was greater than or less than 0.61. In contrast, the AUC of the BIS was low (0.66), with sensitivity and specificity of 0.78 and 0.47, respectively. In terms of the AUC, moderate accuracy was obtained for both the unaffected- and affected-handgrip ratio. However, the specificity of the handgrip ratio of the affected hand was relatively low because of large individual differences due to intraoperative paralysis.
Receiver operating characteristic (ROC) analysis of the Japan Coma Scale (JCS). The red curves indicate JCS 0 or 1, (A) The predicted plasma concentration (Cp) shows an area under the curve (AUC) of 0.78 (p < .001). (B) The BIS shows an AUC of 0.66 (p < .001). (C) Hand strength on the unaffected side shows an AUC of 0.75 (p < .001). D: Hand strength on the affected side shows an AUC of 0.73 (p < .001).
Illustrative case
Chronological changes of the indicators (Cp of propofol, BIS, hand strength) in patient No. 17 (43-year-old man) are shown in Fig. 4. The Cp of propofol decreased as time passed after extubation and was clearly correlated with the consciousness level. Specifically, the Cp was 1.01 at JCS 10, and it decreased to 0.62 at JCS 1 (33 min after extubation) (Fig. 4A). The BIS was over 80 immediately after extubation and remained between 70 and 90 afterwards (Fig. 4B). The hand strength of the unaffected hand increased sharply over time, from 40% at JCS 2 (20 min after extubation) to 80% at JCS 1 (30 min after extubation), showing a positive correlation with the level of consciousness. The hand strength remained high around 1.0 afterwards (Fig. 4C). The hand strength on the affected side also increased as time passed and showed a positive correlation with the level of consciousness, but the slope was shallow with a maximum of < 80% (Fig. 4D).
Illustrative case. Plots are each measurement in time after extubation of patient 17. The black curve shows the quadratic regression equation. The orange plot represents Japan Coma Scale (JCS) 10, the green is 2, and the red is 1. (A) The predicted concentration (Cp) of propofol decreases with time and the JCS. (B) The BIS changes very little with time and the JCS. (C) Hand strength on the unaffected side increases with time and approaches 100% with JCS 1. (D) Hand strength on the affected side increases with time but does not reach 100%.
Discussion
Hand strength is useful as an intraoperative consciousness indicator during awake craniotomy
In the present study, it was demonstrated that hand strength relative to the preoperative maximum strength on the unaffected side showed a significant correlation with the Cp of propofol, which represents the depth of anesthesia. It was also correlated with the JCS. ROC curve analysis demonstrated that discrimination ability between JCS 0–1 and JCS ≥ 2 was satisfactory, with an AUC of 0.75 for the unaffected-hand grip strength, which was better than that of the BIS (0.66) and comparable to that of the Cp of propofol (0.78). With a cut-off value of 75% of hand strength, sensitivity was 0.76, and specificity was 0.67, indicating that hand strength is a good indicator of patients’ arousal and provides supportive information about whether the patient has reached a level of consciousness sufficient for monitoring motor and language functions12,13,15.
Relationship between hand strength and depth of anesthesia has previously been reported. A study showed that hand strength decreases in a dose-dependent manner when the Cp of propofol exceeds 0.8 μg/ml21. Another study showed that hand strength can be used to monitor the depth of anesthesia during induction with propofol20. Hand strength on the unaffected side is also a useful indicator of overall limb strength22. This is applicable to hemiplegic stroke patients23, suggesting that hand strength on the unaffected side can be used for patients with brain tumors, as well to assess overall limb strength. In the present study, unaffected hand strength of 75% or more relative to the preoperative level indicated a good level of consciousness. If this is not achieved, it will be difficult to determine whether the motor dysfunction is due to insufficient recovery from anesthesia or actual motor paralysis, making physiological monitoring unreliable. This would also be the case with verbal functions. Mapping and monitoring of brain functions should be initiated after we confirm sufficient recovery of hand strength, which reflects sufficient arousal and motor function.
