Abstract
Temporal and spatial resolution of dynamic contrast-enhanced MR imaging (DCE-MRI) is critical to reproducibility, and the reproducibility of high-resolution (HR) DCE-MRI was evaluated. Thirty consecutive patients suspected to have brain tumors were prospectively enrolled with written informed consent. All patients underwent both HR-DCE (voxel size, 1.1 × 1.1 × 1.1 mm3; scan interval, 1.6 s) and conventional DCE (C-DCE; voxel size, 1.25 × 1.25 × 3.0 mm3; scan interval, 4.0 s) MRI. Regions of interests (ROIs) for enhancing lesions were segmented twice in each patient with glioblastoma (n = 7) to calculate DCE parameters (Ktrans, Vp, and Ve). Intraclass correlation coefficients (ICCs) of DCE parameters were obtained. In patients with gliomas (n = 25), arterial input functions (AIFs) and DCE parameters derived from T2 hyperintense lesions were obtained, and DCE parameters were compared according to WHO grades. ICCs of HR-DCE parameters were good to excellent (0.84–0.95), and ICCs of C-DCE parameters were moderate to excellent (0.66–0.96). Maximal signal intensity and wash-in slope of AIFs from HR-DCE MRI were significantly greater than those from C-DCE MRI (31.85 vs. 7.09 and 2.14 vs. 0.63; p < 0.001). Both 95th percentile Ktrans and Ve from HR-DCE and C-DCE MRI could differentiate grade 4 from grade 2 and 3 gliomas (p < 0.05). In conclusion, HR-DCE parameters generally showed better reproducibility than C-DCE parameters, and HR-DCE MRI provided better quality of AIFs.
Similar content being viewed by others
Introduction
Dynamic contrast-enhanced (DCE) magnetic resonance (MR) imaging is a well-validated MR imaging technique due to its usefulness in neuroimaging research. For example, several studies have suggested that DCE MR imaging-derived parameters (DCE parameters) have potential to differentiate the grades of gliomas1,2,3,4,5,6 and predict genetic mutation status7,8,9 or pseudoprogression after standard treatments10,11. In addition, DCE MR imaging has a potential role in other brain diseases, such as acute ischemic stroke12, multiple sclerosis13, dementia14, and traumatic brain injury15.
However, there are some limitations to apply DCE parameters directly to real clinical practice because of their low reproducibility16, primarily arising from the reproducibility of arterial input function (AIF) used in DCE MR imaging analysis17,18,19,20. Previously, You et al21 reported that the accuracy and reproducibility of DCE MR imaging parameters could be improved by using AIFs derived from dynamic susceptibility contrast (DSC) MR imaging. This improvement might be achieved by obtaining AIF with accelerated scan intervals, but gadolinium-based contrast agents (GBCAs) need to be injected twice to use DSC MR imaging-based AIF on DCE MR imaging analysis21.
Recently, Park et al22 introduced high resolution DCE (HR-DCE) MR imaging, in which z-axis resolution and temporal resolution were markedly improved compared to conventional DCE (C-DCE) MR imaging. This refinement in resolution of DCE MR imaging might improve the reproducibility of this imaging technique.
To the best of our knowledge, no previous study has evaluated HR-DCE MR imaging in glioma patients. Thus, this study aimed to evaluate the reproducibility and validate the usage of HR-DCE MR imaging in patients with gliomas compared with C-DCE MR imaging.
Results
Table 1 shows demographics and pathologic results of study population. Examples of histograms and parametric maps of Ktrans and Ve are displayed in Supplementary Fig. 1.
Primary study
DCE parameters using population-based AIF showed excellent intraclass correlation coefficients (ICCs) of both C-DCE and HR-DCE MR imaging (range, 0.96–0.99) (Supplementary Fig. 2). When individual AIFs were used, ICCs of HR-DCE parameters showed good to excellent agreement (range, 0.84–0.95), while C-DCE parameters showed moderate to excellent agreement (range, 0.66–0.96) (Table 2).
