Abstract
In elderly ICU patients, the prevalence of skeletal muscle loss is high. Longitudinal effect of thoracic muscles, especially in elderly ICU patients, are unclear although skeletal muscle loss is related with the short- and long-term outcomes. This study aimed to evaluate whether pectoralis muscle mass loss could be a predictor of prognosis in elderly ICU patients. We retrospectively evaluated 190 elderly (age > 70 years) patients admitted to the ICU. We measured the cross-sectional area (CSA) of the pectoralis muscle (PMCSA) at the fourth vertebral region. CT scans within two days before ICU admission were used for analysis. Mortality, prolonged mechanical ventilation, and longitudinal changes in Sequential Organ Failure Assessment (SOFA) scores were examined. PMCSA below median was significantly related with prolonged ventilation (odds ratio 2.92) and a higher SOFA scores during the ICU stay (estimated mean = 0.94). PMCSA below median was a significant risk for hospital mortality (hazards ratio 2.06). In elderly ICU patients, a low ICU admission PMCSA was associated with prolonged ventilation, higher SOFA score during the ICU stay, and higher mortality. Adding thoracic skeletal muscle CSA at the time of ICU admission into consideration in deciding the therapeutic intensity in elderly ICU patients may help in making medical decisions.
Similar content being viewed by others
Introduction
As the population is rapidly aging, the proportion of elderly patients admitted to the intensive care unit (ICU) is increasing. Currently, the median age of critically ill patients approaches 65 years in many countries1. Elderly patients have a higher mortality rate in the ICU, and patients surviving the ICU often suffer from sequalae2. Caring for older patients frequently poses ethical and practical challenges, both prior to and during ICU admission3. In order to determine the level of treatment for elderly patients, we need to consider functional recovery as well as their chances of survival in the ICU.
Loss of muscle mass is a component of sarcopenia and is associated with a risk of adverse outcomes, such as disability, poor quality of life, and death4. Loss of muscle mass is also related to negative outcomes in patients with various lung diseases, cholangiocarcinoma, and breast cancer5,6,7,8. Bioelectrical impedance analysis, dual energy X-ray absorptiometry, magnetic resonance imaging, and B-mode ultrasound are widely used to quantify both total and local skeletal muscle mass4. Measurement of the cross-sectional area (CSA) of skeletal muscles on single-slice axial computed tomography (CT) is an alternative method to assess local skeletal muscle mass9. Correlations have been found between the muscle CSA at a single thoracic level on chest CT and total muscle volume10,11,12. Previous studies reported that at the level of the fourth vertebral region, the CSAs of the skeletal muscles are associated with frailty markers in lung transplantation and cardiac surgery patients8,13. Based on these studies, we have shown that loss of skeletal muscle mass measured by quantifying the thoracic muscle is related to a higher mortality in idiopathic pulmonary fibrosis patients14.
A recent study showed that in ICU patients, the thoracic muscle mass is related to mortality15. Evidence suggests that skeletal muscle mass and strength decline in a linear fashion, with up to 50% of mass being lost by the eighth decade of life16. The consequences of loss of muscle mass can be severe in older adults, as the associated strength and functional declines can contribute to a number of adverse health outcomes17,18,19. In a previous study, the prevalence of sarcopenia was 71% among the elderly ICU patients20.
We, therefore, sought to determine whether a low skeletal muscle mass adversely affects the outcome in elderly patients admitted to the ICU. In this study, we focused on pectoralis muscles at the fourth vertebral region in elderly (aged > 70 years) ICU patients and hypothesized that skeletal muscle mass, measured by analyzing the major and minor pectorals muscles’ CSAs from chest CT images, could be a predictor of prognosis in elderly medical ICU patients.
Results
Baseline characteristics
Baseline characteristics of the study subjects stratified by PMCSA are provided in Table 1. The distribution of the CSAs of the pectoralis muscles is shown in Supplementary Fig. 1, and sequential organ failure assessment (SOFA) scores during the follow-up period are shown in Supplementary Fig. 2. The cutoff value corresponds to the median of the CSA of the pectoralis muscles (male = 24.5 cm2, female = 18.3 cm2). Of the 190 patients, 113 were male and 77 were female. The most common reason for ICU admission was respiratory failure (48.9%). Overall, 121 patients (63.7%) were intubated, 43 (22.6%) died in the ICU, and 71 (37.4%) died in the hospital.
