Abstract
Comprehensive population-based data on myeloid neoplasms (MNs) are limited, mainly because some subtypes were not recognized as hematological cancers prior to the WHO publication in 2001, and others are too rare to allow robust estimates within regional studies. Herein, we provide incidence data of the whole spectrum of MNs in Spain during 2002–2013 using harmonized data from 13 population-based cancer registries. Cases (n = 17,522) were grouped following the HAEMACARE groupings and 2013-European standardized incidence rates (ASRE), incidence trends, and estimates for 2021 were calculated. ASRE per 100,000 inhabitants was 5.14 (95% CI: 5.00–5.27) for myeloproliferative neoplasms (MPN), 4.71 (95% CI: 4.59–4.84) for myelodysplastic syndromes (MDS), 3.91 (95% CI: 3.79–4.02) for acute myeloid leukemia, 0.83 (95% CI: 0.78–0.88) for MDS/MPN, 0.35 (95% CI: 0.32–0.39) for acute leukemia of ambiguous lineage, and 0.58 (95% CI: 0.53–0.62) for not-otherwise specified (NOS) cases. This study highlights some useful points for public health authorities, such as the remarkable variability in incidence rates among Spanish provinces, the increasing incidence of MPN, MDS, and MDS/MPN during the period of study, in contrast to a drop in NOS cases, and the number of cases expected in 2021 based on these data (8446 new MNs).
Similar content being viewed by others
Introduction
Hematological malignancies are the fourth most frequently diagnosed group of cancers worldwide1, with an annual incidence rate of 39.37 per 100,000 inhabitants in Europe in 2000–20022. They encompass a heterogeneous group of diseases with diverse etiology, presentation, and outcomes. Our understanding of these neoplasms has evolved rapidly during the recent decades, resulting in multiple classification updates. Currently, the World Health Organization (WHO) classification of hematologic malignancies, first published in 20013 and later updated in 20084 and 20165, is the gold standard for the study of these neoplasms. Such continuous definition refinements, however, have posed significant problems for population-based cancer registries to present complete and accurate data for the full spectrum of hematological neoplasms6, and particularly for myeloid neoplasms (MNs).
MNs are a group of clonal disorders characterized by excessive proliferation, impaired self-renewal and/or altered differentiation of hematopoietic stem cells and myeloid progenitor cells. Broadly, MNs are classified into four large categories: acute myeloid leukemia (AML), myeloproliferative neoplasms (MPN), myelodysplastic syndromes (MDS), and MDS/MPN overlap syndromes. However, MDS and several MPN subtypes were first recognized as malignant disorders—and thus, reportable to cancer registries—in 2000, with the publication of the third revision of the International Classification of Disease for Oncology (ICD-O-3)7. Regarding AML, past epidemiological studies were prone to group all leukemias together, at best differencing by age range (i.e. pediatric/adult) and chronicity (i.e. acute/chronic), but ignoring cell lineage differences (i.e. lymphoid/myeloid). This situation changed after correspondence was established between the WHO classifications and the ICD-O-3 codes. Since then, several studies using the largest European2,8 and North American9,10,11,12 datasets, as well as from hematology-specialized cancer registries13,14 have reported detailed epidemiological data of MNs. Still, and given that most entities are too rare to make power robust estimates within regional studies, population-based data of several clinically meaningful histological subtypes are limited. Particularly in Spain, there are no available comprehensive nationwide estimates, with only one previous study providing regional data15.
The aim of this study was to assess the incidence of MNs subtypes in Spain over the period 2002–2013, and to estimate the number of MNs expected in 2021, using harmonized data from the Spanish Network of Cancer Registries (REDECAN).
Methods
REDECAN was created in 2010 in order to develop standards for cancer registration, undertake quality audits, and promote the use of cancer surveillance data within Spain16,17. Currently, it comprises 15 population-based cancer registries, 14 of them covering the entire population and one of them only children. In particular, 13 population based-cancer registries (i.e. Asturias, Canary Islands, Castellón, Ciudad Real, Cuenca, Euskadi, Girona, Granada, La Rioja, Mallorca, Murcia, Navarra, and Tarragona) contributed to this study (Table 1), covering ∼26% of the total Spanish population in January 2013 (12,143,157 out of 47,129,783)18.