Correlations between C-DCE and HR-DCE parameters were not statistically significant (p > 0.05) in patients with glioblastoma (GBM). Both C-DCE and HR-DCE parameters were not statistically associated with pathologic results, such as Ki-67 values and O6-methylguanine-DNA-methyltransferase (MGMT) promoter methylation status (p > 0.05), in patients with GBM (Supplementary Table 1).
AIF analyses
The 95th percentile of bolus arrival time (BAT) in C-DCE MR imaging was 20 s and that of wash-in time was 16 s. Thus, delayed bolus arrival error was defined when BAT was longer than 40 s (n = 1). In addition, delayed washout error was defined when time from maximal signal intensity (MSI) to half of MSI in wash-out slope (Twash-out) was longer than 32 s (n = 7).
The MSI and wash-in slope (WIS) of AIFs from HR-DCE MR imaging were significantly greater than those of AIFs from C-DCE MR imaging (median, 31.85 vs. 7.09 and 2.14 vs. 0.63, respectively; p < 0.001) (Table 3), even when suboptimal AIFs were included. After exclusion of suboptimal AIFs, HR-DCE MR imaging still demonstrated greater MSI and steeper WIS of AIFs than C-DCE MR imaging (44.60 vs. 5.87 and 3.01 vs. 0.57, respectively; p < 0.001) (Table 3). All AIFs from 25 patients are plotted in Fig. 1.
AIFs from C-DCE and HR-DCE MR imaging: (a) Individual AIFs of C-DCE MR imaging, (b) virtual AIFs derived from C-DCE MR imaging using median, Q1 and Q3 values, (c) individual and (d) virtual AIFs derived from HR-DCE MR imaging, (e) individual and (f) virtual AIFs derived from HR-DCE MR imaging after exclusion of suboptimal cases.
Extended study
ICCs of C-DCE and HR-DCE MR imaging were described in Supplementary Table 2. The 95th percentile Ktrans and Ve from C-DCE imaging were correlated with those from HR-DCE MR imaging in patients with gliomas (Supplementary Fig. 3). Both 95th percentile Ktrans and 95th percentile Ve derived from HR-DCE and C-DCE MR imaging could differentiate grade 4 gliomas from grade 2 and 3 gliomas (p < 0.05) (Fig. 2). Areas under the receiver operating characteristic curves (AUROC) were not significantly different (95th percentile Ktrans, C-DCE = 0.902 vs. HR-DCE = 0.964; p = 0.539; 95th percentile Ve, C-DCE = 0.964 vs. HR-DCE = 0.982; p = 0.738) (Fig. 2).
DCE MR parameters as differentiators of WHO tumor grades. (a) 95th percentile Ktrans and (b) 95th percentile Ve according to tumor grades (grade 4 vs. grade 2 and 3) using C-DCE MR imaging, (c) 95th percentile Ktrans and (d) 95th percentile Ve according to tumor grades (grade 4 vs. grade 2 and 3) using HR-DCE MR imaging, (e) ROC curves derived from Ktrans and (f) ROC curves derived from Ve for differentiators of tumor grades.
Discussion
In this prospective study, we evaluated the reproducibility and clinical applicability of HR-DCE MR imaging in glioma patients, which demonstrated superior spatial (especially z-axis) and temporal resolutions compared to C-DCE MR imaging22. As many previous studies mentioned, acquisition of reliable AIF is a key factor in DCE MR imaging16,17,18,19,20,21, and improvement in the temporal resolution of DCE MR imaging21 could be a possible strategy to overcome the low reproducibility of this technique.
Several previous studies highlighted the importance of DCE MR imaging with higher temporal resolution in the prostate or breast23,24,25. Indeed, accelerated temporal resolution of DCE MR imaging in our study demonstrated the improved reproducibility of DCE parameters and superior ability to detect MSI of AIF in patients with gliomas. The effect of higher spatial resolution on the reproducibility of DCE parameters seems to be less significant.
