Abstract
Measurement of glomerular filtration rate (GFR) in children by iohexol injection and blood sampling from the contralateral arm is widely used. A single intravenous access for iohexol injection and subsequent blood sampling has the obvious advantages of being less painful and easier to perform. The purpose of our study was to determine if blood samples drawn from the injection access are feasible and accurate for iohexol GFR (iGFR) measurements. Thirty-one children, median age 10.5 (range 6–17) years, with chronic kidney disease were given a bolus of iohexol followed by extended saline flushing and subsequent venous blood samples collected from the injection access as well as from a cannula in the contralateral arm, the latter serving as the reference method. Paired venous blood samples were collected at four time points (2, 3, 3.5 and 4 h) after the iohexol bolus. Blood sample discarding preceded and saline flushing followed each blood sampling to avoid marker contamination. iGFR based on samples drawn from the injection access at 2 and 3 h showed significantly lower iGFR than measurement from the contralateral arm (p < 0.01). Singlepoint iGFR did not differ significantly after 3–4 repeated procedures of blood discarding and saline flusing (3.5 and 4 h). Despite thorough saline flushing there is still a relatively high risk of falsely low iGFR due to marker contamination in blood samples from the injection site. Hence, blood sampling from a second intravenous access is recommended for routine iohexol GFR measurements in children.
Clinical trial registration: ClinicalTrials.gov, Identifier NCT01092260, https://clinicaltrials.gov/ct2/show/NCT01092260?term=tondel&rank=2.
Similar content being viewed by others
Introduction
Renal inulin clearance is considered the gold standard method for measurement of glomerular filtration rate (GFR) in children. This method is, however, complicated, and inulin is not easily available anymore1,2,3. Several exogenous markers have been evaluated, and multipoint pharmacokinetics methods have been adopted as new gold standard procedures for measured GFR (mGFR) using markers like iohexol and 51CrEDTA2,3,4. In clinical practice, serum creatinine based calculation of estimated GFR (eGFR) is the most common method for evaluating renal function as it is simple and has low cost, although clear limitations with low accuracy is well known especially in paediatrics due to the correlation to muscle mass5,6. Hence, accurate mGFR methods based on external markers is of great importance for children with renal diseases and for children treated with nephrotoxic drugs due to malignant diseases where muscle mass can change considerably during the treatment7.
Iohexol plasma clearance was introduced as a method to measure GFR in the 1980s3,8, corresponds well to inulin clearance9,10,11 and is increasingly used in clinical practice16,17. Major advantages by using iohexol bolus injections for GFR measurement include safety, simplicity, tolerance, stability, low cost and low inter-laboratory variations3,12,13,14,15,16,17.
Most laboratories use blood samples drawn from the contralateral arm to measure iohexol plasma concentration. To eliminate any risk of contamination, guidelines recommend that blood samples should not be taken from the site of injection18. However, it is often challenging to gain intravenous access in children. As a consequence, a method allowing injection and blood sampling from a single intravenous access would be highly welcomed and would potentially simplify and facilitate the monitoring of iohexol GFR in children.
Brøchner-Mortensen stated, based on their own unpublished data, no significant contamination of plasma samples drawn from the injection cannula when 15 mL isotonic saline was injected following the administration of the marker 51CrEDTA in one of the taps of the cannula and 10 mL saline through the other tap19. Stake et al. used this procedure to establish a new single-plasma sample method in children based on injecting iohexol and drawing blood samples from the same cannula20,21. Brändström et al. stated also based on unpublished data no difference in GFR values when comparing blood samples drawn from the injection arm to samples from the contralateral arm in 14 adult patients after post-injection flushing of the intravenous access with 30 mL physiologic saline22. To our knowledge, there are no published studies comparing the agreement of iohexol plasma concentration sampled from the injection cannula to iohexol plasma concentration in samples from a contralateral venous access.