All incident MNs registered from 2002 to 2013 (or the available period) were included in the analyses. Cases were codified using the ICD-O-37,19, and classified following the HAEMACARE scheme2, a European project aimed to improve standardization of epidemiological information on hematological malignancies (Table 2). In brief, MNs were grouped into six broad categories: MPN, MDS/MPN, MDS, AML, acute leukemia with ambiguous lineage, and not otherwise specified (NOS). Within AML, the following groupings were considered: AML with recurrent cytogenetic abnormalities, AML with multilineage dysplasia, AML and MDS therapy-related, AML not otherwise categorized (NOC), and AML NOS. In the 2001 WHO classification3, the number of blast cells to define AML decreased from 30 to 20% so that some conditions previously considered MDS, such as refractory anemia with excess blasts in transformation (9984/3), were included with the AMLs. In line with previous studies20, we grouped these cases as AML with multilineage dysplasia (9895/3) as this cytological property is characteristic of MDS. Likewise, therapy related-MDS (9987/3) were included in the AML therapy related (9920/3) subgroup, and cases of chronic myeloid leukemia NOS (CML NOS) (9863/3) were grouped with CML BCR-ABL positive (9875/3). Finally, in cases of hematological transformation, only the first tumor was considered for incidence21.
Crude rate (CR) and age-standardized incidence rate using the 2013 European standard population (ASRE) were calculated using population data provided by the National Statistics Institute (Instituto Nacional de Estadística—INE)18, and expressed per 100,000 person-years. Poisson regression models were used to analyze the overall incidence time trends and to estimate the annual percent change (APC). The number of cases in Spain for 2021 was determined by applying to the 2021 Spanish population18 the age-specific rates estimated for the year 2021. The latter were obtained by applying the APC (period 2002–2013) to the last quinquennium of known incidence (i.e. 2009–2013). All analyses were performed using R software (version 3.6.1)22.
Ethics approval
This study is based on data from cancer registries gathered in the Spanish network of cancer registries (REDECAN). The public health administration of each autonomous community/province* authorized the collection and use of this data for its analysis without requirement of informed consent and ethical approval, covered by the Spanish general and public health laws 14/1986 and 33/2011.
*The authorizing bodies for each autonomous community/province are listed below: Asturias: Sección de Información Sanitaria. Servicio de Evaluación de la Salud, Calidad y Programas de la Dirección General de Salud Pública. Consejería de Sanidad; Canary Islands: Servicio de Epidemiología y Prevención. Dirección General de Salud Pública. Servicio Canario de la Salud; Castellón: Conselleria de Sanitat. Dirección General de Salud Pública; Ciudad Real: Consejería de Sanidad y Asuntos Sociales de la Junta de Comunidades de Castilla-La Mancha; Cuenca: Consejería de Sanidad y Asuntos Sociales. Junta de Comunidades de Castilla la Mancha; Euskadi: Dirección de Planificación, Ordenación y Evaluación Sanitarias. Departamento de Salud. Gobierno Vasco; Girona: Plan Director de Oncología-Instituto Catalán de Oncología; Granada: Consejería de Salud de la Junta de Andalucía, adscrito para su desarrollo a la Escuela Andaluza de Salud Pública (EASP); La Rioja: Servicio de Epidemiología y Prevención Sanitaria de la Consejería de Salud y Servicios Sociales del Gobierno de La Rioja; Mallorca: Dirección General de Salud Pública y Participación; Murcia: Consejería de Salud de Murcia; Navarra: Departamento de Salud del Gobierno de Navarra. Instituto de Salud Pública y Laboral de Navarra; Tarragona: Hospital Universitario Sant Joan de Reus.
Results
MNs accounted for 30.81% (n = 17,522) of all hematologic malignancies (n = 56,777) diagnosed in Spanish population covered by cancer registries between 2002 and 2013. The quality and completeness of each registry, together with the study period and the total cases are detailed in Table 1. Of the total, 96.3% of the cases had microscopic confirmation, 3.6% were NOS cases, and 1.9% were recorded exclusively by death certificate (DCO). In particular, 33.5% of cases were MPN, 29.8% MDS, 25.7% AML, 5.2% MDS/MPN, 2.3% acute leukemia of ambiguous lineage, and the remaining 3.6% were NOS cases.
