Abstract
We compared the effect of commercial vaginal douching products on Lactobacillus crispatus, L. jensenii, L. gasseri, L. iners, E. coli, and immortalized vaginal epithelial cells (VK2). All studied douching products (vinegar, iodine and baking soda based) induced epithelial cell death, and all inhibited growth of E. coli. Co-culture of vaginal epithelial cells with any of the lactobacilli immediately following exposure to douching products resulted in a trend to less human cell death. However, co-culture of epithelial cells with L. iners was associated with higher production of IL6 and IL8, and lower IL1RA regardless of presence or type of douching solution. Co-culture with L. crispatus or L. jensenii decreased IL6 production in the absence of douches, but increased IL6 production after exposure to vinegar. Douching products may be associated with epithelial disruption and inflammation, and may reduce the anti-inflammatory effects of beneficial lactobacilli.
Similar content being viewed by others
Introduction
Between 10 and 40% of American women use vaginal douching products to treat vaginal discharge, odor or discomfort1,2,3,4. Intravaginal washing is associated with decreased vaginal colonization with beneficial lactobacilli5. Cross-sectional studies have demonstrated an association between douching and bacterial vaginosis (BV)5,6. Although women with recurrent vaginal infections are advised not to douche, studies of vaginal washing cessation have not demonstrated dramatic restoration of a Lactobacillus-dominant vaginal microbiota in women who stop the practice7,8,9. It is unclear whether douching causes the alteration in vaginal microbiota, is initiated as a response to a change in microbiota and symptoms or is simply a confounder associated with an unknown risk factor.
Urinary tract infections (UTI) are diagnosed in over 7 million women annually, and are most commonly caused by E. coli10,11,12. Vaginal Lactobacillus spp. are thought to be protective against E. coli due to bactericidal activity and there is an inverse association between the presence of vaginal lactobacilli and E. coli in women with recurrent UTI13,14. Women with BV (and thus, low levels of vaginal lactobacilli) are at an increased risk for UTI15,16. However, douching itself has not been well studied as a risk factor for UTI.
One way to assess the potential role of douching in altering the risk for UTI is to determine the impact of douching products on E. coli and commensal vaginal lactobacilli. A study examining the effect of vinegar-based douching products in vitro showed no inhibitory effects on either L. gasseri or L. jensenii, though it did demonstrate inhibition with iodine-based solutions17. However, there are no in vitro studies looking at the inhibitory effects of douching products on the most common vaginal Lactobacillus species (L. crispatus and L. iners), or E. coli, the most common pathogen isolated in UTI. In an effort to better understand how douching products impact the vaginal environment and potential risk for UTI, we co-cultured commercially available douches with the four most common vaginal Lactobacillus spp., E. coli and vaginal epithelial cells and measured the effect of douches on bacterial growth and human cell viability and cytokine production.
Results
Effect of douching products on bacterial growth
We co-cultured E. coli and the four most common vaginal Lactobacillus species (L. crispatus, L. iners, L. gasseri, L. jensenii) with vinegar-, iodine-, and baking soda-based commercial douching products (Table 1). E. coli was grown aerobically in nutrient broth to mid-exponential phase, diluted to a standard starting OD600 of 0.1 and mixed with the different douching solutions or sterile saline. After 2 h at 37C, E. coli growth was inhibited by all products (p < 0.01) compared to the saline control (Fig. 1). Lactobacillus species were cultured anaerobically in MRS broth (L. crispatus, L. gasseri, L. jensenii) or MRS broth plus Isovitalex (L. iners), and co-incubated with douching solutions for 24 h at 37C. The only douching solution which inhibited growth of any Lactobacillus species was the baking soda-based product (Fig. 1). E. coli growth was inhibited by more dilute solutions of iodine and baking soda douching products, but Lactobacillus species were only inhibited by the undiluted product (Supplemental Fig. 1).
Impact of douching products on bacterial growth. Full strength vinegar (a), iodine (b) and baking soda-based (c) douching products were mixed 1:2 with broth culture of E. coli or one of four Lactobacillus species and growth compared by OD600 over 2 h (E. coli) or 24 h (lactobacilli). Results are presented as mean ± SD from at least two separate experiments.