The purpose of this study was, given extensive post-injection flushing and pre-sampling blood discarding procedures, to determine if blood samples drawn from the same intravenous access as injection of iohexol are feasible and accurate for measurement of the iohexol plasma concentration, compared to samples from the contralateral arm. To validate the agreement between the venous blood samples drawn from both arms, we compared four different time points for each of the methods i.e. pharmacokinetic (PK) sampling after saline flushing and blood discarding one, two, three or four times.
Results
Iohexol concentrations for each individual patient at various time points are illustrated in Fig. 1. The discrepancy between the paired measurements is in general larger at 2 h than at the other three time points. Still, some variation is observed also in the samples at 3.5 and 4 h.
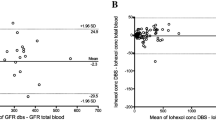
Agreement between calculated iohexol-GFR (iGFR) from paired samples drawn at 2, 3, 3.5 and 4 h using Bland–Altman plots is illustrated in Fig. 2. All the one-sample iGFR were based on the 1-point Fleming formula due to previous shown best performance23,24. The results show smaller bias, less variation and fewer outliers in the samples drawn at later time points, suggesting more consistent iGFR for all patients. Calculations of iGFR from the paired samples drawn 2 h after injection showed clear lack of agreement, with unreliable measurements (lower mGFR) from the injection site compared to the reference method. Patient nine is a clear outlier at the 2-h time point, with 43% lower iGFR estimate for the measurement from the ipsilateral arm (60 mL/min/1.73 m2) than from the contralateral arm (104 mL/min/1.73 m2). For the other time points, the iGFR estimate ranged from 99 to 103 mL/min/1.73 m2 for both arms.
Figure 3 shows the corresponding differences when using two or four time points and the JBM formula26,27. Measuring GFR using multiple time points gives a more reliable estimate than using one time point, but there is still a notable bias, mainly caused by differences in measured concentration at 2 h.
The performances at four different time points are shown in Table 1. The analysis shows best agreement between the two single-point procedures is found at the 3.5-h time point with the least amount of bias, the tightest limits of agreement and the highest p-value (0.24). For measurements at 2 and 3 h, the results with the Fleming formula are statistically significantly different between the samples drawn from the injection cannula and the reference method, suggesting unreliable results due to marker contamination.
Comparisons between the two intravenous accesses for two time points and four time points using the JBM formula are also shown in the same table. There were little overall differences in agreement between two and four time points, but both procedures had poor agreement, with generally lower iGFR estimates from the ipsilateral arm, likely due to both procedures including the 2-h time point.
Discussion
Poor agreement was found between paired samples from injection site and contralateral arm collected at time point 2 h after injection, with an unacceptable discrepancy in the calculated iohexol-GFR values (Table 1). Likewise, the samples drawn at time point 3 h after injection shows a suboptimal agreement between the test method and reference method, with a risk of underestimating the iGFR. However, the GFR 1-point measurements using the Fleming formula shows a smaller discrepancy between paired iGFR by samples drawn from the cannulae at 3.5 and 4 h after injection. At these time points, the results indicate more agreement between the two sampling methods, with lower bias and better limits of agreement (Table 1, Fig. 2). The reason for better agreement after multiple samplings is likely the increased rinsing of the cannula through movement of the rubber inside the cannula due to repeated procedures with blood discarding and saline injection. This is in agreement with results observed in the study showing less discrepancy between the paired samples collected at 3.5 and 4 h after injection of iohexol. The persistent minor difference is likely due to analytical variation as previous studies show an intra-individual coefficient of variation of 5.6%28 and 5.4%29 using iohexol clearance.