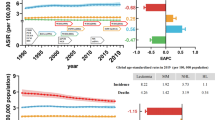
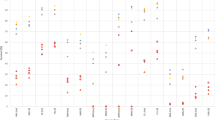
Table 2 shows the number of cases, median age and incidence rates of all MNs according to histological subtype. The overall CR was 13.97 (95% CI 13.77; 14.18), and the overall ASRE was 15.52 (95% CI: 15.29; 15.75), being 19.92 (95% CI 19.51; 20.33) in men and 12.39 (95% CI 12.12; 12.67) in women. There was a marked male predominance (9,650 cases in men (55.1%), sex ratio = 1.61), and the median age at diagnosis was 73 years (interquatile range (IQR) 60–81 years). Moreover, incidence increased markedly with age, reaching a maximum around 75–79 years in most subgroups (Fig. 1). ASRE of MNs by cancer registry are displayed in Fig. 2. There were significant differences across different cancer registries (especially regarding MDS, MPN, and MDS/MPN), with the highest overall rates in Girona (21.14, 95% CI 20.09; 22.19), and the lowest rates observed in Asturias (11.61, 95% CI 10.95; 12.26) and Granada (12.11, 95% CI 11.38; 12.83).
Age-standardized incidence rates (ASRE) of myeloid neoplasms diagnosed in Spain during 2002–2013 by region. AML acute myeloid leukemia, AL acute leukemia of ambiguous lineage, MDS myelodysplastic syndromes, MPN myeloproliferative neoplasms, NOS not-otherwise specified. Rates are expressed per 100,000 person-years.
Myeloproliferative neoplasms
The CR and ASRE for MPN were 4.68 (95% CI 4.56; 4.80) and 5.14 (95% CI 5.00; 5.27), respectively. The most frequent subtype was essential thrombocythemia (41% of cases, ASRE = 2.10 (95% CI 2.01; 2.18)), followed by chronic myeloid leukemia (23% of cases, ASRE = 1.13 (95% CI 1.06; 1.19)), and polycythemia vera (18%, ASRE = 0.95 (95% CI 0.89; 1.01)) (Table 2). Median age (IQR) at diagnosis was 69 (54–78) years, being slightly lower in cases with mastocytosis, 54 (36–66) years. Higher rates in men than in women were reported in almost all subtypes, being particularly higher in primary myelofibrosis (sex ratio = 2.83), while no difference by sex was seen in essential thrombocythemia (sex ratio = 1.02). The incidence of MPN increased across the period in 2002–2013, with an APC of 1.6% (95% CI 0.8; 2.4) (Table 3). Moreover, results by specific subtype indicate that this increment in the incidence was namely due to the contribution of essential thrombocythemia (4.6%, 95% CI 3.3; 5.9), primary myelofibrosis (3.5%, 95% CI 0.6; 6.5), and polycythemia vera (2.2%, 95% CI 0.3; 4.1). Conversely, we evidenced a decrease in the incidence of chronic myeloid leukemia (− 2.1%, 95% CI − 3.7; − 0.4) and MPN NOS (− 5.0%, 95% CI − 7.6; − 2.4).
Myelodysplastic/myeloproliferative neoplasms
The CR and ASRE for MDS/MPN were 0.73 (95% CI 0.68; 0.77) and 0.83 (95% CI 0.78; 0.88), respectively. By far, the most common subtype was chronic myelomonocytic leukemia (84% of cases), with an ASRE of 0.70 (95% CI 0.65; 0.75) and a marked male predominance (sex ratio = 3.1) (Table 2). The median (IQR) age at diagnosis was 77 (70–83) years. The incidence of MDS/MPN rose markedly throughout 2002–2013, with an APC of 6.9% (95% CI: 4.8; 9.1) (Table 3).
Myelodysplastic syndromes
The CR and ASRE for MDS were 4.16 (95% CI 4.04; 4.27) and 4.71 (95% CI 4.59; 4.84), respectively (Table 2). The most frequent subtype was MDS with excess of blasts (17%, ASRE = 0.82 (95% CI 0.77; 0.88)), closely followed by MDS with multilineage dysplasia (15%, ASRE = 0.70 (95% CI 0.65; 0.75)) and MDS with single lineage dysplasia (14%, ASRE = 0.66 (95% CI: 0.61; 0.71)], while 42% of the cases were MDS unclassifiable. Median age (IQR) at diagnosis was 78 (71–83) years, and incidence rates were higher in men, except in MDS associated with isolated del(5q), in which we noted a reverse sex ratio (0.43). There was a positive incidence trend of overall MDS throughout the period (APC = 1.3%, 95% CI 0.4; 2.1), mostly due to MDS with multilineage dysplasia (APC = 19.1%, 95% CI 16.3; 21.9) and MDS associated with isolated del(5q) (APC = 13.7%, 95% CI 7.2; 20.6). Conversely, cases of MDS with single lineage dysplasia decreased across the period of study (APC = − 11.9%, − 13.9; − 9.8) (Table 3).