Effect of douching products on vaginal epithelial cell viability and immune response
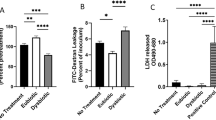
We then examined the effect of douching products on the vaginal epithelium by co-culturing immortalized vaginal epithelial cells (VK2) with douching solution diluted 1:4 in the cell culture media (keratinocyte serum-free (KSF) medium) for one hour. After the hour, media was aspirated and replaced with fresh culture medium and cultured for an additional 23 h. Human cell death, measured using a lactate dehydrogenase (LDH) assay, was higher after exposure to douching products, though this effect was not statistically significant (Fig. 2a). We evaluated cytokine expression in culture supernatant using ELISA. When compared to saline control, a one-hour exposure to iodine and baking soda suppressed production of IL8 (Fig. 2b). Both vinegar and iodine douching products induced greater production of IL6 and IL1β, while baking soda suppressed production (Fig. 2c,d). IL1RA was not significantly different between KSF alone and douching products (Fig. 2e).
Impact of douching products on vaginal epithelial cells. An immortalized vaginal epithelial cell line was cultured with a 25% solution of douching product diluted in the cell culture medium for 1 h, followed by replacement with fresh media for 23 h. Cell death (a) was measured using an LDH assay and IL6 (b), IL8 (c), IL1β (d) and IL1-RA (e) were measured in supernatant by ELISA. Results are presented as mean ± SD from at least two separate experiments.
Effect of lactobacilli on epithelial cell viability and immune response
Lactobacilli were resuspended in KSF at an OD of 0.1 and added to the human vaginal epithelial cell cultures for 24 h. There was no difference in cell death after exposure to any of the Lactobacillus spp. (Fig. 3a). When compared to KSF alone, co-culture with L. iners induced production of IL6 and IL8 (Fig. 3b,c) and suppressed production of IL1-RA (Fig. 3e). The other three species did not induce cytokine production (Fig. 3b–d), but all suppressed production of IL1-RA (Fig. 3e).
Impact of bacteria on vaginal epithelial cells. An immortalized vaginal epithelial cell line was cultured with a suspension of Lactobacillus iners, L. crispatus, L. gasseri or L. jensenii in cell culture medium (OD 0.1) for 24 h. Cell death (a) was measured using an LDH assay and IL6 (b), IL8 (c), IL1β (d) and IL1-RA (e) were measured in supernatant by ELISA. Results are presented as mean ± SD from at least two separate experiments.
Impact of lactobacilli on epithelial cell response to douching products
We then examined how exposure to a douching solution impacted the epithelial cell response to commensal lactobacilli. Co-culture with either L. crispatus or L. iners for 23 h after a 1-h exposure to douching solutions resulted in a trend to less human cell death compared to cell culture media alone, though this did not reach statistical significance (Fig. 4).
Impact of bacteria on vaginal epithelial cell death with douching exposure. An immortalized vaginal epithelial cell line was cultured with a 25% solution of douching product diluted in the cell culture medium for 1 h, followed by replacement with a suspension of L. crispatus or L. iners (OD 0.1) in cell culture media for 23 h. Cell death was measured with an LDH assay and values compared by ANOVA. Results are presented as mean ± SD from at least two separate experiments.
Whether bacteria were present or not, baking soda douche suppressed production of IL6 and IL8 compared to media alone (Fig. 5a,b). L. crispatus suppressed production of IL1b across conditions, though this did not reach statistical significance (Fig. 5c). When baking soda douche was followed by L. iners or L. crispatus, cells produced higher levels of IL1RA (Fig. 5d). Pre-incubation with vinegar induced higher levels of IL6, most significantly with subsequent exposure to L. crispatus. Pre-treatment with douching products suppressed production of IL8 after exposure to L. iners, though overall values were still higher than after exposure to other lactobacilli.