Unexpectedly, HR-DCE MR imaging-specific errors (delayed wash-out error, delayed bolus arrival error) were noted. Even after thorough review and discussion with developers of HR-DCE MR imaging, we could not determine the exact cause of those errors. We believe there might be some setting errors during MR imaging that were not identified retrospectively. Nonetheless, HR-DCE MR imaging demonstrated the superior ability to detect greater MSI and steeper WIS of AIF. According to the study by You et al21, DSC MR imaging-based AIF showed improvement in the reproducibility of DCE MR imaging. Although this improvement was achieved by demonstrating higher signal intensity changes caused by the T2* effect21, it is also plausible that accelerated scan interval (1.6 s vs. 4.0 s) enables us to catch the MSI of AIF more precisely.
Perfusion MRI has a role in treatment response evaluation in patients who underwent antiangiogenic agents (i.e., bevacizumab)26,27,28, but DCE parameters are not routinely used in real clinical practice because of their low reproducibility. Because the reproducibility of imaging parameters is a critical issue in clinical practice and trials17,29,30, our results of improved reproducibility of HR-DCE MR imaging could increase the possibility of implementing this technique in real clinical practice.
Interestingly, it seemed that histogram of DCE parameters could be different when temporal and spatial resolutions had been changed. Differences in z-axis resolution as well as AIF might affect the shape of histogram. Further evaluation investigating the effect of spatial and temporal resolutions on histograms is needed because histogram analysis has added prognostic value in patients with GBM31.
According to the results for spatial resolution, ICCs were excellent in both C-DCE and HR-DCE MR imaging, even though the z-axis resolution was significantly different. This might suggest that increased spatial resolution had a less significant effect on the reproducibility of DCE parameters. Indeed, HR-DCE MR images comprise 33,984 DICOM files in each patient, and heavy data size of HR-DCE MR imaging requires excessive analysis time. Although high spatial resolution of DCE MR imaging has an advantage in superior image quality32, clinical usefulness of DCE MR imaging might be enhanced with lower spatial resolution, given our results showing a less significant effect of spatial resolution on the reproducibility of DCE parameters.
In terms of clinical usefulness, the 95th percentile Ktrans and Ve were significantly higher in grade 4 gliomas than in grade 2 and 3 gliomas. However, the superiority of HR-DCE MR imaging for tumor grading was not proven because AUCs were not different in C-DCE and HR-DCE MR imaging. These findings might be related to the small size of the study population.
There are some limitations to our study. First, our study population was rather small because of several suboptimal cases, and this might be why our study failed to prove correlation between HR-DCE parameters and pathologic results in patients with GBM. Second, we could not identify the exact cause resulting in suboptimal AIFs. This finding might urge validation of HR-DCE MR imaging in different study populations. Third, our study did not evaluate whether HR-DCE parameters could predict survival better or differentiate pseudoprogression from true progression after treatment more precisely. Thus, our study has little clinical impact, but we focused on the clinical applicability of HR-DCE MR imaging in terms of reproducibility. Further studies pursuing the clinical importance of HR-DCE MR imaging in a larger population is needed. Finally, it is difficult to set a gold standard in DCE MR imaging studies. Thus, we chose C-DCE parameters as reference standards, and HR-DCE and C-DCE parameters did not always correlate with each other. Thus, DCE parameters should be interpreted with clinical context, which often failed to be demonstrated in our study, possibly due to the small study population. This also suggests the importance of clinical validation of HR-DCE MR imaging in a larger study population.
In conclusion, HR-DCE parameters showed better reproducibility than C-DCE parameters, and AIF derived from HR-DCE MR imaging exhibited higher MSI and steeper WIS in prospectively enrolled patients with gliomas. Further studies investigating clinical importance using HR-DCE MR imaging should be conducted with a larger study population.
Materials and Methods
This prospective study was approved by the institutional review board of Seoul National University Hospital, and written informed consent was obtained from all patients, and all experiments were performed in accordance with relevant guidelines and regulations.