Acute myeloid leukemia
The CR and ASRE for AML were 3.59 (95% CI 3.48; 3.69) and 3.91 (95% CI 3.79; 4.02), respectively (Table 2). Among the 4498 AML cases, 47% were AML NOC, 14% were AML with recurrent cytogenetic abnormalities, 11% were AML with multilineage dysplasia, 3% were therapy-related, and 25% were NOS. Within AML with recurrent cytogenetic abnormalities (n = 608), the most frequent subtype was AML with t(15;17), followed by AML with t(8;21). A male predominance was shared across all subgroups (overall sex ratio = 1.45), with the exception of AML and MDS therapy related (sex ratio = 0.83). The median (IQR) age of AML patients was 68 (50–78) years, being lower in AML with cytogenetic abnormalities (49 (35–67) years). The incidence of overall AML was relatively stable over time, yet there was an increase in the incidence of specified cases in detriment of AML NOS cases (APC = − 5.2%, 95% CI − 6.9; − 3.4) (Table 3).
Acute myeloid leukemia with ambiguous lineage and NOS cases
There were 395 cases of acute myeloid leukemia with ambiguous lineage, 12% of them being biphenotypic acute leukemia. The overall CR and ASRE were 0.32 (95% CI 0.28; 0.35) and 0.35 (95% CI 0.32; 0.39), respectively. Finally, there were 632 NOS cases (3.6% of the overall dataset), with a higher median age than that of the MNs as a whole [81 (73–86) years]. We evidenced a marked negative incidence trend across the period of study, both for acute leukemia of ambiguous lineage (APC = − 9.2. 95% CI − 12.0; − 6.3), and NOS cases (APC = − 14.3%, 95% − 16.5; − 12.1) (Table 3).
Projections for 2021
Predicted incidence of MNs for 2021, overall and by sex, are detailed in Table 4. According to our projections, 8446 new incident cases of MNs will be diagnosed in Spain in 2021, of which 2835 will be MPN, 650 MSD/MPN, 2670 MDS, 2060 AML, and 106 acute leukemia of ambiguous lineage, and 126 NOS cases.
Discussion
Limited epidemiological data are available on the whole spectrum of hematologic disorders of the myeloid lineage. Within Europe, the most relevant data comes from two large European datasets, the RARECARE8 (1995–2002, n = 69,212) and HAEMACARE2 (2000–2002, n = 21,796) collaborative projects, and from two hemato-specialized registries, the Coté d’Or (France)13 (1980–2004, n = 5,086) and the UK Haematological Malignancy Research Network (HMRN)14 (2004–2015, n = 5,231). Our large-population-based study, which includes 17,522 cases diagnosed after the introduction of the WHO classification breakthrough, further complements these data by providing complete estimates of MNs burden in Spain during 2002–2013, with predictions for 2021.
In line with previous studies, incidence of MNs was markedly higher in men than in women for most subtypes. Notable exceptions included MDS associated with isolated del(5q), AML and MDS therapy related, and essential thrombocythemia, already reported in the literature. Likewise, the incidence of all MNs increased with advancing age, being particularly marked in NOS cases, in which the incidence rose sharply from age 70 years. This might suggest a decline in the quality of the diagnostic workup in the elderly, who are less likely to receive aggressive diagnostic tests due to comorbidity and/or frailty, and may therefore receive a suboptimal treatment for their conditions23.
Regarding specific MNs subtypes, lower incidence rates for most entities were reported in our study in comparison to the most recent data provided by the HMRN14. This could be partly explained by the specialized nature of the HMRN (with all diagnoses made and coded by clinical specialists working at a single integrated hematopathology laboratory), and by the lack of concordance in the recording of progressions/transformations. In contrast, incidence rates of overall MPN, MDS, and MDS/MPN in our region were markedly higher in comparison to European2,8,13 and US9,10,11 datasets, most of them covering years before/close to the implementation of the ICD-O-3 and the 2001 WHO classification. As far as MPN are regarded, disparities were mainly attributed to polycythemia vera and essential thrombocythemia, while rates of chronic myeloid leukemia (consistently documented since 1970’s with the identification of its causal chromosome transition), primary myelofibrosis, and mastocytosis were similar across studies. Such differences may be linked to the identification of the JAK2 mutation in 200524 and the derived 2008 WHO guidelines for MPN, whose impact is not documented in series covering only previous years.