Impact of bacteria on vaginal epithelial cell death immune response to douching exposure. An immortalized vaginal epithelial cell line was cultured with a 25% solution of douching product diluted in the cell culture medium for 1 h, followed by replacement with a suspension of L. iners, L. crispatus, L. gasseri or L. jensenii (OD 0.1) in cell culture media for 23 h. Supernatant was used to measure IL6 (a), IL8 (b), IL1β (c) and IL1-RA (d) with ELISA, and values compared by ANOVA. Results are presented as mean ± SD from at least two separate experiments.
Discussion
Between 9 and 48% of women in the US report use of vaginal douching products, despite recommendations from health care providers against douching1,3. Washing practices have been associated with increased risk for BV, persistent human papillomavirus (HPV) infection, and decreased vaginal colonization with hydrogen peroxide-producing Lactobacillus5,6,18. In our in vitro model, all three types of commercial douching solutions tested inhibited growth of the common uropathogen E. coli, while vinegar- and iodine-based douches did not inhibit commensal lactobacilli. All douches were toxic to vaginal epithelial cells, but co-culture with commensal Lactobacillus spp. decreased that effect. Our study is one of the first to examine the common species, L. iners, which does not grow in the traditional Lactobacillus-selective medium MRS. We found that L. iners is more inflammatory than other species, which is in keeping with the finding that L. iners-dominant communities have more inflammation in vivo than those dominated by L. crispatus19,20. However, all Lactobacillus species mitigated the toxicity of douching products on epithelial cells.
While many cross-sectional studies show an association between bacterial vaginosis and douching4,21,22, some do not23,24, which has raised questions about the causal association between douching and BV. Cross-sectional studies demonstrate lower detection of vaginal lactobacilli in women who douche25. A small longitudinal study of a douching cessation intervention demonstrated an increase in vaginal lactobacilli after cessation, though this finding was not statistically significant7. Douching has been associated with increased risk for adverse reproductive health outcomes such as pelvic inflammatory disease and endometritis26, or HIV acquisition27. Recent studies demonstrate higher numbers of HPV types or higher prevalence of high risk subtypes in women who douched in the past 6 months18,28. Few studies have examined the contribution of douching to risk for UTI. In a case control study of young Seattle women with and without recurrent UTI, douching was reported by only 11% of participants and was not different between cases and controls29.
Few studies have examined the effect of vaginal douching solutions on uropathogens and commensal vaginal bacteria and none have tested baking soda-based douching solutions. A previous in vitro study demonstrated that vinegar based douching solutions inhibited multiple vaginal pathogens but had no impact on vaginal lactobacilli. However, the same study showed that iodine-based douching products inhibited L. gasseri but not L. jensenii, which contrasts with our findings17. A separate evaluation of a vinegar-based douche also demonstrated no impact on growth of L. crispatus in vitro30. An in vivo comparison of vinegar vs. iodine-based douching solutions, used daily for 7 days, demonstrated a decrease in detection of Lactobacillus species by culture after iodine-based douche but not vinegar-based. E. coli was infrequently detected in all participants, regardless of douching31. The acidity of a vinegar-based douche could be hypothesized to be beneficial for the vaginal environment, mimicking the low pH associated with a Lactobacillus-dominant bacterial community. However, a study of a lactic acid based vaginal douching product demonstrated higher prevalence of a diverse vaginal microbial community after use, which is the opposite of what one might expect32. In vitro, lactic acid-based vaginal douches, which also included several other ingredients, inhibited clinical Lactobacillus strains33. As commensal lactobacilli have shown to have bactericidal activity against common uropathogens such as E. coli, loss of lactobacilli due to douching could increase UTI risk, though this might be balanced by the negative effects of many douching products on E. coli growth34,35.