The advantage of hand strength is that it can be measured with a small device that is a convenient tool for checking the results immediately. Therefore, hand strength can be measured repeatedly at a high time resolution and provides continuous values, making it a useful indicator for the assessment of the level of consciousness. Given that hand strength on the unaffected side can be used for monitoring the level of consciousness and that on the affected side can be used to monitor motor function, continuous measurement of hand strength would have substantial clinical benefits in awake craniotomy.
Consciousness level of JCS1 or higher is required for language monitoring
Assessment of the level of consciousness includes use of the GCS and JCS, which are assessed from the patient's motor and verbal responses in general clinical practice. The Japanese Awake Craniotomy Guidelines do not specify a scale for assessing the level of consciousness at the beginning of neurological monitoring, especially language mapping24. In other studies, the level of consciousness to determine when language mapping can be initiated has not been clearly defined, since “mapping was initiated at the discretion of the neuropsychologist present”10 or no detailed description of the patient's level of consciousness is available25,26. Thus, the level of consciousness at which neurological mapping can be initiated has not been clearly defined. In other words, assessment of the level of consciousness during the awake phase has been subjective, and an objective assessment should be made immediately before starting the language task27.
When a patient breathes spontaneously and can follow simple verbal commands21,22,23, the patient can be extubated and shifted to the awake phase, even if not fully awake. However, to perform motor and verbal monitoring, nearly full consciousness is needed. To evaluate such subtle changes in consciousness, we used the JCS, which can evaluate consciousness in five linear steps (JCS 10, 3, 2, 1, and 0). JCS 3 and 2 indicate disorientation and do not indicate that the patient is ready to cooperate with monitoring. To accurately assess motor and language functions, reaching JCS1 (mostly conscious but slow to respond) or 0 (completely conscious) is needed. Thus, we considered that JCS 0–1 was a suitable condition for neurophysiological monitoring. Mapping and monitoring of higher brain functions during awake craniotomy have been recommended in the dominant hemisphere, as well as the non-dominant hemisphere28. In this context, hand strength is useful because we can measure it in both hands simultaneously.
A discrepancy between consciousness and hand strength can occur. When consciousness is disturbed despite a sufficient increase in hand strength, other adverse events may occur, such as seizures, discomfort, and/or language impairment. When the hand strength decreases despite a good level of consciousness, brain damage or ischemia may have occurred in the motor area or corticospinal tract. Therefore, a discrepancy between the level of consciousness and hand strength is helpful for early detection of adverse events during awake craniotomy.
Reaching a sufficient consciousness level for language mapping takes time
In this study, an average of 19.4 min was required from anesthetic cessation to extubation, and an average of 26.4 min was required from extubation to JCS 1. Studies from Europe and the United States showed that the mean time from cessation of anesthesia to the initiation of mapping was 17.6 minutes21, and the median time from extubation to the initiation of mapping was 11 minutes27, both of which were shorter than those of the present study. This may be because of different pharmacokinetics between the Japanese and the European and US populations. The pharmacokinetic parameters used for propofol TCI have been validated in Japan4,5, but the times from anesthetic cessation to initial response are different between Europeans and Japanese (10.5 ± 6.37 min in Europeans, 20.1 ± 12.69 min in Japanese)5,29. Differences in height, weight, and other physical characteristics may have contributed to the ethnic differences in the time to full recovery of consciousness. To monitor language and higher brain functions accurately, the patient must be fully awake and cooperative. Patients should be assessed with a reliable measure of their level of consciousness to ensure that they are able to respond accurately and with appropriate speed to the task, and they should be allowed sufficient time to become fully conscious.