On the other hand, the incidence of AML, which is a long-established entity, was more homogeneous across different regions. Indeed, overall rates were consistent with European2,8 and US25 findings, as well as with smaller European series13,26,27,28, while slightly lower rates were reported in Canada29 and Switzerland30. Karyotypic information was not available for many of our cases, and thus, the proportion of AML with cytogenetic abnormalities (14%) was slightly lower in comparison with more specific studies31,32,33. However, rates of AML with t(15;17)(q22;q12) were still higher compared to the European average, further supporting the hypothesis that such entity might be more prevalent among individuals with Spanish ancestry34. Finally, most of these studies included AML of ambiguous lineage within AML-NOS subgroups, although it is placed as a distinct category from AML since the introduction of the 2008 WHO classification. Further studies are warranted to clarify the epidemiology of these entities owing their clinical relevance.
We evidenced increasing incidence trends of MDS/MPN, MDS, and several MPN, previously reported in the literature and mostly linked to refinements in the diagnostic, classification, and registration practices. Within the latter, this was particularly seen in the three most frequent Philladelphia chromosome negative subtypes, and thus, may be linked to the implementation of screening for JAK2 mutation. In the same vein, Girodon et al.35 documented an almost twofold increase in the incidence of essential thrombocythemia after 2005, but not in the remaining MPN subtypes. In agreement with the few European studies examining AML incidence trends13,15, we found a stable incidence of overall AML across the period of study. In contrast, an increasing trend was found in a Dutch pediatric study (1990–2015)36 and in Canada (1992–2010)29 and US from 2009 to 201025 in the general population, the latter mainly attributed to changes in the registration of transformations in the Surveillance, Epidemiology, and End Results (SEER) program. Finally, NOS cases decreased remarkably across the period of study, which could be attributed both to a more specific clinical diagnosis and/or to an improved codification in Spanish cancer registries.
The etiology of MNs, in line with most hematological malignancies, is still uncertain. Several subtypes have been consistently associated with treatments (i.e. radiation, alkylating agents or topoisomerase II inhibitors)37, while environmental epidemiological studies suggest a potential role of obesity, tobacco exposure, autoimmune disorders, and infections in myelodisplastic38 or myeloproliferative diseases39. However, neither these factors, nor the genetic alterations currently described40, can explain the large variability in the incidence of these neoplasms2. In addition, drawing etiological hypothesis based on geographic heterogeneity in incidence rates is hampered by heterogeneity in accuracy and completeness in the registration of several subtypes. Several medical-claims-based studies have shown an underreporting of MNs41,42,43,44, namely MDS and MPN (which are often diagnosed and managed in an outpatient setting, and might be missed by surveillance systems relying on hospital registration), and among the elderly (in which diagnostic evaluation might not be as aggressively sought as in younger individuals). Indeed, we evidenced marked differences in incidence rates across Spanish provinces, with the highest incidence rates of MDS, MPN, and MDS/MPN reported in the Girona cancer registry, which has started several initiatives15,45 to cope with these challenges. Following the example of the French Network of Cancer Registries (FRANCIM)46, training programs to improve the codification and registration of hematological neoplasm have been boosted in the REDECAN during the last few years, which are expected to start to bear fruits in future studies.
Since 2008, there have been numerous advances in the identification of genetic biomarkers associated with specific MNs, which led to the release of an updated WHO classification in 20165. The impact of these changes will be noticeable within the next years, when they become routinely distinguished in clinical practice and consistently coded in cancer registries. The incorporation of these updates at a cancer registry level will be eased with the release of the ICD-O-3, second revision47, which is recommended for use from 2020. Further studies with contemporary data including these classification changes are warranted.
The number of expected MNs in 2021 depicts the present cancer burden of these malignancies in Spain. However, these data should be interpreted with caution due to several factors. First, some subtypes are extremely rare, making estimates less robust. Furthermore, the estimates provided herein do not reflect the impact of the new 2016 WHO classification5, nor that of coronavirus disease 2019 (COVID-19)48, as they are based on extrapolations of cancer data collected in previous years. Regarding the latter, although the full extent of the impact of the COVID-19 pandemic remains unknown, delays in cancer diagnosis are expected to cause a short-term decline in cases followed by an increasing incidence of advanced-stage diagnosis49,50. In addition, if, over the period 2002–2013, there had been an increase in the completeness in the registration of MN cases, with the corresponding positive effect on the APC, this would cause an overestimation in the number of cases predicted for the year 2021. Nonetheless, these results are still interesting for clinicians and public health specialists in evaluating the cost of management and new treatments for these pathologies, and to account for the gap between the expected and the observed cases after the COVID-19 pandemic.