Our study, and others, demonstrate in vitro that vaginal douching products increase vaginal epithelial cell death and secretion of pro-inflammatory cytokines, suggesting the potential for epithelial disruption30. Additionally, and of concern, is our finding that after exposure to vinegar-based douche, L. crispatus and L. jensenii—two classic beneficial lactobacilli—induce greater production of the pro-inflammatory cytokine IL6. This result may be due to less cell death, and so greater ability to produce cytokines, but if so we would expect to see a similar pattern with L. iners and L. gasseri. In vivo, analysis of vaginal fluid cytokine levels demonstrates higher levels of pro-inflammatory cytokines in women who use douching products. However, the type of vaginal douching solution was not specified36.
Other common vaginal personal care products, such as lubricants, have also been shown to limit growth of both uropathogens and commensal lactobacilli34,37. Unlike douches, many of those products contain antimicrobial preservatives such as chlorhexidine or parabens, or detergents such as nonoxynol-9. Lubricant products have also been shown to impact epithelial barrier integrity, and reduce adherence of lactobacilli to vaginal epithelial cells37,38,39. The douching products we tested also include other compounds, which may contribute to the effects seen; citric acid (included in the iodine-based douche) has been associated with epithelial toxicity, as has benzoic acid (included in both iodine- and vinegar-based douches) to a lesser extent40.
Limitations of this study include the lack of a robust model for human vaginal microbial colonization. The vaginal epithelium is composed of layers of cells with basal, parabasal, squamous flat cells and intermediate cells. Disruption of the epithelium could lead to exposure of stroma or antigen-presenting cells which might have different immune responses. Our co-culture model does not mimic this multilayer system. Also, our co-culture model does not include the mucous normally found in the in vivo setting, which could serve as a protective barrier to the effects of douching products—for both epithelial cells and bacteria. However, there are no animal models of the vaginal ecosystem that include Lactobacillus-dominant microbiota in which to conduct these studies41.
Our study highlights the potential negative impacts of douching solutions through induction of epithelial cell death and inflammation. Douches at either end of the pH spectrum (i.e. baking soda and vinegar) had different but consistently negative effects on either human cells or commensal lactobacilli. Our results suggest possible mechanisms through which vaginal douching could potentially increase acquisition of all genitourinary infections, including UTI, and support clinical recommendations to avoid douching.
Materials and methods
Douche/bacteria co-culture
Three vaginal douching products with different active ingredients were purchased online or at local drug stores within the United States. The ingredients of the douching solutions as well as their pH values are listed in Table 1. Five species of bacteria were grown in broth to mid-exponential phase: E. coli (clinical strain, obtained from the clinical lab at MGH, reported as from a woman with acute cystitis), Lactobacillus crispatus (obtained through BEI Resources, NIAID, NIH as part of the Human Microbiome Project: Lactobacillus crispatus, Strain JV-V01, HM-103), L. jensenii (ATCC 25258), L. gasseri (ATCC 33323), L. iners (ATCC 55195). E. coli was grown in nutrient broth to mid-exponential phase, diluted to a standard starting OD600 and mixed with the different douching solutions or sterile saline. E. coli experiments were conducted aerobically, at 37C with gentle agitation. Lactobacillus species were cultured in MRS broth (L. crispatus, L. gasseri, L. jensenii) or MRS broth plus Isovitalex (L. iners)42. Co-culture experiments were performed by mixing 400 uL of the bacterial broth cultures and 200 uL of douches in varying concentrations (100%, 75%, 50%, and 25%, diluted with saline) in a 48 well plate. Lactobacillus experiments were conducted in an anaerobic chamber, at 37C, and the plates were kept still. Each combination was tested in triplicate and compared to a saline control. Change in optical density (OD600) was measured over 2 h for E. coli and 24 h for Lactobacilli to assess growth using a Gen 5 2.09 Biotek microplate reader.