Due to need of one relatively early sample using the slope methodology4,25,26,27, JBM calculations with both two sampling points and four sampling points resulted in unreliable iGFR-values from the ipsilateral cannula (Table 1, Fig. 3). Both calculation methods included the samples drawn 2 h after injection, which is shown to be unreliable and inaccurate (Fig. 1). Yet, the risk of outliers is an issue in every test. If using a single-point procedure it would therefore be necessary to redo the test if the result seems unreliable, whereas a multi-point GFR procedure would normally be more accurate and have the possibility to remove an outlier reviewing the examination of the elimination curve. However, using blood sampling from the ipsilateral cannula, the risk of contamination of the sample with remnants of marker is the highest at the first samples, i.e. on a time point where it is not possible on the eliminations curve to see that it is an outlier (Fig. 1). In a real-world clinical caring perspective the benefit of single access sometimes may outweigh the methodological inaccuracy. However, if the clinician deem the highest accuracy of GFR of critical importance in specific cases, we recommend a standard GFR method sampling from a contralateral intravenous access.
Endogenous substances, such as creatinine, are widely used to estimate kidney function in clinical practice. However, formulas used to estimate GFR in children often show low accuracy, with studies reporting less than 50% of eGFR based on serum creatinine, cystatin C and/or urea within ± 10% of the reference method5,6. Feasible methods to measure GFR using an infused marker with purely renal clearance is therefore of great importance. In our study iohexol clearance has been used for GFR determination and shows good agreement for single access procedure at the time points at 3.5 and 4 h, provided repeated saline flushing and blood discarding at earlier time points.
Previous publications on both children and adults have stated no contamination by using the same cannula for marker injection and blood sampling when flushing the taps with similar amounts of physiologic saline as in our study19,20,21. To our knowledge, this statement has not been confirmed using systematic statistical analysis in any publication. Stake et al. used this method to establish a single plasma sample method with iohexol to estimate GFR20,21. In their study, concern about the possible marker contamination of the blood sample using such method was raised21. Despite their concern, they still used this method to measure the renal function in the patients. They showed that GFR estimates based on the first sample drawn 1 h after iohexol injection were clearly inaccurate compared to samples collected at later time points, which is consistent with the results for the first sampling point found in our study. The findings from Stake et al. may be explained due to sampling outside the time limits of the formula used for calculation, but it is likely that contamination by the marker itself also may have influenced results20,21.
Small remnants of iohexol particles in the cannula used for injection is a likely explanation for the iGFR discrepancies found in our study, even after flushing considerable amounts of physiologic saline through the taps of the cannula. For the third and fourth sampling time points, i.e. at 3.5 and 4 h, however, where blood sampling procedures including blood discarding and saline flushing had already been repeated several times, there was no significant difference between the paired samples (Table 1).
A limitation of our study are the small amounts of blood drawn for discarding, however 0.5 mL equals multiple fillings of the cannula and should therefore secure elimination of administered saline and heparin. The low blood volume both for discarding as well as for PK-sampling, totally 1 mL, were used to minimize the blood loss for the patients and was sufficient for several HPLC analysis (Table 2). Using a radioactive substance as GFR-marker would potentially reduce the probability of marker remnants30. However, the fact that iohexol is a non-radioactive substance is important due to safety by repeated use in CKD children.
The findings in our study have significant clinical value. The potential benefit of using a minimal intravenous access procedure and fewer blood samples paying attention to sufficient saline flushing are of great value in patient groups consisting of children who have difficult venous access and are subject to regular testing and treatment. In such cases a single intravenous access method may be an alternative. However, our study highlights the importance of awareness of the risk of falsely low iGFR, especially in high risk patients in need of GFR-dependent dosing of drugs as well in studies of GFR-methodology19,20,21,22.
In summary, the most reliable method to measure iohexol concentration and iGFR requires two venous accesses, with blood sampling from the contralateral arm. With a single intravenous access method when blood is collected from the same cannula as the iohexol injection there is still a risk of marker contamination and underestimation of GFR, despite thorough saline flushing, In cases where there is difficulty in establishing two intravenous accesses, blood samples from the injection site may be an clinical acceptable alternative provided awareness of the limitations of this method.