Among the strengths of this population-based study is the large number of MNs that allowed us to assess and compare incidence rates not only for common but also for relatively rare entities. However, several limitations must be considered when interpreting our results. First, the changing classification and diagnostic criteria (and consequent heterogeneity in disease definitions across countries, clinical centers, and cancer registries) hamper the interpretation of our incidence rates and trends, as well as comparisons with previous studies. In addition, we cannot exclude the aforementioned underreporting of cases, particularly documented in MDS and MPN, and among the elderly. In addition, we lacked a centralized pathology and clinical review, which could have decreased the proportion of NOS cases and improved the quality of our data. This is particularly relevant for MDS, due to the poor inter-observed concordance in diagnosis and the numerous non-neoplastic conditions that can mimic such neoplasms51,52. Nevertheless, in spite of the unavoidable biases due to variability and variation in registration quality and coding practices, over 95% of cases had adequate morphology specification.
In conclusion, this study presents the first comprehensive population-based analysis of MNs incidence in Spain. It highlights some useful points for public health authorities, such as the increasing incidence of several subtypes, the remarkable variability in incidence rates (especially of MDS, MPN, and MDS/MPN) among provinces, and the number of cases expected in 2021 based on these data. The negative trend in the incidence of NOS cases suggests a more specific diagnosis and/or improvements in the registration of these cases across the study period, however, additional efforts should be made to improve the quality of MNs data in future studies.
Data availability
The dataset analyzed during the current study is not publicly available due to national regulations of cancer registry data. However, it is available anonymized from Dr. Rafael Marcos-Gragera (rmarcos@iconcologia.net) on reasonable request.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 71, 209–249 (2021).
Sant, M. et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood 116, 3724–3734 (2010).
Jaffe, E. S., Harris, N. L., Stein, H. & Wardiman, J. W. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (IARC Press, 2001).
Swerdlow, S. H. et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (IARC Press, 2008).
Swerdlow, S. H. et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (IARC Press, 2017).
Sant, M., Karjalainen-Lindsberg, M. L., Maynadié, M., & HAEMACARE Working Group. Manual for coding and reporting haematological malignancies. Tumori 96(4), (2010).
Fritz, A. et al. International Classification of Diseases for Oncology 3rd edn, 357 (World Health Organization, 2000).
Visser, O. et al. Incidence, survival and prevalence of myeloid malignancies in Europe. Eur. J. Cancer 48, 3257–3266 (2012).
Srour, S. A. et al. Incidence and patient survival of myeloproliferative neoplasms and myelodysplastic/myeloproliferative neoplasms in the United States, 2001–12. Br. J. Haematol. 174, 382–396 (2016).
Ma, X., Does, M., Raza, A. & Mayne, S. T. Myelodysplastic syndromes: incidence and survival in the United States. Cancer 109, 1536–1542 (2007).
Rollison, D. E. et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood 112, 45–52 (2008).
Bailey, C. et al. Adult leukemia survival trends in the United States by subtype: a population-based registry study of 370,994 patients diagnosed during 1995–2009. Cancer 124, 3856–3867 (2018).
Maynadié, M. et al. Twenty-five years of epidemiological recording on myeloid malignancies: data from the specialized registry of hematologic malignancies of Cote d’Or (Burgundy, France). Haematologica 96, 55–61 (2011).
Roman, E. et al. Myeloid malignancies in the real-world: Occurrence, progression and survival in the UK’s population-based Haematological Malignancy Research Network 2004–15. Cancer Epidemiol. 42, 186–198 (2016).
Osca-Gelis, G. et al. Population-based incidence of myeloid malignancies: fifteen years of epidemiological data in the province of Girona, Spain. Haematologica 98, e95–e97 (2013).
Navarro, C. et al. Population-based cancer registries in Spain and their role in cancer control. Ann. Oncol. 21, 3–13 (2010).
Spanish Network of Cancer Registries (REDECAN). Available at: https://stage.redecan.org/es. (Accessed: 17th March 2021)
Instituto Nacional de Estadística (INE) [Available at: https://www.ine.es, last accessed on April 16, 2020].
World Health Organization. International classification of diseases for oncology (ICD-O) – 3rd edition, 1st revision, 3rd ed.; 2013. Available at: https://apps.who.int/iris/handle/10665/96612.
Maynadié, M. et al. Survival of European patients diagnosed with myeloid malignancies: a HAEMACARE study. Haematologica 98, 230–238 (2013).
Gavin, A. et al. Towards optimal clinical and epidemiological registration of haematological malignancies: Guidelines for recording progressions, transformations and multiple diagnoses. Eur. J. Cancer 51, 1109–1122 (2015).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria 2020. Available at: https://www.R-project.org/.