Patient selection
From October 2018 to March 2019, 30 consecutive patients with presumed primary brain tumors who underwent diagnostic MR imaging were included in our study, and the details of diagnostic MR imaging protocol were previously described by You et al21. During diagnostic MR imaging, C-DCE MR images were routinely obtained. In these patients with informed consent, HR-DCE MR images were additionally obtained during navigation MR imaging for surgery. Because HR-DCE MR imaging was performed with GBCAs that used for navigation MR imaging, additional administration of GBCAs did not need. Median of time interval between diagnostic and navigation MR imaging was 2 days (interquartile range, 1–3 days).
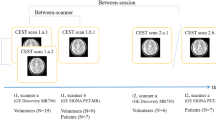
The study design was abbreviated on Fig. 3 and Supplementary Fig. 4. The final pathological diagnosis was confirmed based on the WHO 2016 classification. For the primary study, we included patients whose final pathologic diagnosis was GBM (n = 14) and one patient was finally excluded whose HR-DCE MR imaging could not be analyzed. Thus, HR-DCE MR imaging data from 13 patients were included in the primary study. Among them, 6 patients were additionally excluded from the primary analysis, because of HR-DCE specific suboptimal AIFs (Supplementary Fig. 5). Other pathologies in 16 patients were as follows: gliosarcomas = 2, anaplastic astrocytomas = 5, diffuse astrocytomas = 2, diffuse midline glioma = 1, oligodendrogliomas = 3, metastasis = 1, lymphocytic infiltration = 1, and lymphoma = 1. For an extended study, we also included patients with other types of gliomas.
Study flow diagram. This study consists of (1) AIF analysis using individual AIFs, (2) analysis of the effect on in-plane (xy-plane) spatial resolution using population-based AIF, and the effect on the z-axis and temporal resolution using individual AIFs in patients with GBM, and (3) correlation between DCE parameters and clinical parameters using individual AIFs and VOIs for T2 hypersignal intense lesions.
MR imaging parameters
Glioma study MR imaging studies were performed by using 3.0 T MR imaging units (Verio or Skyra, Siemens Healthineers, Erlangen, Germany; Ingenia, Philips Heathcare, Best, Netherlands) with a 32-channel head coil, and HR-DCE MR imaging was performed by using either Verio or Skyra (Siemens Healthineers, Erlangen, Germany).
Glioma study MR imaging protocols included pre- and postcontrast three dimensional magnetization-prepared rapid acquisition with gradient echo (MPRAGE) sequences (Repetition time[TR]/Echo time[TE] = 1670/2.8 ms, Flip angle[FA] = 9°, Matrix = 256 × 232, Field of view[FOV] = 226 × 250, Section thickness = 1.0 mm, Number of excitation[NEX] = 1.0 for Siemens MR imaging machines; TR/TE = 8.1–8.2/3.7 ms, FA = 8°, Matrix = 240 × 240, FOV = 240 × 240, Section thickness = 1.0 mm, NEX = 1.0 for Philips MR imaging machine), transverse T2-weighted image (T2WI) with turbo spin echo (TSE) sequence (TR/TE = 2000–4630/80–299 ms, FA = 90–150°, Matrix = 252 × 252 or 512 × 464 or 640 × 297, FOV = 185 × 220 to 250 × 250, Section thickness = 4.0–5.0 mm, NEX = 1.0–2.0). Navigation MR imaging protocols consisted of postcontrast axial T1-weighted image (T1WI) with gradient echo (TR/TE = 1600/2.3 ms, FA = 9°, Matrix = 256 × 256, FOV = 240 × 240, Section thickness = 2.0 mm, NEX = 1.0), axial T2WI with TSE (TR/TE = 5700–6160/93.0 ms, FA = 130°, Matrix = 256 × 256, FOV = 240 × 240, Section thickness = 2.0 mm, NEX = 1.0), and HR-DCE MR imaging.