Methods
Patients
Thirty-one children with chronic kidney disease (CKD) at Haukeland University Hospital were included in the study and had complete data sets for analysis. One additional patient was initially included, but due to problems with intravenous access, this patient was excluded from the data analyses. The median age of the 31 children was 10.5 years (range 6–17 years), with median height 142 cm (range 114–177 cm) and median weight was 41 kg (19–85 kg). Written informed consent by parents/legal guardians was obtained from for all included children as well as from the included patients > 16 years of age. The study was approved by the Western Norway Regional Committee for Medical and Health Research Ethics (REK2009/741) and was in accordance with the Helsinki Declaration. The study was registered at ClinicalTrials.gov with the identifier NCT01092260, and other sub-studies on intravenous GFR methodology from the study have been published elsewhere24,25.
GFR measurements
The GFR procedure was performed in three steps (Table 2). Following the first blood sample extraction, iohexol (Omnipaque 300 mg iodine/mL (GE Healthcare)) was injected. The doses of Omnipaque were calibrated according to the patient’s body weight: 10–20 kg: 2 mL; 20–30 kg: 3 mL; 30–40 kg: 4 mL; ≥ 40 kg: 5 mL. The syringe used in the injection was weighed before and after injection to calculate exact amount of Omnipaque injected24. After administration of iohexol, the intravenous access was flushed with a total of 30 mL saline (Table 2).
Timed blood samples of 0.5 mL were drawn from the injection access and a second cannula in the contralateral arm. Parallel samples were drawn 2, 3, 3.5 and 4 h after the injection of iohexol. Blood discarding (0.5 mL) preceded and saline flushing (1 mL) followed each blood sampling. After the saline injection 0.2 mL Heparin 100 IE/mL was administered in the veneflon.
The blood was centrifuged at 1000–1300 G for 10 min and then stored at −20 °C until analysis. Analysis of the iohexol concentration was performed by the Laboratory of Clinical Biochemistry at Haukeland University Hospital. A high performance liquid chromatography (HPLC) was used to determine the iohexol plasma concentration in the samples. The area under the largest iohexol peak was used to calculate the concentration of iohexol by comparing it to an internal calibration curve. Using this method, the coefficient of variation was 4.1% at 10 mg/L, 3.8% at 25–290 mg/L and 3.3% at > 290 mg/L.
The GFR values were calculated using Fleming’s single-point formula23,24 for all four time points and Jødal–Brøchner-Mortensen’s (JBM) multipoint formula25,26,27 for both two and four time points. This was done separately for the ipsilateral and contralateral cannulae.
Statistical analysis
Agreement between GFR values from the ipsilateral and contralateral cannulae was evaluated using Bland–Altman plots31 (Figs. 2 and 3, Table 1). We report the bias—i.e., the mean difference in GFR based on data from the ipsilateral and contralateral arms—the 95% confidence intervals for the bias and the limits of agreement. Systematic differences in GFR values between the two arms/cannulae were tested using the Wilcoxon signed rank test, which tests for symmetry around zero. R version 4.0.4 (https://www.R-project.org/) was used for all statistical analyses and figure preparation.
References
Arant, B. S. Jr., Edelmann, C. M. Jr. & Spitzer, A. The congruence of creatinine and inulin clearances in children: Use of the Technicon AutoAnalyzer. J. Pediatr. 81(3), 559–561. https://doi.org/10.1016/s0022-3476(72)80191-4 (1972).
Schwartz, G. J. & Work, D. F. Measurement and estimation of GFR in children and adolescents. Clin. J. Am. Soc. Nephrol. 4(11), 1832–1843. https://doi.org/10.2215/CJN.01640309 (2009).
Delanaye, P. et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: A review. Part 2: Why to measure glomerular filtration rate with iohexol?. Clin. Kidney J. 9(5), 700–704. https://doi.org/10.1093/ckj/sfw071 (2016).
Delanaye, P. et al. Iohexol plasma clearance for measuring glomerular filtration rate in clinical practice and research: A review. Part 1: How to measure glomerular filtration rate with iohexol?. Clin. Kidney J. 9(5), 682–699. https://doi.org/10.1093/ckj/sfw070 (2016).