Vaginal epithelial cell/douche/bacteria co-culture
Vaginal epithelial cells (VK2 E6/E7, ATCC) were seeded at 40,000 cells/well in a 96-well plate, in Keratinocyte-Serum Free medium (KSF) (with BPE and EGF, but no antibiotics), and allowed to grow overnight to confluence. Vaginal cells were then exposed to dilute (25%) douching product or fresh media for 1 h. This concentration and duration of exposure was chosen because of prior data showing that exposure to undiluted douching products inhibited vaginal epithelial cell viability30, and our own data showing significant human cell death at higher concentrations or for longer duration (Supplemental Fig. 2). Supernatant was aspirated, and vaginal cells were then exposed to a fresh KSF or a suspension of one of the four commensal Lactobacillus species in KSF for 23 h (at OD 0.1). At the end of the exposure, supernatant was aspirated and used to measure LDH or frozen until used for ELISA. Vaginal epithelial cell death was quantified using a lactate dehydrogenase assay (Invitrogen, Waltham, MA). Pro-inflammatory cytokine levels (IL6, IL8, IL1β, IL1RA) were measured using ELISA (R&D Systems, Minneapolis, MN).
Statistical analysis
Change in OD600 between time 0 and the end of the bacterial/douche co-culture was compared across concentrations and media control for each product using ANOVA. Cell death was compared between individual douching product or Lactobacillus species and KSF control by student’s T-test. Cytokine values were compared between conditions using one-way or two-way ANOVA followed by Tukey’s multiple comparison test, as appropriate.
References
Brown, J. M. et al. Motivations for intravaginal product use among a cohort of women in Los Angeles. PLoS ONE 11(3), e0151378. https://doi.org/10.1371/journal.pone.0151378 (2016).
Diclemente, R. J. et al. Prevalence and correlates of recent vaginal douching among African American adolescent females. J. Pediatr. Adolesc. Gynecol. 25(1), 48–53. https://doi.org/10.1016/j.jpag.2011.07.017 (2012).
Ding, N., Batterman, S. & Park, S. K. Exposure to volatile organic compounds and use of feminine hygiene products among reproductive-aged women in the United States. J. Womens Health (Larchmt). 29(1), 65–73. https://doi.org/10.1089/jwh.2019.7785 (2020).
Muzny, C. A., Sunesara, I. R., Austin, E. L., Mena, L. A. & Schwebke, J. R. Bacterial vaginosis among African American women who have sex with women. Sex. Transm. Dis. 40(9), 751–755. https://doi.org/10.1097/OLQ.0000000000000004 (2013).
Lokken, E. M. et al. Association between vaginal washing and detection of Lactobacillus by culture and quantitative PCR in HIV-seronegative Kenyan women: A cross-sectional analysis. Sex. Transm. Infect. 95(6), 455–461. https://doi.org/10.1136/sextrans-2018-053769 (2019).
Sabo, M. C. et al. Association between vaginal washing and vaginal bacterial concentrations. PLoS ONE 14(1), e0210825. https://doi.org/10.1371/journal.pone.0210825 (2019).
Masese, L. et al. A pilot study of the feasibility of a vaginal washing cessation intervention among Kenyan female sex workers. Sex. Transm. Infect. 89(3), 217–222. https://doi.org/10.1136/sextrans-2012-050564 (2013).
Brotman, R. M. et al. The effect of vaginal douching cessation on bacterial vaginosis: A pilot study. Am. J. Obstet. Gynecol. 198(6), 628. https://doi.org/10.1016/j.ajog.2007.11.043 (2008).
Klebanoff, M. A. et al. A pilot study of vaginal flora changes with randomization to cessation of douching. Sex. Transm. Dis. 33(10), 610–613. https://doi.org/10.1097/01.olq.0000216050.41305.c1 (2006).
Echols, R. M., Tosiello, R. L., Haverstock, D. C. & Tice, A. D. Demographic, clinical, and treatment parameters influencing the outcome of acute cystitis. Clin. Infect. Dis. 29(1), 113–119. https://doi.org/10.1086/520138 (1999).
Foxman, B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N. Am. 28(1), 1–13. https://doi.org/10.1016/j.idc.2013.09.003 (2014).
Schappert, S. M. & Rechtsteiner, E. A. Ambulatory medical care utilization estimates for 2007. Vital Health Stat. 13 169, 1–38 (2011).
Gupta, K. et al. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli colonization in women with recurrent urinary tract infections. J. Infect. Dis. 178(2), 446–450 (1998).