DCE MR imaging was performed by intravenous administration of gadobutrol (Gadovist, Bayer Schering Pharma, Berlin, Germany) at a dose of 0.1 mmol/kg of body weight. The specific imaging parameters for C-DCE MR imaging were as follows: TR/TE = 2.8/1.0 ms, FA = 10°, Matrix = 192 × 192, FOV = 240 × 240, Section thickness = 3.0, Voxel size = 1.25 × 1.25 × 3 mm3, NEX = 1.0, Scan interval = 4.0 s, Total images = 40 × 60 phases for Verio and Skyra scanners; and TR/TE = 4.2/2.1 ms, FA = 10°, Matrix = 192 × 192, FOV = 240 × 240, Section thickness = 3.0, Voxel size = 1.25 × 1.25 × 3.0 mm3, NEX = 1.0, Scan interval = 4.0 s, Total images = 40 × 60 phases for Ingenia Scanner. The specific imaging parameters for HR-DCE MR imaging were as follows: TR/TE = 3.2/1.4 ms, FA = 15°, matrix = 192 × 144, FOV = 229 × 172, section thickness = 1.1, voxel size = 1.1 × 1.1 × 1.1 mm3, NEX = 1.0, scan interval = 1.6 s, and total images = 192 × 177 phases.
Image analysis for primary study
All image analyses were performed using commercially available software, Nordic ICE (NordicNeuroLab, Bergen, Norway), by a single radiologist (5 years of experience in neuroradiology) under the supervision of an expert neuroradiologist (18 years of experience in neuroradiology).
For C-DCE MR image analyses, parametric maps of Ktrans, Vp, and Ve were generated based on extended Tofts model33, using C-DCE MR image digital imaging and communications in medicine (DICOM) files. AIF search box was drawn at the level of the middle cerebral artery transverse segment34, and AIF was generated as a mean of five different AIFs from 5 automatically selected pixels. The second AIF was generated by using an AIF search box to draw subsequent axial images in the same manner to evaluate the reproducibility of AIF. After coregistration between DCE parametric maps and postcontrast T1WI images21,34, regions of interest (ROI) were drawn to cover contrast-enhancing portion and avoid cystic portions or vascular structures for each transverse image. Using these ROIs with enhancing foci, volumetric information about DCE parameters was calculated on a pixel-by-pixel basis. HR-DCE MR image analyses were performed in the same manner using postcontrast T1WI from navigation MR imaging and HR-DCE MR image DICOM files.
After a month, AIF selections in two consecutive image planes were repeated to evaluate intraobserver reproducibility. Previously designated ROIs for enhancing lesions were reused to exclude the effect of ROI selection and assess interobserver reproducibility primarily arising from z-axis and temporal resolutions. DCE parametric maps and AIFs obtained from these measurements were used to evaluate the intraobserver reproducibility and AIF analysis.
In addition, to evaluate the reproducibility originating from spatial resolution, DCE parametric maps using population-based AIF were generated to reduce the effect of AIF selection, and a second ROI for measurable enhancing foci was drawn for each patient. Using two individual ROIs and DCE parametric maps derived from population-based AIF, intraobserver reproducibility related to in-plane resolution was evaluated.
AIF analysis
As in a previous study21, five parameters from AIF were evaluated: (a) BAT, (b) time to peak (TTP), (c) baseline signal intensity (BSI), (d) MSI, and (e) WIS. AIF analyses were performed both with and without suboptimal AIFs. Virtual AIFs from the median value were plotted with 25th (Q1) and 75th percentile (Q3) values21.
In cases in which AIFs were significantly different from others, AIFs were only used for AIF analysis, while DCE parameters derived from those AIFs were not included for DCE parameter comparison and correlation with clinical parameters. In our study, two types of suboptimal AIFs were detected in HR-DCE MR imaging. To identify these findings, Twash-out was calculated to define delayed wash-out error: Twash-out > two times more than 95th percentile value of wash-in time (time from BAT to TTP) using C-DCE MR images (Supplementary Fig. 5). Similarly, delayed bolus arrival error was defined when BAT was significantly delayed (two times more than the 95th percentile value of BAT using C-DCE MR images) (Supplementary Fig. 5).
Extended study
For extended study, T2WI images instead of postcontrast T1WI images were used as structural images. Using T2WI images, T2 hypersignal intense areas and contrast-enhancing lesions on postcontrast T1WI were included for ROI designation. DCE parametric map generation and coregistration were performed in the same manner as previously described. DCE parameters as differentiators for tumor grading were evaluated 21 in these patients. When there was a technical problem in coregistration of DCE and structural images, DCE parameter comparison or correlation with clinical parameters were not performed (Supplementary Fig. 4).