Limitations
There are several limitations in the present study. First, the study population was small, and the results were not validated in either internal or external independent cohorts. Second, individuals who did not respond well to verbal commands due to somnolence were excluded from the analysis. Left-handed individuals were also excluded due to their history of dominant hand correction. Therefore, the results of the present study may not be applicable to all individuals. Third, inter-rater reliability analysis for the assessment of consciousness by the JCS was not performed. Fourth, multiple analyses were performed to show the robustness of hand strength to predict the level of consciousness, but this is susceptible to multiple testing error. However, the positive correlations were confirmed in all of the analyses, and therefore, multiplicity of analyses is unlikely to affect the interpretation of the results of the present study. Last, the correlation between the success rate of neuromonitoring and the recovery of hand strength was not examined. Further study is needed to address these limitations.
Conclusions
Hand strength on the unaffected side was significantly correlated with the Cp of propofol and the BIS, supporting our assumption that hand strength is correlated with depth of anesthesia. It also showed a significant correlation with the JCS, and ROC curve analysis showed that JCS 0–1 and JCS ≥ 2 were efficiently distinguished by hand strength with a cutoff value of 75%. Routine measurement of hand strength on the unaffected side is easy and reliable. Thus, hand strength appears to be a good indicator of the level of consciousness during awake craniotomy. Further study is needed to validate these results and to test the generalizability to a larger population, including left-handed individuals.
Methods
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Graduate School of Medicine and Faculty of Medicine, Kyoto University, Japan (R1843-1). Written, informed consent was obtained preoperatively from all participants. All procedures were performed in accordance with relevant named guidelines and regulations including the Declaration of Helsinki and Ethical Guidelines for Medical and Biological Research Involving Human Subjects in Japan.
Study population
The study population was prospectively collected from February 2020. Consecutive patients who underwent awake craniotomy at our hospital with a diagnosis of any brain tumor were included in the study. Thirty-two patients fulfilled the criteria between February 2020 and April 2021, and all of them gave their informed consent. According to the exclusion criteria, nine were excluded: 1) two patients were left-handed, 2) four patients were unable to communicate due to intraoperative seizures or delirium, 3) three patients had poor BIS measurements, the primary parameter of this study. Accordingly, 23 patients were finally analyzed in the present study.
Anesthesia and the predicted concentration of propofol in the blood
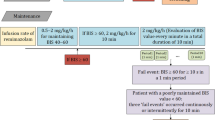
Anesthesia during the awake craniotomy through all of the asleep-awake-awake phase was conducted based on a standardized institutional protocol, which was reported previously30. Anesthesia is induced and maintained by continuous infusion of propofol and remifentanil; muscle relaxants were not administered. In the analysis, the Cp of propofol in the blood was calculated from the propofol dosage in anesthesia records using Marsh’s pharmacokinetic model31.
BIS monitoring
The BIS has routinely been used to monitor the depth of anesthesia since it was introduced by Aspect Medical Systems, Inc. in 19947. Patch electrodes were positioned from the contra- and ipsilateral nasal bones to the ipsilateral zygomatic bone to avoid any noise due to craniotomy. The BIS was recorded before the craniotomy and throughout the operating procedure. The BIS score represents the electroencephalography activity during the immediately previous 60 s, and it was automatically calculated and recorded every 5 s as a unitless whole number between 0 and 100. In this study, the BIS values were stored in the surgical record every minute.
Japan Coma Scale (JCS) assessments
The JCS levels are shown in Table 115. During the awake phase of craniotomy, immediately after extubation, most patients show a level of consciousness that allows them to open their eyes in response to a call, understand verbal commands, and perform automatic movements (JCS: 10, GCS: E3V4M6; total score 13). If the eyes can be kept open, the JCS becomes a single digit, and the GCS becomes E4V4M6 (total score 14). Changes leading to better consciousness are judged by the verbal response, which is divided into two levels (E4V4M6; total score 14 or E4V5M6; total score 15) based on the presence or absence of confused speech in the GCS16, and four levels (3, 2, 1, 0) based on the presence or absence of disorientation and delayed response in the JCS (Table 1). In this study, level of consciousness of patients was assessed using the JCS, which can distinguish the level of consciousness in the awake phase more precisely. The evaluation was done by evaluators who knew the patient's condition before surgery. In order to avoid interactions, the evaluators of hand strength and those of the JCS were different.