Schwartz, G. J. et al. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 20(3), 629–637. https://doi.org/10.1681/ASN.2008030287 (2009).
Salvador, C. L. et al. Estimating glomerular filtration rate in children: Evaluation of creatinine- and Cystatin C based equations. Pediatr. Nephrol. 34(2), 301–311. https://doi.org/10.1007/s00467-018-4067-3 (2019).
Smets, E. M., Garssen, B., Schuster-Uitterhoeve, A. L. & de Haes, J. C. Fatigue in cancer patients. Br. J. Cancer 68(2), 220–224. https://doi.org/10.1038/bjc.1993.319 (1993).
Krutzén, E., Bäck, S. E., Nilsson-Ehle, I. & Nilsson-Ehle, P. Plasma clearance of a new contrast agent, iohexol: A method for the assessment of glomerular filtration rate. J. Lab Clin. Med. 104(6), 955–961 (1984).
Brown, S. C. & O’Reilly, P. H. Iohexol clearance for the determination of glomerular filtration rate in clinical practice: Evidence for a new gold standard. J. Urol. 146(3), 675–679. https://doi.org/10.1111/j.1464-410x.1995.tb07340.x (1991).
Lindblad, H. G. & Berg, U. B. Comparative evaluation of iohexol and inulin clearance for glomerular filtration rate determinations. Acta Paediatr. 83(4), 418–422. https://doi.org/10.1111/j.1651-2227.1994.tb18133.x (1994).
Gaspari, F. et al. Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J. Am. Soc. Nephrol. 6(2), 257–263. https://doi.org/10.1681/ASN.V62257 (1995).
Tøndel, C., Ramaswami, U., Aakre, K. M., Wijburg, F., Bouwman, M., & Svarstad, E. Monitoring renal function in children with Fabry disease: comparisons of measured and creatinine-based estimated glomerular filtration rate. Nephrol Dial Transplant. 25(5), 1507–1513. https://doi.org/10.1093/ndt/gfp658 (2010).
Stake, G. & Smevik, B. Iohexol and metrizamide for urography in infants and children. Invest. Radiol. 20(1), 115–116. https://doi.org/10.1097/00004424-198501002-00027 (1985).
Hill, J. A. et al. Multicenter trial of ionic versus nonionic contrast media for cardiac angiography. Am. J. Card. 72(11), 770–775. https://doi.org/10.1016/0002-9149(93)91061-l (1993).
Nilsson-Ehle, P. & Grubb, A. New markers for the determination of GFR: Iohexol clearance and cystatin C serum concentration. Kidney Int. Suppl. 47(11), S17-19 (1994).
Rudnick, M. R. et al. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: A randomized trial. Kidney Int 47(1), 254–261. https://doi.org/10.1038/ki.1995.32 (2021).
Schwartz, G. J., Furth, S., Cole, S. R., Warady, B. & Muñoz, A. Glomerular filtration rate via plasma iohexol disappearance: Pilot study for chronic kidney disease in children. Kidney Int. 69(11), 2070–2077. https://doi.org/10.1038/sj.ki.5000385 (2006).
Fleming, J. S., Zivanovic, M. A., Blake, G. M., Burniston, M. & Cosgriff, P. S. British Nuclear Medicine Society: Guidelines for the measurement of glomerular filtration rate using plasma sampling. Nucl. Med. Commun. 25(8), 759–769. https://doi.org/10.1097/01.mnm.0000136715.71820.4a (2004).
Bröchner-Mortensen, J., Haahr, J. & Christoffersen, J. A simple method for accurate assessment of the glomerular filtration rate in children. Scand. J. Clin. Lab. Invest. 33(2), 140–143 (1974).
Stake, G. & Monclair, T. A single plasma sample method for estimation of the glomerular filtration rate in infants and children using iohexol, I: Establishment of a body weight-related formula for the distribution volume of iohexol. Scand. J. Clin. Lab. Invest. 51(4), 335–342. https://doi.org/10.1080/00365519109091624 (1991).