Statistical analysis
ICC and Bland–Altman plotting were performed to evaluate the intraobserver reproducibility of DCE MR imaging according to spatial and temporal resolution. For ICC interpretation, ICC values were considered (a) poor (ICC < 0.50), (b) moderate (ICC, 0.50–0.75), (c) good (ICC, 0.75–0.90), and (d) excellent (ICC > 0.90)35. Linear correlation coefficient was evaluated between mean pharmacokinetic parameters derived from C-DCE MR imaging and those derived from HR-DCE MR imaging. Relationship between DCE parameters and pathologic results such as Ki-67 or MGMT promoter methylation status were compared with linear and logistic regression analyses.
Normality of the parameters was assessed using Kolmogorov–Smirnov test, and subsequently, comparisons of AIFs or DCE parameters were performed by paired t test or Wilcoxon test, as appropriate. Unpaired parametric and nonparametric data were compared by independent samples t test or Mann–Whitney test, as appropriate. Comparison of area of receiver operating characteristic curve (AUROC) was performed between grade 4 tumors and others.
The statistical analysis was performed using statistical software (MedCalc version 15.2, Ostend, Belgium), and p-values less than 0.05 were considered statistically significant.
Data availability
Data of our study population could be accessed as a separate excel file.
References
Choi, H. S. et al. Glioma grading capability: comparisons among parameters from dynamic contrast-enhanced MRI and ADC value on DWI. Korean J. Radiol. 14, 487–492. https://doi.org/10.3348/kjr.2013.14.3.487 (2013).
Li, X. et al. Glioma grading by microvascular permeability parameters derived from dynamic contrast-enhanced MRI and intratumoral susceptibility signal on susceptibility weighted imaging. Cancer Imaging 15, 4. https://doi.org/10.1186/s40644-015-0039-z (2015).
Santarosa, C. et al. Dynamic contrast-enhanced and dynamic susceptibility contrast perfusion MR imaging for glioma grading: Preliminary comparison of vessel compartment and permeability parameters using hotspot and histogram analysis. Eur. J. Radiol. 85, 1147–1156. https://doi.org/10.1016/j.ejrad.2016.03.020 (2016).
Zhao, J., Yang, Z. Y., Luo, B. N., Yang, J. Y. & Chu, J. P. Quantitative evaluation of diffusion and dynamic contrast-enhanced MR in tumor parenchyma and peritumoral area for distinction of brain tumors. PLoS ONE 10, e0138573. https://doi.org/10.1371/journal.pone.0138573 (2015).
Jung, S. C. et al. Glioma: application of histogram analysis of pharmacokinetic parameters from T1-weighted dynamic contrast-enhanced MR imaging to tumor grading. AJNR Am. J. Neuroradiol. 35, 1103–1110. https://doi.org/10.3174/ajnr.A3825 (2014).
Nguyen, T. B. et al. Comparison of the diagnostic accuracy of DSC- and dynamic contrast-enhanced MRI in the preoperative grading of astrocytomas. AJNR Am. J. Neuroradiol. 36, 2017–2022. https://doi.org/10.3174/ajnr.A4398 (2015).
Arevalo-Perez, J. et al. T1-weighted dynamic contrast-enhanced MRI as a noninvasive biomarker of epidermal growth factor receptor VIII status. AJNR Am. J. Neuroradiol. 36, 2256–2261. https://doi.org/10.3174/ajnr.A4484 (2015).
Ahn, S. S. et al. Prediction of methylguanine methyltransferase promoter methylation in glioblastoma using dynamic contrast-enhanced magnetic resonance and diffusion tensor imaging. J. Neurosurg. 121, 367–373. https://doi.org/10.3171/2014.5.JNS132279 (2014).