Stapleton, A. E. et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin. Infect. Dis. 52(10), 1212–1217. https://doi.org/10.1093/cid/cir183 (2011).
Hillebrand, L., Harmanli, O. H., Whiteman, V. & Khandelwal, M. Urinary tract infections in pregnant women with bacterial vaginosis. Am. J. Obstet. Gynecol. 186(5), 916–917. https://doi.org/10.1067/mob.2002.123987 (2002).
Sharami, S. H., Afrakhteh, M. & Shakiba, M. Urinary tract infections in pregnant women with bacterial vaginosis. J. Obstet. Gynaecol. 27(3), 252–254. https://doi.org/10.1080/01443610701194846 (2007).
Pavlova, S. I. & Tao, L. In vitro inhibition of commercial douche products against vaginal microflora. Infect. Dis. Obstet. Gynecol. 8(2), 99–104. https://doi.org/10.1155/S1064744900000090 (2000).
Bui, T. C. et al. Association between vaginal douching and genital human papillomavirus infection among women in the United States. J. Infect. Dis. 214(9), 1370–1375. https://doi.org/10.1093/infdis/jiw388 (2016).
Anahtar, M. N. et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity 42(5), 965–976. https://doi.org/10.1016/j.immuni.2015.04.019 (2015).
Gosmann, C. et al. Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African Women. Immunity 46(1), 29–37. https://doi.org/10.1016/j.immuni.2016.12.013 (2017).
Marconi, C. et al. Characterization of the vaginal microbiome in women of reproductive age from 5 regions in Brazil. Sex. Transm. Dis. 47(8), 562–569. https://doi.org/10.1097/OLQ.0000000000001204 (2020).
Ness, R. B. et al. Douching in relation to bacterial vaginosis, lactobacilli, and facultative bacteria in the vagina. Obstet. Gynecol. 100(4), 765. https://doi.org/10.1016/s0029-7844(02)02184-1 (2002).
Forcey, D. S. et al. Factors associated with bacterial vaginosis among women who have sex with women: A systematic review. PLoS ONE 10(12), e0141905. https://doi.org/10.1371/journal.pone.0141905 (2015).
Marrazzo, J. M. et al. Characterization of vaginal flora and bacterial vaginosis in women who have sex with women. J. Infect. Dis. 185(9), 1307–1313. https://doi.org/10.1086/339884 (2002).
Beigi, R. H., Wiesenfeld, H. C., Hillier, S. L., Straw, T. & Krohn, M. A. Factors associated with absence of H2O2-producing Lactobacillus among women with bacterial vaginosis. J. Infect. Dis. 191(6), 924–929. https://doi.org/10.1086/428288 (2005).
Gondwe, T. et al. Novel bacterial vaginosis-associated organisms mediate the relationship between vaginal douching and pelvic inflammatory disease. Sex. Transm. Infect. 96(6), 439–444. https://doi.org/10.1136/sextrans-2019-054191 (2020).
McClelland, R. S. et al. Vaginal washing and increased risk of HIV-1 acquisition among African women: A 10-year prospective study. AIDS 20(2), 269–273. https://doi.org/10.1097/01.aids.0000196165.48518.7b (2006).
Tounkara, F. K. et al. Human papillomavirus genotype distribution and factors associated among female sex workers in West Africa. PLoS ONE 15(11), e0242711. https://doi.org/10.1371/journal.pone.0242711 (2020).
Scholes, D. et al. Risk factors for recurrent urinary tract infection in young women. J. Infect. Dis. 182(4), 1177–1182. https://doi.org/10.1086/315827 (2000).
Fashemi, B., Delaney, M. L., Onderdonk, A. B. & Fichorova, R. N. Effects of feminine hygiene products on the vaginal mucosal biome. Microb. Ecol. Health Dis. https://doi.org/10.3402/mehd.v24i0.19703 (2013).