Stake, G., Monn, E., Rootwelt, K. & Monclair, T. A single plasma sample method for estimation of the glomerular filtration rate in infants and children using iohexol, II: Establishment of the optimal plasma sampling time and a comparison with the 99Tcm-DTPA method. Scand. J. Clin. Lab. Invest. 54(1), 343–348. https://doi.org/10.1080/00365519109091625 (1991).
Brändström, E. et al. GFR measurement with iohexol and 51Cr-EDTA. A comparison of the two favoured GFR markers in Europe. Nephrol. Dial. Transplant. 13(5), 1176–1182. https://doi.org/10.1093/ndt/13.5.1176 (1998).
Tøndel, C. et al. Iohexol plasma clearance in children: Validation of multiple formulas and single-point sampling times. Pediatr. Nephrol. 33(4), 683–696. https://doi.org/10.1007/s00467-017-3841-y (2018).
Fleming, J. S., Persaud, L. & Zivanovic, M. A. A general equation for estimating glomerular filtration rate from a single plasma sample. Nucl. Med. Commun. 26(8), 743–748. https://doi.org/10.1097/01.mnm.0000171783.18650.80 (2005).
Tøndel, C. et al. Iohexol plasma clearance in children: Validation of multiple formulas and two-point sampling times. Pediatr. Nephrol. 32(2), 311–320. https://doi.org/10.1007/s00467-016-3436-z (2017).
Jødal, L. & Brøchner-Mortensen, J. Reassessment of a classical single injection 51Cr-EDTA clearance method for determination of renal function in children and adults. Part I: Analytically correct relationship between total and one-pool clearance. Scand. J. Clin. Lab Invest. 69(3), 305–313. https://doi.org/10.1080/00365510802566882 (2009).
Brøchner-Mortensen, J. & Jødal, L. Reassessment of a classical single injection 51Cr-EDTA clearance method for determination of renal function in children and adults. Part II: Empirically determined relationships between total and one-pool clearance. Scand. J. Clin. Lab. Invest. 69(3), 314–322. https://doi.org/10.1080/00365510802653680 (2009).
Gaspari, F. et al. Precision of plasma clearance of iohexol for estimation of GFR in patients. J. Am. Soc. Nephrol. 9(2), 310–313. https://doi.org/10.1681/ASN.V92310 (1998).
James, T. J. et al. Validity of simplified protocols to estimate glomerular filtration rate using iohexol clearance. Ann. Clin. Biochem. 44(4), 369–376. https://doi.org/10.1258/000456307780945804 (2007).
Mekonnen, B. W., Warwick, J. M. & Holness, J. L. Validation of glomerulat filtration rate measurement with blood sampling from the injection site. J. Nucl. Med. Technol. 47(4), 305–308 (2019).
Bland, J. M. & Altman, D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476), 307–310 (1986).
Acknowledgements
We are very grateful to the pediatric study nurses Hildur Grindheim and Renathe Håpoldøy at the Pediatric Clincial Trial Unit, Haukeland University Hospital for their high-quality assistance in collecting the data, and to laboratory engineer Kjersti Carstensen for the iohexol analyses. The study was supported by grants from the Health Trust of Western Norway and Haukeland University Hospital.
Funding
The study was supported by grants from the Health Trust of Western Norway, The Norwegian Society of Nephrology and Haukeland University Hospital.
Author information
Authors and Affiliations
Contributions
All co-authors have provided intellectual content of critical importance to the work presented and approve this manuscript. T.T.E. analyzed data and drafted the article together with C.T, K.O.H. analyzed data, made figures and revised the manuscript, A.B., D.B., E.S. and C.T. designed the study, collected data and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eide, T.T., Hufthammer, K.O., Brun, A. et al. Accuracy of single intravenous access iohexol GFR in children is hampered by marker contamination. Sci Rep 11, 23224 (2021). https://doi.org/10.1038/s41598-021-02759-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-02759-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.