Male patients had higher PMCSA values (P < 0.01). In comparison, patients whose PMCSA values were below the median exhibited a significantly lower body mass index (BMI) (P < 0.01), a higher nutritional risk screening 2002 (NRS-2002) score (P < 0.01), a higher rate of prolonged mechanical ventilation (P = 0.02), and a higher ICU and hospital mortality (P = 0.04 and P = 0.01, respectively) than patients whose PMCSA values were above the median.
Prolonged mechanical ventilation and SOFA score
In the multivariate logistic regression analysis for the relation of multiple clinical variables with prolonged mechanical ventilation (Table 2), a PMCSA below the median (OR 2.92, 95% confidence interval (95% CI) 1.06–8.06, P = 0.04) and a higher SOFA score at the time of ICU admission (OR 1.20, 95% CI 1.02–1.42, P = 0.03) were related to prolonged ventilation. In the regression analysis for the association of the PMCSA with the longitudinal change of the SOFA score, adjusted for age, sex, BMI, and the Charlson Comorbidity Index (CCI), a PMCSA below the median was related to a higher SOFA score during the ICU stay (estimated mean = 0.94, standard error = 0.44, P = 0.03).
Survival and clinical factors associated with mortality
Hospital mortality at 30, 60, and 90 days after ICU admission was 23.4%, 25.5%, and 26.6%, respectively, in patients whose PMCSA values were above the median, whereas it was 34.4%, 41.7%, and 43.8%, respectively, in patients whose PMCSA values were below the median. Kaplan–Meier survival curves stratified by the CSA of the muscles at the level of T4 are shown in Fig. 1; patients were stratified by the PMCSA. Patients whose PMCSA values were below the median showed worse survival rates than those whose PMCSA values were above the median (P = 0.034).
The relationships between hospital mortality and clinical parameters were evaluated using Cox proportional hazards analysis (Table 3). Univariate analysis showed that a PMCSA below the median (P = 0.03) and intubation (P < 0.01) were significantly correlated with hospital mortality.
Additional multivariate Cox proportional hazards analysis was performed to compare the contributions of these indices. In the multivariate analysis, we included the PMCSA, age, sex, BMI, CCI, whether the patient underwent intubation, and SOFA score at the time of ICU admission as explanatory variables. Stepwise Cox proportional hazards analysis demonstrated that PMCSA values below the median [hazards ratio (HR) 2.06; 95% CI 1.23–3.47; P = 0.01], intubation (HR 2.32; 95% CI 1.33–4.05; P < 0.01), a higher CCI (HR 1.12; 95% CI 1.02–1.23; P = 0.02), and a higher SOFA score (HR 1.1; 95% CI 1.02–1.18; P = 0.02) were related to hospital mortality.
Discussion
This study showed that in elderly ICU patients, a low PMCSA at ICU admission was associated with prolonged mechanical ventilation, a higher SOFA score during the ICU stay, and lower mortality.
The present findings agree with previous studies15,21, which showed that low CSAs from CTs were correlated with mortality, whereas this study focused on mortality, longitudinal changes in organ dysfunction, namely the SOFA score, and prolonged ventilation. Not only the initial SOFA scores but also the trends in SOFA scores are known to be helpful in predicting the outcomes of ICU patients22. Prolonged mechanical ventilation is associated with increased health costs, morbidity, and mortality23. As skeletal muscle mass is related to the short- and long-term outcomes in various diseases24,25, it would be meaningful to focus on longitudinal changes in prolonged mechanical ventilation and the SOFA scores.
We believe that the CSAs of the pectoralis muscles reflect the patient’s general health and nutritional status and are markers of frailty before ICU admission. Skeletal muscles are important in regulating immune function, glucose disposal, cytokine signaling, and protein synthesis20. Skeletal muscle loss is associated with an increased risk of developing nosocomial infections and experiencing falls in the hospital26. There are also studies indicating that skeletal muscle loss is associated with depression27. It is possible that the factors described above contributed to these findings.
In previous studies on skeletal muscle mass in the ICU using CT as an assessment tool, lumbar muscle at the level of L3 had been the main concern28,29. In contrast, this study focused on the thoracic skeletal muscles. According to a previous study, up to 65% of all ICU chest X-rays (CXR) had unexpected or abnormal findings, many of which affected management. The problem is further compounded as CT scanning is being used in critically ill patients who may have multiple medical problems that may not be easily discriminated by the CXR30. Without any additional radiation exposure, CSA analysis through existing chest CT scans provides an objective index of future prognosis in elderly ICU patients.