Marcos-Gragera, R. et al. Survival of European patients diagnosed with lymphoid neoplasms in 2000–2002: Results of the HAEMACARE project. Haematologica 96, 720–728 (2011).
Baxter, E. J. et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 365, 1054–1061 (2005).
Polednak, A. P. Recent improvement in completeness of incidence data on acute myeloid leukemia in US cancer registries. J. Registry Manag. 41, 77–84 (2014).
Derolf, Å. R. et al. Improved patient survival for acute myeloid leukemia: a population-based study of 9729 patients diagnosed in Sweden between 1973 and 2005. Blood 113, 3666–3672 (2009).
Broccia, G. et al. Hematological malignancies in the island of Sardinia, 1974–1993: age and sex distributions and temporal changes in incidence. Hematol. Oncol. 22, 91–109 (2004).
Smith, A., Howell, D., Patmore, R., Jack, A. & Roman, E. Incidence of haematological malignancy by sub-type: a report from the haematological malignancy research network. Br. J. Cancer 105, 1684–1692 (2011).
Ghazawi, F. M. et al. Analysis of acute myeloid leukemia incidence and geographic distribution in Canada from 1992 to 2010 reveals disease clusters in Sarnia and other industrial US border cities in Ontario. Cancer 125, 1886–1897 (2019).
Schnegg-Kaufmann, A. et al. Improvement of relative survival in elderly patients with acute myeloid leukaemia emerging from population-based cancer registries in Switzerland between 2001 and 2013. Cancer Epidemiol. 52, 55–62 (2018).
Sanderson, R. N. et al. Population-based demographic study of karyotypes in 1709 patients with adult Acute Myeloid Leukemia. Leukemia 20, 444–450 (2006).
Bacher, U. et al. Population-based age-specific incidences of cytogenetic subgroups of acute myeloid leukemia. Haematologica 90, 1502–1510 (2005).
Preiss, B. S. et al. Cytogenetic findings in adult de novo acute myeloid leukaemia. A population-based study of 303/337 patients. Br. J. Haematol. 123, 219–234 (2003).
Dinmohamed, A. G. & Visser, O. Incidence of acute promyelocytic leukemia across Europe: results of RARECAREnet-a population-based study. Stem cell Investig. 6, 37 (2019).
Girodon, F. et al. Significant increase in the apparent incidence of essential thrombocythemia related to new WHO diagnostic criteria: a population-based study. Haematologica 94, 865–869 (2009).
Reedijk, A. M. J. et al. Improved survival for children and young adolescents with acute myeloid leukemia: a Dutch study on incidence, survival and mortality. Leukemia 33, 1349–1359 (2019).
Arber, D. A. et al. The 2016 revision to the world health organization classification of myeloid neoplasms and acute leukemia. Blood 127, 2391–2405 (2016).
Sweeney, M. R. et al. Medical conditions and modifiable risk factors for myelodysplastic syndrome: a systematic review. Cancer Epidemiol. Biomarkers Prev. 28, 1502–1517 (2019).
Duncombe, A. S. et al. Modifiable lifestyle and medical risk factors associated with myeloproliferative neoplasms. HemaSphere 4, e327 (2020).
Taylor, J., Xiao, W. & Abdel-Wahab, O. Diagnosis and classification of hematologic malignancies on the basis of genetics. Blood 130, 410–423 (2017).
Craig, B. M., Rollison, D. E., List, A. F. & Cogle, C. R. Underreporting of myeloid malignancies by United States cancer registries. Cancer Epidemiol. Biomarkers Prev. 21, 474–481 (2012).
Cogle, C. R., Craig, B. M., Rollison, D. E. & List, A. F. Incidence of the myelodysplastic syndromes using a novel claims-based algorithm: high number of uncaptured cases by cancer registries. Blood 117, 7121–7125 (2011).
Dinmohamed, A. G. et al. The use of medical claims to assess incidence, diagnostic procedures and initial treatment of myelodysplastic syndromes and chronic myelomonocytic leukemia in the Netherlands. Leuk. Res. 39, 177–182 (2015).
McQuilten, Z. K. et al. Underestimation of myelodysplastic syndrome incidence by cancer registries: results from a population-based data linkage study. Cancer 120, 1686–1694 (2014).
Solans, M. et al. Challenges in assessing the real incidence of chronic lymphocytic leukemia: 16 years of epidemiological data from the province of Girona, Spain. Cancer Causes Control 29, 379–382 (2018).
Monnereau, A. et al. Unbiased estimates of long-term net survival of hematological malignancy patients detailed by major subtypes in France. Int. J. cancer 132, 2378–2387 (2013).