Measurement of hand strength
A hand dynamometer (MG4800, Charder Medical, Taiwan) was used to measure hand strength preoperatively and intraoperatively. Measurements were taken with both unaffected and affected hands. The affected hand was defined as the hand on the opposite side of the brain lesion. For preoperative measurements, hand strength was measured three times using the Max mode of the hand dynamometer on the day before surgery, and the average of these measurements was used as the preoperative value shown in Table 2. The evaluator encouraged the patient to exert maximum hand strength and instructed him/her to continue exerting hand strength for about 5 s. The measurement was performed in the supine position (same as the surgical position), with the elbow extended and the forearm and wrist in the intermediate position. This position is reproducible compared with the normal sitting position to evaluate hand strength32. To exclude the weight of the hand dynamometer, the dynamometer was placed on the operating table. For intraoperative measurements, hand strength during the awake state was measured intermittently with an interval of approximately 5 min to avoid the effects of muscle fatigue, and measurement was started after confirming that the patient had no nausea or delirium. The limb position and the settings of the hand dynamometer were performed under the same conditions as preoperative measurement. Intraoperative hand strength was presented as a percentage of the preoperative hand strength and used in the analysis.
Statistical analysis
The continuous variables used in this study were the Cp of propofol, the BIS, and percentages of hand strength on the unaffected and affected sides; the JCS was treated as a categorical variable. The mean ± SD was used for descriptive statistics of continuous variables. The normal distribution was tested by the Shapiro–Wilk test. Since all variables were confirmed to not be normally distributed, a nonparametric approach was chosen for further analyses. Spearman's rank correlation coefficient was used to examine correlations among continuous variables (BIS, Cp of propofol, and hand strength), and Kendall's rank correlation coefficient was used to examine correlations between categorical variables and continuous variables (JCS and Cp of propofol, BIS, and grip strength). ROC analysis was used to evaluate the discrimination capacity between JCS 0–1 and JCS ≥ 2. To accurately show changes in the level of consciousness and to exclude the effects of noise and outliers in the data, Kendall's correlation analysis was performed using representative values of the Cp of propofol, the BIS, and hand strength, which were averaged for each level of JCS. All data were collected 1–75 min after extubation. All statistical analyses were performed using JMP Pro 15.2.0., and p < 0.05 was considered significant.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under the curve
- BIS:
-
Bispectral index
- Cp:
-
Predicted plasma concentration
- GCS:
-
Glasgow Coma Scale
- JCS:
-
Japan Coma Scale
- MEP:
-
Motor-evoked potential
- ROC:
-
Receiver operating characteristic
- SD:
-
Standard deviation
- TCI:
-
Target-controlled infusion
References
De Witt Hamer, P. C., Robles, S. G., Zwinderman, A. H., Duffau, H. & Berger, M. S. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J. Clin. Oncol. 30, 2559–2565. https://doi.org/10.1200/JCO.2011.38.4818 (2012).
Gerritsen, J. K. W., Arends, L., Klimek, M., Dirven, C. M. F. & Vincent, A. J. E. Impact of intraoperative stimulation mapping on high-grade glioma surgery outcome: a meta-analysis. Acta Neurochir. 161, 99–107. https://doi.org/10.1007/s00701-018-3732-4 (2019).
Stalnacke, M., Bergenheim, T. & Sjoberg, R. L. Neuropsychological function and quality of life after resection of suspected lower-grade glioma in the face primary motor area. J. Clin. Med. https://doi.org/10.3390/jcm10040580 (2021).