Previous studies15,19 included a younger patient population, whereas this study focused on the elderly medical patient population. As the skeletal muscle loss increases with aging and the proportion of elderly patients in the ICU is increasing1, it is important to accurately stratify the risk of sarcopenia in elderly ICU patients.
The most appropriate treatment for elderly ICU patients may not necessarily mean maximal treatment, and in patients without improvement in their clinical situation, the therapeutic intensity level may no longer be in accordance with patients’ chances of long-term survival with acceptable quality of life, during which a clinical decision might need to be made2. In the algorithm on the decision-making process for caring for critically ill older patients proposed by Bertrand et al.2, it is recommended to assess the patient’s condition and arrange a meeting with the family on day 2 or 3 of the ICU admission. Considering the thoracic skeletal muscle CSA at the time of ICU admission when deciding the therapeutic intensity for elderly ICU patients may help in making medical decisions.
In abdominal CT, the muscle area at the L3 vertebra level, divided by the patient height2, is accepted as a surrogate marker of loss of skeletal muscle quantity and cachexia31. However, at the thoracic level, the method of assessing skeletal muscle loss has not been established, and the muscle areas measured for each study were different. For example, a study by Ariel et al.15 assessed the pectoralis muscle CSA, a study by Florian et al.32 assessed the thoracic skeletal muscles at the level of T5, a study by Lee et al.33 assessed diaphragm thickness, and a study by Fuseya et al.10 used the erector spinae muscle CSA at the level of T12. In this study, we analyzed the CSA of the pectoralis at the level of T4. Muscles at the level of T4 have shown clinical significance in various diseases12,14,34; among these muscles, the pectoralis muscle CSA at the level of T4 is easy to identify, and its area can be standardized across cohorts12. However, more studies are needed to clarify which muscles among the thoracic muscles best reflect sarcopenia.
Our study has limitations. First, the nature of this study was retrospective. Second, the study population was from a single center, consisted only of Asian patients, and involved no replication cohort. Thus, our results may not be fully generalizable to other populations. However, validity of the reported prognostic factors in ICU patients, such as the CCI and SOFA scores, was confirmed in this study population, which supported the present findings. Third, the physical activity levels of all subjects were not directly evaluated. We could not include data regarding physical function testing. However, based on other reports6,7,11, we suspected that the skeletal muscles of the chest may reflect both the physical activity and physiological parameters. Further analysis is needed to verify these assumptions. Fourth, we could not perform pectoralis muscle mean density measurements because less than half of the patients received non-contrast CTs.
In conclusion, low CSAs of the pectoralis muscle obtained from single-slice axial chest CT were associated with prolonged mechanical ventilation, higher SOFA scores during ICU stays, and a higher mortality. Considering the thoracic skeletal muscle CSA at the time of ICU admission when deciding the therapeutic intensity in elderly ICU patients may help in making medical decisions.
Methods
Study design and population
We retrospectively reviewed the medical records of patients aged > 70 years admitted to the ICU between August 2016 and December 2018 in a tertiary care hospital in South Korea. The flow diagram of the subjects in this study is depicted in Supplementary Fig. 3. Initially, 1085 patients who were admitted to the medical ICU were included. Patients were excluded if (1) the patient was admitted from another hospital or ICU; (2) the patient did not perform a chest CT scan within two days before ICU admission; (3) the patient was aged < 70 years; (4) data on the performance status and death were unavailable; or (5) the quality of the chest CT scan was poor. After meeting these criteria, 190 (113 men and 77 women) subjects were included in the analysis. As our hospital operates a cardiac ICU, an oncology ICU, a pediatric ICU, and a surgical ICU, patients from these units were not included in our study.
Age, body mass index (BMI), underlying diseases (CCI), NRS-2002 points, mechanical ventilation, ICU and hospital discharge, the serial weekly SOFA score up to 4 weeks, and mortality data were collected for all patients; data regarding the pectoralis muscle cross-sectional area at the level of the fourth thoracic vertebra (PMCSA) were available in all patients. The NRS-2002 used in this study was calculated according to the study by Kondrup et al.35. The CCI used in this study was calculated according to the study by Charlson et al.36. The SOFA score was calculated according to the study by Jones et al.37. Prolonged mechanical ventilation was defined as greater than 21 days of mechanical ventilation required for at least six hours per day38. Follow-up data, including mortality data, were collected until June 2019. This study was performed in accordance with the provisions of the Declaration of Helsinki. This research protocol was approved by the Institutional Review Board of Severance Hospital. (IRB No. 2019-2889-001) The study design was approved by the appropriate ethics review boards. Due to the retrospective nature of this study, the requirement to obtain informed patient consent was waived. (The Institutional Review Board of Severance Hospital waived the need to provide informed consent).