Onderdonk, A. B., Delaney, M. L., Hinkson, P. L. & DuBois, A. M. Quantitative and qualitative effects of douche preparations on vaginal microflora. Obstet. Gynecol. 80(3 Pt 1), 333–338 (1992).
van der Veer, C. et al. Effects of an over-the-counter lactic-acid containing intra-vaginal douching product on the vaginal microbiota. BMC Microbiol. 19(1), 168. https://doi.org/10.1186/s12866-019-1545-0 (2019).
Juliano, C., Piu, L., Gavini, E., Zanetti, S. & Fadda, G. In vitro antibacterial activity of antiseptics against vaginal lactobacilli. Eur. J. Clin. Microbiol. Infect. Dis. 11(12), 1166–1169. https://doi.org/10.1007/BF01961138 (1992).
Hung, K. J. et al. Effect of commercial vaginal products on the growth of uropathogenic and commensal vaginal bacteria. Sci. Rep. 10(1), 7625. https://doi.org/10.1038/s41598-020-63652-x (2020).
Vagios, S., Hesham, H. & Mitchell, C. Understanding the potential of lactobacilli in recurrent UTI prevention. Microb. Pathog. 148, 104544. https://doi.org/10.1016/j.micpath.2020.104544 (2020).
Alcaide, M. L. et al. High levels of inflammatory cytokines in the reproductive tract of women with BV and engaging in intravaginal douching: A cross-sectional study of participants in the women interagency HIV study. AIDS Res. Hum. Retrovir. 33(4), 309–317. https://doi.org/10.1089/AID.2016.0187 (2017).
Dezzutti, C. S. et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV-1 activity. PLoS ONE 7(11), e48328. https://doi.org/10.1371/journal.pone.0048328 (2012).
Laniewski, P., Owen, K. A., Khnanisho, M., Brotman, R. M. & Herbst-Kralovetz, M. M. Clinical and personal lubricants impact the growth of vaginal Lactobacillus species and colonization of vaginal epithelial cells: An in vitro study. Sex. Transm. Dis. 48(1), 63–70. https://doi.org/10.1097/OLQ.0000000000001272 (2021).
Wilkinson, E. M., Laniewski, P., Herbst-Kralovetz, M. M. & Brotman, R. M. Personal and clinical vaginal lubricants: Impact on local vaginal microenvironment and implications for epithelial cell host response and barrier function. J. Infect. Dis. 220(12), 2009–2018. https://doi.org/10.1093/infdis/jiz412 (2019).
Hu, M., Zhou, T., Dezzutti, C. S. & Rohan, L. C. The effect of commonly used excipients on the epithelial integrity of human cervicovaginal tissue. AIDS Res. Hum. Retrovir. 32(10–11), 992–1004. https://doi.org/10.1089/AID.2016.0014 (2016).
Herbst-Kralovetz, M. M., Pyles, R. B., Ratner, A. J., Sycuro, L. K. & Mitchell, C. New systems for studying intercellular interactions in bacterial vaginosis. J. Infect. Dis. 214 Suppl 1, S6–S13. https://doi.org/10.1093/infdis/jiw130 (2016).
Bloom, S. et al. Cysteine dependence in Lactobacillus iners constitutes a novel therapeutic target to modify the vaginal microbiota. biorxivorg. https://doi.org/10.1101/2021.06.12.448098 (2021).
Funding
This work was supported by the Vincent Memorial Research Funds.
Author information
Authors and Affiliations
Contributions
H.H. and C.M. conceived of the project. H.H., K.H., A.B. performed or designed experiments. H.H., A.M., A.B., C.M. performed analysis of results. H.H., A.M., C.M. wrote the manuscript. All authors reviewed and contributed to the final version.
Corresponding author
Ethics declarations
Competing interests
Dr. Mitchell receives Grant funding from Merck, royalties from Up to Date and has served as a consultant for Scynexis, Inc. The other authors report no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hesham, H., Mitchell, A.J., Bergerat, A. et al. Impact of vaginal douching products on vaginal Lactobacillus, Escherichia coli and epithelial immune responses. Sci Rep 11, 23069 (2021). https://doi.org/10.1038/s41598-021-02426-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-02426-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.