International Agency for Cancer Registration (IACR). International Classification of Diseases for Oncology. ICD-O-3.2. (2021). Available at: http://www.iacr.com.fr/index.php?option=com_content&view=category&layout=blog&id=100&Itemid=577. (Accessed: 3rd March 2021).
Hu, B., Guo, H., Zhou, P. & Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 19, 141–154 (2021).
Petrova, D., Pérez-Gómez, B., Pollán, M. & Sánchez, M.-J. Implications of the COVID-19 pandemic for cancer in Spain. Med. Clin. (Barc) 155, 263–266 (2020).
Maringe, C. et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 21, 1023–1034 (2020).
Steensma, D. P. Dysplasia has A differential diagnosis: distinguishing genuine myelodysplastic syndromes (MDS) from mimics, imitators, copycats and impostors. Curr. Hematol. Malig. Rep. 7, 310–320 (2012).
Parmentier, S. et al. Assessment of dysplastic hematopoiesis: lessons from healthy bone marrow donors. Haematologica 97, 723–730 (2012).
Acknowledgements
REDECAN Working Group: Girona Cancer Registry—Coordination of haematology neoplasms in REDECAN: Rafael Marcos-Gragera, Montse Puigdemont, Anna Vidal-Vila, Arantza Sanvisens; Tarragona Cancer Registry—Data coordination in REDECAN: Marià Carulla, Alberto Ameijide, Clàudia Pla, Jaume Galceran; Álava Cancer Registry: Arantza López de Munain, Patricia Sancho, Mª Luisa Iruretagoyena; Albacete Cancer Registry: Katia del Pozo; Asturias Cancer Registry: Susana Merino Perera, Virginia Menéndez-García, Marta Rodríguez-Camblor; Bizkaia Cancer Registry: Visitación de Castro, Marta De La Cruz, Joseba Bidaurrazaga; Castellón Cancer Registry: Emilia Banqueri, Consol Sabater, Javier Peñalver; Ciudad Real Cancer Registry: Matilde Chico; Cuenca Cancer Registry: Ana-Isabel Marcos, Rosario Jimenez-Chillarón; Gipuzkoa Cancer Registry: Leire Gil, Amaia Aizurura, Nerea Larrañaga; Gran Canaria Cancer Registry: Mª Dolores Rojas-Martin, Emilio De Miguel, Maria- Carmen Gabas; Granada Cancer Registry: Maria-José Sánchez, Daysi Yoe-Ling Chang-Chan; Rafael Rios-Tamayo; La Rioja Cancer Registry: Josefina Perucha; Mallorca Cancer Registry: Patricia Ruiz-Armengol, Carmen Sánchez-Contador; Murcia Cancer Registry: Mª Dolores Chirlaque, Antonia Sánchez-Gil, Ricardo-José Vaamonde; Navarra Cancer Registry: Marcela Guevara, Eva Ardanaz; Tenerife Cancer Registry: Mª Araceli Alemán Herrera, Leonor-Olga Veláquez, Mª Magdalena Ramos-Marrero; Castilla y León Cancer Registry: Pilar Gutiérrez, Rufino Álamo, Lorena Estévez; Registro Español de Tumores Infantiles: Rafael Peris, Adela Cañete; Valencia Autonomous Community Registry: Ana Vizcaino, Fernando Almela.
Funding
This work was partially funded by the Josep Carreras Leukaemia Research Institute (Grant No.: FIJC1100); the Ministry of Science, Innovation and Universities, Carlos III Health Institute (ISCIII), Spain (Grant No.: PI15/00966); the Agency for Management of University and Research Grants, Government of Catalonia (Grant No.: 2017SGR00733); the CIBER of Epidemiology and Public Health (CIBERESP) (‘Cohort-Real World Data’ subprogram).
Author information
Authors and Affiliations
Contributions
RMG, MS, and AS contributed to the study conception and design. Data collection was performed by SM, DR, AA, EB, MC, AIM, VC, LG, ALM, MP, MJS, JP, PRA, MDC, MG, and MC. Data curation and data analysis were performed by AS and AA. The first draft of the manuscript was written by MS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Solans, M., Sanvisens, A., Ameijide, A. et al. Incidence of myeloid neoplasms in Spain (2002–2013): a population-based study of the Spanish network of cancer registries. Sci Rep 12, 323 (2022). https://doi.org/10.1038/s41598-021-03734-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-03734-6
This article is cited by
-
Myelodysplastic syndromes mortality in Spain: a comprehensive age-period-cohort and joinpoint analysis
Clinical and Translational Oncology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.