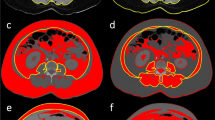
Measurement of CSA of the skeletal muscle at the level of T4
CT within 2 days before the admission to the ICU was used for the analysis because approximately 10% of the total body muscle mass may be lost in hospitalized elderly patients from only 3 days of immobility39. All scans were performed with the following scanning parameters: a tube voltage of 120 kVp, an average tube current of 300 mA, an average pitch of 0.9, and a volume CT dose index less than 6.0 mGy. After scanning, axial images were reconstructed at a slice thickness of 1 mm and a slice increment of 1 mm with a medium-smooth convolution kernel. Measurements of the major and minor pectoralis muscles were performed as in our previous study15. Quantitative assessment of the CSA was performed semi-automatically using the Aquarius iNtuition Viewer (ver. 4.4.11, TeraRecon Inc., San Mateo, CA, USA), as shown in Fig. 2. The T4 level was defined as the slice including the middle of the fourth thoracic vertebrae by comparing the sagittal and transverse views; the observers visually identified single cross-sectional images in which the borders of the thoracic and back muscles were outlined. Tissue CSAs in slices were computed automatically by summation of the pixel attenuation of − 30 to + 150 Hounsfield units for skeletal muscle. After applying the threshold method (with a predefined Hounsfield unit threshold) to slices, boundaries between different tissues were manually corrected. Contrast-enhanced and non-contrast CT scans were used, as there was no difference in muscle CSA measurements between these in the previous study40. The measurement of the CSAs was performed independently by two radiology technicians with 4 and 6 years of experience. Differences greater than 5% were settled by a third radiology technician who had 10 years of experience. Radiology technicians performed measurements of the CSAs without access to patient information. CSA values below the median value in the respective sexes were considered to indicate loss of skeletal muscle mass.
Statistical analysis
Descriptive statistics are reported as numbers with proportions or medians with interquartile ranges. Fisher's exact tests were conducted to compare categorical variables between the survivor and non-survivor groups; Mann–Whitney tests were conducted to compare continuous variables between the two groups. To analyze the effect of muscle CSAs on the SOFA score, the linear mixed model analysis for the continuous longitudinal dataset was used. To evaluate the relationship between prolonged mechanical ventilation and multiple clinical parameters while controlling potential confounding factors, multivariate logistic regression models were used. Survivals were estimated with the Kaplan–Meier method and compared with the log-rank test. Multivariate Cox proportional hazards models were performed to investigate relationships between clinical parameters and mortality. NRS-2002 points were excluded from the multivariate analyses since the BMIs were included in the calculation of the NRS-2002 points. An adjusted P-value < 0.05 was considered statistically significant. All statistical analyses were performed with SPSS version 25.0 (SPSS Inc., Chicago, IL, USA, https://www.ibm.com/support/pages/downloading-ibm-spss-statistics-25).
Data availability
The datasets used and/or analyzed are available from corresponding author upon reasonable request.
References
Flaatten, H. et al. The status of intensive care medicine research and a future agenda for very old patients in the ICU. Intensive Care Med. 43, 1319–1328. https://doi.org/10.1007/s00134-017-4718-z (2017).
Guidet, B. et al. Caring for the critically ill patients over 80: A narrative review. Ann. Intensive Care. 8, 114. https://doi.org/10.1186/s13613-018-0458-7 (2018).
Nguyen, Y. L., Angus, D. C., Boumendil, A. & Guidet, B. The challenge of admitting the very elderly to intensive care. Ann. Intensive Care. 1, 29. https://doi.org/10.1186/2110-5820-1-29 (2011).
Muscaritoli, M. et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 29, 154–159. https://doi.org/10.1016/j.clnu.2009.12.004 (2010).
Deluche, E. et al. Impact of body composition on outcome in patients with early breast cancer. Support Care Cancer. https://doi.org/10.1007/s00520-017-3902-6 (2017).