Wang, X. et al. Simplified perfusion fraction from diffusion-weighted imaging in preoperative prediction of IDH1 mutation in WHO grade II-III gliomas: comparison with dynamic contrast-enhanced and intravoxel incoherent motion MRI. Radiol. Oncol. https://doi.org/10.2478/raon-2020-0037 (2020).
Suh, C. H., Kim, H. S., Choi, Y. J., Kim, N. & Kim, S. J. Prediction of pseudoprogression in patients with glioblastomas using the initial and final area under the curves ratio derived from dynamic contrast-enhanced T1-weighted perfusion MR imaging. AJNR Am. J. Neuroradiol. 34, 2278–2286. https://doi.org/10.3174/ajnr.A3634 (2013).
Yun, T. J. et al. Glioblastoma treated with concurrent radiation therapy and temozolomide chemotherapy: differentiation of true progression from pseudoprogression with quantitative dynamic contrast-enhanced MR imaging. Radiology 274, 830–840. https://doi.org/10.1148/radiol.14132632 (2015).
Villringer, K. et al. DCE-MRI blood-brain barrier assessment in acute ischemic stroke. Neurology 88, 433–440. https://doi.org/10.1212/WNL.0000000000003566 (2017).
Ingrisch, M. et al. Quantification of perfusion and permeability in multiple sclerosis: dynamic contrast-enhanced MRI in 3D at 3T. Invest Radiol 47, 252–258. https://doi.org/10.1097/RLI.0b013e31823bfc97 (2012).
Raja, R., Rosenberg, G. A. & Caprihan, A. MRI measurements of Blood-Brain Barrier function in dementia: a review of recent studies. Neuropharmacology 134, 259–271. https://doi.org/10.1016/j.neuropharm.2017.10.034 (2018).
Li, W. et al. A quantitative MRI method for imaging blood-brain barrier leakage in experimental traumatic brain injury. PLoS ONE 9, e114173. https://doi.org/10.1371/journal.pone.0114173 (2014).
Heye, T. et al. Reproducibility of dynamic contrast-enhanced MR imaging. Part I. Perfusion characteristics in the female pelvis by using multiple computer-aided diagnosis perfusion analysis solutions. Radiology 266, 801–811. https://doi.org/10.1148/radiol.12120278 (2013).
Rata, M. et al. Assessment of repeatability and treatment response in early phase clinical trials using DCE-MRI: comparison of parametric analysis using MR- and CT-derived arterial input functions. Eur Radiol 26, 1991–1998. https://doi.org/10.1007/s00330-015-4012-9 (2016).
Filice, S. & Crisi, G. Dynamic contrast-enhanced perfusion mri of high grade brain gliomas obtained with arterial or venous waveform input function. J. Neuroimaging 26, 124–129. https://doi.org/10.1111/jon.12254 (2016).
Port, R. E., Knopp, M. V. & Brix, G. Dynamic contrast-enhanced MRI using Gd-DTPA: interindividual variability of the arterial input function and consequences for the assessment of kinetics in tumors. Magn. Reson. Med. 45, 1030–1038. https://doi.org/10.1002/mrm.1137 (2001).
Li, X. et al. Relative sensitivities of DCE-MRI pharmacokinetic parameters to arterial input function (AIF) scaling. J. Magn. Reson. 269, 104–112. https://doi.org/10.1016/j.jmr.2016.05.018 (2016).
You, S. H. et al. Differentiation of high-grade from low-grade astrocytoma: improvement in diagnostic accuracy and reliability of pharmacokinetic parameters from DCE MR imaging by using arterial input functions obtained from DSC MR imaging. Radiology 286, 981–991. https://doi.org/10.1148/radiol.2017170764 (2018).
Park, J. S. et al. Model-based high-definition dynamic contrast enhanced MRI for concurrent estimation of perfusion and microvascular permeability. Med. Image Anal. https://doi.org/10.1016/j.media.2019.101566 (2020).
Chatterjee, A. et al. Performance of ultrafast DCE-MRI for diagnosis of prostate cancer. Acad. Radiol. 25, 349–358. https://doi.org/10.1016/j.acra.2017.10.004 (2018).