Hunt-Smith, J. et al. Safety and efficacy of target controlled infusion (Diprifusor (TM)) vs manually controlled infusion of propofol for anaesthesia. Anaesth. Intens. Care 27, 260–264. https://doi.org/10.1177/0310057x9902700306 (1999).
Kazama, T. et al. Assessment of use of “Diprifusor” target controlled infusion (TCI) of 1% diprivan(ICI 35, 868). Anesth. Resuscit. 34, 121–139 (1998).
Sebel, P. S. et al. A multicenter study of bispectral electroencephalogram analysis for monitoring anesthetic effect. Anesth. Analg. 84, 891–899. https://doi.org/10.1097/00000539-199704000-00035 (1997).
Sigl, J. C. & Chamoun, N. G. An introduction to bispectral analysis for the electroencephalogram. J. Clin. Monit. 10, 392–404. https://doi.org/10.1007/BF01618421 (1994).
Glass, P. S. et al. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology 86, 836–847. https://doi.org/10.1097/00000542-199704000-00014 (1997).
Nossek, E. et al. Failed awake craniotomy: A retrospective analysis in 424 patients undergoing craniotomy for brain tumor. J. Neurosurg. 118, 243–249. https://doi.org/10.3171/2012.10.JNS12511 (2013).
Conte, V., L’Acqua, C., Rotelli, S. & Stocchetti, N. Bispectral index during asleep-awake craniotomies. J. Neurosurg. Anesthesiol. 25, 279–284. https://doi.org/10.1097/ANA.0b013e3182913afd (2013).
Ohtaki, S. et al. The influence of depth of anesthesia on motor evoked potential response during awake craniotomy. J. Neurosurg. 126, 260–265. https://doi.org/10.3171/2015.11.JNS151291 (2017).
Teasdale, G. & Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 2, 81–84. https://doi.org/10.1016/s0140-6736(74)91639-0 (1974).
Teasdale, G. & Jennett, B. Assessment and prognosis of coma after head injury. Acta Neurochir. 34, 45–55. https://doi.org/10.1007/BF01405862 (1976).
Teasdale, G. et al. The Glasgow Coma Scale at 40 years: Standing the test of time. Lancet Neurol. 13, 844–854. https://doi.org/10.1016/S1474-4422(14)70120-6 (2014).
Ohta, T., Kikuchi, H., Hashi, K. & Kudo, Y. Nizofenone administration in the acute stage following subarachnoid haemorrhage. Results of a multi-center controlled double-blind clinical study. J. Neurosurg. 64, 420–426. https://doi.org/10.3171/jns.1986.64.3.0420 (1986).
Namiki, J. et al. Difficulty and inaccuracy of assessment of the consciousness level by the Glasgow Coma Scale: Comparison with the Japan Coma Scale. J. Jpn. Soc. Emerg. Med. 10, 20–25 (2007).
Meskelevicius, D. et al. Determination of optimal time window for cortical mapping in awake craniotomy: Assessment of intraoperative reaction speed. Neurosurg. Rev. 43, 633–642. https://doi.org/10.1007/s10143-019-01094-4 (2020).
Kumabe, T. et al. Summary of 15 years experience of awake surgeries for neuroepithelial tumors in tohoku university. Neurol. Med. Chir. (Tokyo) 53, 455–466. https://doi.org/10.2176/nmc.53.455 (2013).
Kwinta, B. M., Myszka, A. M., Bigaj, M. M., Krzyewski, R. M. & Starowicz-Filip, A. Intra- and postoperative adverse events in awake craniotomy for intrinsic supratentorial brain tumors. Neurol. Sci. 42, 1437–1441. https://doi.org/10.1007/s10072-020-04683-0 (2021).
Guay, C. S. & Plourde, G. Handgrip dynamometry for continuous assessment of volitional control during induction of anesthesia: a prospective observational study. Can. J. Anaesth. 66, 48–56. https://doi.org/10.1007/s12630-018-1224-x (2019).