Kim, E. Y. et al. Prognostic significance of CT-determined sarcopenia in patients with small-cell lung cancer. J. Thorac. Oncol. 10, 1795–1799. https://doi.org/10.1097/jto.0000000000000690 (2015).
Kim, E. Y. et al. Evaluation of sarcopenia in small-cell lung cancer patients by routine chest CT. Support Care Cancer. 24, 4721–4726. https://doi.org/10.1007/s00520-016-3321-0 (2016).
Zuckerman, J. et al. Psoas muscle area and length of stay in older adults undergoing cardiac operations. Ann. Thorac. Surg. 103, 1498–1504. https://doi.org/10.1016/j.athoracsur.2016.09.005 (2017).
Heymsfield, S. B., Gonzalez, M. C., Lu, J., Jia, G. & Zheng, J. Skeletal muscle mass and quality: Evolution of modern measurement concepts in the context of sarcopenia. Proc. Nutr. Soc. 74, 355–366. https://doi.org/10.1017/s0029665115000129 (2015).
Fuseya, Y. et al. Quantitative assessment of erector spinae muscles in patients with chronic obstructive pulmonary disease. Novel chest computed tomography-derived index for prognosis. Ann. Am. Thorac. Soc. https://doi.org/10.1513/AnnalsATS.201507-446OC (2016).
Mathur, S., Rodrigues, N., Mendes, P., Rozenberg, D. & Singer, L. G. Computed tomography-derived thoracic muscle size as an indicator of sarcopenia in people with advanced lung disease. Cardiopulm. Phys. Ther. J. 28, 99–105. https://doi.org/10.1097/cpt.0000000000000054 (2017).
McDonald, M. L. et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann. Am. Thorac. Soc. 11, 326–334. https://doi.org/10.1513/AnnalsATS.201307-229OC (2014).
Rozenberg, D. et al. Thoracic muscle cross-sectional area is associated with hospital length of stay post lung transplantation: A retrospective cohort study. Transpl. Int. 30, 713–724. https://doi.org/10.1111/tri.12961 (2017).
Moon, S. W. et al. Thoracic skeletal muscle quantification: Low muscle mass is related with worse prognosis in idiopathic pulmonary fibrosis patients. Respir. Res. 20, 35. https://doi.org/10.1186/s12931-019-1001-6 (2019).
Jaitovich, A. et al. ICU admission muscle and fat mass, survival, and disability at discharge: A prospective cohort study. Chest 155, 322–330. https://doi.org/10.1016/j.chest.2018.10.023 (2019).
Metter, E. J., Conwit, R., Tobin, J. & Fozard, J. L. Age-associated loss of power and strength in the upper extremities in women and men. J. Gerontol. A Biol. Sci. Med. Sci. 52, B267–276 (1997).
Dufour, A. B., Hannan, M. T., Murabito, J. M., Kiel, D. P. & McLean, R. R. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: The Framingham Study. J. Gerontol. A Biol. Sci. Med. Sci. 52, B267-276. https://doi.org/10.1093/gerona/52a.5.b267 (1997).
Walston, J. D. Sarcopenia in older adults. Curr. Opin. Rheumatol. 24, 623–627. https://doi.org/10.1097/BOR.0b013e328358d59b (2012).
Xue, Q. L., Walston, J. D., Fried, L. P. & Beamer, B. A. Prediction of risk of falling, physical disability, and frailty by rate of decline in grip strength: The women’s health and aging study. Arch. Intern. Med. 171, 1119–1121. https://doi.org/10.1001/archinternmed.2011.252 (2011).
Moisey, L. L. et al. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit. Care 17, R206. https://doi.org/10.1186/cc12901 (2013).
Joyce, P. R., O’Dempsey, R., Kisby, G. & Anstey, C. A retrospective observational study of sarcopenia and outcomes in critically ill patients. Anaesth. Intensive Care 48, 229–235. https://doi.org/10.1177/0310057X20922234 (2020).
Ferreira, F. L., Bota, D. P., Bross, A., Mélot, C. & Vincent, J.-L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 286, 1754–1758. https://doi.org/10.1001/jama.286.14.1754 (2001).
Lone, N. I. & Walsh, T. S. Prolonged mechanical ventilation in critically ill patients: Epidemiology, outcomes and modelling the potential cost consequences of establishing a regional weaning unit. Crit. Care 15, R102. https://doi.org/10.1186/cc10117 (2011).