Othman, A. E. et al. Effect of temporal resolution on diagnostic performance of dynamic contrast-enhanced magnetic resonance imaging of the prostate. Invest. Radiol. 51, 290–296. https://doi.org/10.1097/RLI.0000000000000234 (2016).
Georgiou, L. et al. Estimating breast tumor blood flow during neoadjuvant chemotherapy using interleaved high temporal and high spatial resolution MRI. Magn. Reson. Med. 79, 317–326. https://doi.org/10.1002/mrm.26684 (2018).
Choi, S. H. et al. Perfusion MRI as the predictive/prognostic and pharmacodynamic biomarkers in recurrent malignant glioma treated with bevacizumab: a systematic review and a time-to-event meta-analysis. J. Neurooncol 128, 185–194. https://doi.org/10.1007/s11060-016-2102-4 (2016).
Kickingereder, P. et al. Evaluation of dynamic contrast-enhanced MRI derived microvascular permeability in recurrent glioblastoma treated with bevacizumab. J Neurooncol 121, 373–380. https://doi.org/10.1007/s11060-014-1644-6 (2015).
Xue, W. et al. Aberrant glioblastoma neovascularization patterns and their correlation with DCE-MRI-derived parameters following temozolomide and bevacizumab treatment. Sci. Rep. 7, 13894. https://doi.org/10.1038/s41598-017-14341-9 (2017).
Ng, C. S. et al. Reproducibility of perfusion parameters in dynamic contrast-enhanced MRI of lung and liver tumors: effect on estimates of patient sample size in clinical trials and on individual patient responses. AJR Am. J. Roentgenol. 194, W134-140. https://doi.org/10.2214/AJR.09.3116 (2010).
van den Boogaart, V. E. et al. Inter-reader reproducibility of dynamic contrast-enhanced magnetic resonance imaging in patients with non-small cell lung cancer treated with bevacizumab and erlotinib. Lung Cancer 93, 20–27. https://doi.org/10.1016/j.lungcan.2015.12.009 (2016).
Choi, Y. S. et al. The added prognostic value of preoperative dynamic contrast-enhanced MRI histogram analysis in patients with glioblastoma: analysis of overall and progression-free survival. AJNR Am. J. Neuroradiol. 36, 2235–2241. https://doi.org/10.3174/ajnr.A4449 (2015).
Guo, Y. et al. High-resolution whole-brain DCE-MRI using constrained reconstruction: prospective clinical evaluation in brain tumor patients. Med. Phys. 43, 2013. https://doi.org/10.1118/1.4944736 (2016).
Tofts, P. S. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J. Magn. Reson. Imaging 7, 91–101. https://doi.org/10.1002/jmri.1880070113 (1997).
Yoo, R. E. et al. Dynamic contrast-enhanced MR imaging in predicting progression of enhancing lesions persisting after standard treatment in glioblastoma patients: a prospective study. Eur. Radiol. 27, 3156–3166. https://doi.org/10.1007/s00330-016-4692-9 (2017).
Koo, T. K. & Li, M. Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 15, 155–163. https://doi.org/10.1016/j.jcm.2016.02.012 (2016).
Acknowledgements
This study was supported by a grant from the Korea Healthcare Technology R&D Projects, Ministry for Health, Welfare & Family Affairs (HI16C1111), by the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2016M3C7A1914002), by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2020R1A2C2008949 and NRF-2020R1A4A1018714), by the Creative-Pioneering Researchers Program through Seoul National University (SNU), and by the Institute for Basic Science (IBS-R006-A1).
Author information
Authors and Affiliations
Contributions
All authors (W.H.L., J.S.P., J.S.P., S.H.C.) contributed to the study conception and design. Material preparation, data collection and analysis were performed by W.H.L. and J.S.P. (2nd author). The first draft of the manuscript was written by W.H.L. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lim, W.H., Park, J.S., Park, J. et al. Assessing the reproducibility of high temporal and spatial resolution dynamic contrast-enhanced magnetic resonance imaging in patients with gliomas. Sci Rep 11, 23217 (2021). https://doi.org/10.1038/s41598-021-02450-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-02450-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.