Tsai, P. F., Matsuura, N., Kaneko, Y. & Ichinohe, T. Propofol dose-dependently increases bite force during sedation. J. Oral Maxillofac. Surg. 69, 2746–2752. https://doi.org/10.1016/j.joms.2011.02.112 (2011).
Bohannon, R. W., Magasi, S. R., Bubela, D. J., Wang, Y. C. & Gershon, R. C. Grip and knee extension muscle strength reflect a common construct among adults. Muscle Nerve 46, 555–558. https://doi.org/10.1002/mus.23350 (2012).
Takahashi, J., Nishiyama, T. & Matsushima, Y. Does grip strength on the unaffected side of patients with hemiparetic stroke reflect the strength of other ipsilateral muscles?. J. Phys. Ther. Sci. 29, 64–66. https://doi.org/10.1589/jpts.29.64 (2017).
Kayama, T. The guidelines for awake craniotomy guidelines committee of the Japan awake surgery conference. Neurol. Med. Chir. (Tokyo) 52, 119–141. https://doi.org/10.2176/nmc.52.119 (2012).
Chang, W. H. et al. Intraoperative linguistic performance during awake brain surgery predicts postoperative linguistic deficits. J. Neurooncol. 139, 215–223. https://doi.org/10.1007/s11060-018-2863-z (2018).
Kanno, A. & Mikuni, N. Evaluation of language function under awake craniotomy. Neurol. Med. Chir. (Tokyo) 55, 367–373. https://doi.org/10.2176/nmc.ra.2014-0395 (2015).
Aabedi, A. A. et al. Assessment of wakefulness during awake craniotomy to predict intraoperative language performance. J. Neurosurg. 132, 1930–1937. https://doi.org/10.3171/2019.2.JNS183486 (2019).
Duffau, H. Is non-awake surgery for supratentorial adult low-grade glioma treatment still feasible?. Neurosurg. Rev. 41, 133–139. https://doi.org/10.1007/s10143-017-0918-9 (2018).
Russell, D. et al. Manual compared with target-controlled infusion of propofol. Br. J. Anaesth. 75, 562–566. https://doi.org/10.1093/bja/75.5.562 (1995).
Shiraki, A. et al. Effects of low-dose remifentanil infusion on analgesic or antiemetic requirement during brain function mapping: A retrospective cohort study. Acta Anaesth. Scand. 64, 735–741. https://doi.org/10.1111/aas.13554 (2020).
Marsh, B., White, M., Morton, N. & Kenny, G. N. C. Pharmacokinetic model driven infusion of propofol in children. Br. J. Anaesth. 67, 41–48. https://doi.org/10.1093/bja/67.1.41 (1991).
Vargas-Pinilla, O. C. & Rodriguez-Grande, E. I. Reproducibility and agreement between three positions for handgrip assessment. Sci. Rep. https://doi.org/10.1038/s41598-021-92296-8 (2021).
Acknowledgements
The authors would like to thank the staff of Kyoto University Hospital for assistance during awake craniotomy.
Author information
Authors and Affiliations
Contributions
Conception and design: Y.A., C.U., Y.M. Acquisition of data: C.U., T.M., R.Y., M.U., Y.Y., M.N. Analysis and interpretation of data: Y.A., C.U., Y.M., N.L. Drafting the article: Y.A., C.U., Y.M., N.L. Critically revising the article: S.M., S.M., H.I. Reviewed submitted version of manuscript: all authors. Statistical analysis: C.U., Y.M. Administrative/technical/material support: Y.A., Y.M. Study supervision: Y.A.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Umaba, C., Mineharu, Y., Liang, N. et al. Intraoperative hand strength as an indicator of consciousness during awake craniotomy: a prospective, observational study. Sci Rep 12, 216 (2022). https://doi.org/10.1038/s41598-021-04026-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-04026-9
This article is cited by
-
Developing a robust model to predict depth of anesthesia from single channel EEG signal
Physical and Engineering Sciences in Medicine (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.