Vetrano, D. L. et al. Association of sarcopenia with short- and long-term mortality in older adults admitted to acute care wards: Results from the CRIME study. J. Gerontol. 69, 1154–1161. https://doi.org/10.1093/gerona/glu034 (2014).
Voron, T. et al. Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann. Surg. 261, 1173–1183. https://doi.org/10.1097/sla.0000000000000743 (2015).
Cosqueric, G. et al. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br. J. Nutr. 96, 895–901. https://doi.org/10.1017/bjn20061943 (2006).
Chang, K. V., Hsu, T. H., Wu, W. T., Huang, K. C. & Han, D. S. Is sarcopenia associated with depression? A systematic review and meta-analysis of observational studies. Age Ageing. 46, 738–746. https://doi.org/10.1093/ageing/afx094 (2017).
Looijaard, W. G. P. M. et al. Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Crit. Care. 20, 386–386. https://doi.org/10.1186/s13054-016-1563-3 (2016).
Paris, M. & Mourtzakis, M. Assessment of skeletal muscle mass in critically ill patients: Considerations for the utility of computed tomography imaging and ultrasonography. Curr. Opin. Clin. Nutr. Metab. Care. 19, 125–130. https://doi.org/10.1097/mco.0000000000000259 (2016).
Fishman, J. E. & Primack, S. L. Thoracic imaging in the intensive care unit. Appl. Radiol. 34, 8–17 (2005).
Portal, D. et al. L3 skeletal muscle index (L3SMI) is a surrogate marker of sarcopenia and frailty in non-small cell lung cancer patients. Cancer Manag. Res. 11, 2579–2588. https://doi.org/10.2147/CMAR.S195869 (2019).
Fintelmann, F. J. et al. Thoracic skeletal muscle is associated with adverse outcomes after lobectomy for lung cancer. Ann. Thorac. Surg. 105, 1507–1515. https://doi.org/10.1016/j.athoracsur.2018.01.013 (2018).
Lee, G. D. et al. Computed tomography confirms a reduction in diaphragm thickness in mechanically ventilated patients. J. Crit. Care. 33, 47–50. https://doi.org/10.1016/j.jcrc.2016.02.013 (2016).
Go, S. I. et al. Prognostic impact of sarcopenia in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J. Cachexia Sarcopenia Muscle 7, 567–576. https://doi.org/10.1002/jcsm.12115 (2016).
Kondrup, J., Rasmussen, H. H., Hamberg, O. & Stanga, Z. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 22, 321–336. https://doi.org/10.1016/s0261-5614(02)00214-5 (2003).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 40, 373–383. https://doi.org/10.1016/0021-9681(87)90171-8 (1987).
Jones, A. E., Trzeciak, S. & Kline, J. A. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit. Care Med. 37, 1649–1654. https://doi.org/10.1097/CCM.0b013e31819def97 (2009).
MacIntyre, N. R. et al. Management of patients requiring prolonged mechanical ventilation: Report of a NAMDRC consensus conference. Chest 128, 3937–3954. https://doi.org/10.1378/chest.128.6.3937 (2005).
Kizilarslanoglu, M. C., Kuyumcu, M. E., Yesil, Y. & Halil, M. Sarcopenia in critically ill patients. J. Anesth. 30, 884–890. https://doi.org/10.1007/s00540-016-2211-4 (2016).
Derstine, B. A. et al. Quantifying sarcopenia reference values using lumbar and thoracic muscle areas in a healthy population. J. Nutr. Health Aging. 21, 180–185. https://doi.org/10.1007/s12603-017-0983-3 (2017).
Acknowledgements
The authors are grateful to the staff in the intensive care unit and the Radiology Department of Severance Hospital.
Author information
Authors and Affiliations
Contributions
Authors contributed to this work as follows: S.W.M. and K.S.C.—conception, design, analysis and interpretation of data, project administration, drafting the manuscript, revising manuscript, final approval; J.S.C.—acquisition of data, revising manuscript, final approval; S.H.L., M.S.P., & Y.S.K.—formal analysis, revising manuscript, final approval; S.Y.K.—conception, revising manuscript, final approval; A.Y.L.—conception, design, revising manuscript, final approval. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moon, S., Kim, S., Choi, J. et al. Thoracic skeletal muscle quantification using computed tomography and prognosis of elderly ICU patients. Sci Rep 11, 23461 (2021). https://doi.org/10.1038/s41598-021-02853-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-02853-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.