Abstract
Laboulbeniales are a highly specialized group of fungi living only on arthropods. They have high host specificity and spend their entire life-cycle on an arthropod host. Taxonomic characters of Laboulbeniales are based on the architecture of the cells of the parenchymal thallus, i.e. the visible part of the fungus outside the host. The extent of the fungus spreading inside the host—the haustorium—remains largely unknown. The attachment to the arthropod host is fundamental to understand the fungus-animal interaction, but how this truly occurs is unclear. Recent evidences question the strictly parasitic life-style of Laboulbeniales. We used micro-computed tomography (µCT) and 3D reconstructions to visualize, for the first time, the complete structure of Laboulbeniales species in situ on their hosts. We compared the haustoriate species, Arthrorhynchus nycteribiae on an insect host to the non-haustoriate species, Rickia gigas on a millipede host. Our results confirm that some Laboulbeniales species are ectoparasitic and have a haustorial structure that penetrates the host’s cuticle, while others are ectobionts and are only firmly attached to the host’s cuticle without penetrating it. The presence and the morphology of the haustorium are important traits for Laboulbeniales evolution, and key factors for future understanding of host dependence and specificity.
Similar content being viewed by others
Introduction
Parasitism is defined as the relationship between two organisms where one organism, the parasite, benefits while the other organism, the host, is harmed by the relationship1. Typically, the parasite extracts nourishment from the host and thereby reduces its fitness1. A parasite itself can be infected with another parasite, which defines the latter as a hyperparasite2. Understanding parasitism, especially hyperparasitism, requires a complex multidisciplinary approach involving ecology, evolution and behaviour of the three participants in the interaction2,3,4,5.
Laboulbeniales have long been considered ectoparasites of living arthropods, where they can be found on the external surface of their cuticle6,7. Laboulbeniales hosts must combine important properties: (i) successive generations of adult hosts should overlap in time because transmission occurs mainly during copulation, because the vast majority does not live on eggs or larval stages of their host; (ii) their populations must be large and stable; and (iii) they must inhabit moist environments8. Studies on Laboulbeniales have mostly been taxonomic, with a very recent emergence of phylogenetic analysis9,10,11,12,13, and a few recent studies have provided insights into the interaction of Laboulbeniales and their hosts, and the environment2,14,15,16,17.
Morphological studies on Laboulbeniales have focused on the external part of the fungus, the thallus. A recent paper on the histopathology of the genus Rickia started a debate on the absence of the haustorial structures in some genera of Laboulbeniales18, whether this is a secondary loss remains unknown.
We investigate the presence and reveal the structure of Laboulbeniales haustoria in situ in their hosts, using the novel visualization technique based on micro-CT, and also on scanning electron microscopy (SEM).
Results
Micro-computed tomography (µCT)
A 3D reconstruction based on µCT of Arthrorhynchus nycteribiae on a male Penicillidia conspicua, shows host cuticle with attached thalli, attached to an intersegment membrane (Fig. 1 and Supplementary Video S1), and a group of four thalli (of which one is broken at the base) of Arthrorhynchus nycteribiae. At the base of each thallus the cuticle (with a diameter of 25–30 µm) is penetrated by a circular hole with a diameter of 22–28 µm (n = 4). From this penetration a cylindrical lumen, which we interpret as the haustorium extends 45–71 µm (n = 4) into the host’s tissue. Inside the host’s body cavity this structure, the haustorium, tapers distally and gives rise to several side branches. In contrast, at the base of the four thalli of Rickia gigas shown in (Fig. 1 and Supplementary Video S2), no such penetration into the host’s cuticle is visible in the µCT-data.
Comparison of the haustoriate Laboulbeniales Arthrorhynchus nycteribiae (a–g) on the abdomen of the batfly Penicillidia conspicua (SR-Nyct85) and the non-haustoriate Rickia gigas on the leg of the millipede Tropostreptus hamatus (h–l). (a–c,i,h) Segmentation based on µCT data. (d–g,j–l) Virtual sections based on µCT data. (a) External view. (b) Penetration of the hosts cuticle (transparent). (c) Haustorium, internal view. (d) Transverse section trough the fungi and the host’s cuticle. (e) Transverse section trough the host’s cuticle underneath the base of the thallus. (f) Section trough the host’s cuticle underneath the base of the thallus, plane as indicated in (d). (g) Section trough the host’s tissue and haustorium, plane as indicated in (d). (h) External view. (i) Penetration of the hosts cuticle (transparent). (j) Transverse section trough the fungi and the host’s cuticle. (k) longitudinal section through the host’s leg. (l) Cross section trough the host’s leg. Cu host’s cuticle, Ex exterior, Ha haustorium, Pe penetration of cuticle, Th thallus, Ti host’s tissue. Red arrows indicate the base (foot) of the thalli. Figure plate prepared with Gimp 2.10.6 (GIMP Development Team, https://www.gimp.org) and Inkscape 1.0 (Inkscape Developers, https://inkscape.org).
Scanning electron microscope (SEM)
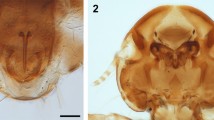
We here present SEMs of the “gigantic” non-haustoriate species Rickia gigas. When a thallus of R. gigas is separated from the host cuticle, an horseshoe-shaped wall remains on the host, surrounding an amorphous structure in the middle (Fig. 2). This structure is mirrored by the foot. On the underside of the foot of the fungus, a similar horseshoe-shaped rim, or impression of a wall, is evident. Within this outer rim/wall a smaller circular structure is evident, which surrounds an amorphous substance in the middle. The inner circle attaches to the foot’s outer margin in a position corresponding to the opening in the horseshoe-shaped ring wall (Fig. 3). SEM images of Arthrorhynchus—a Laboulbeniales species with haustoria—were previously shown2.
Discussion
The micro-CT results from Arthrorhynchus agree perfectly with the previously known light microscope and transmission electron microscope images2. This emphasizes that microtomography is a good technique to visualize the type of fungal attachment to the host and especially the penetration of the cuticle, apart from the study of thallus in amber fossils17. As Jensen et al. (2019) demonstrated the presence of a haustorium in Arthrorhynchus using scanning electron microscopy, we are confident that the lack of penetration and haustorium in Rickia found by micro-CT is real. This is also in agreement with results from the scanning electron microscopical investigation of the attachment sites of R. gigas, which exhibits no indication of penetration and are very similar to those of R. wasmannii previously shown18.
Despite the absence of a haustorium, and hence without any obvious means of obtaining nutrition, Rickia gigas is quite a successful fungus, being often abundant on several species of Afrotropical millipedes of the family Spirostreptidae10. It was originally described from Archispirostreptus gigas, and Tropostreptus (= ‘Spirostreptus’) hamatus20, and was subsequently reported from several other Tropostreptus species19.
A further challenge for Laboulbeniales growing on millipedes is that infected millipedes, in some species even adults, may moult, shedding the exuviae with the fungus, as has been observed by us on an undescribed Rickia species on a millipede of the genus Spirobolus (family Spirobolidae).
The question of how non-haustoriate Laboulbeniales obtain nutrients has been discussed by several authors18, including staining experiments using fungi of the non-haustoriate genus Laboulbenia on various beetles21. Whereas the surface of the main thallus was almost impenetrable to the dye applied (Nile Blue), the smaller appendages could sometimes be penetrated21. The dye injection into the beetle elytra upon which the fungi were sitting, actually spread from the elytron into the fungus, thus indicating that in spite of the lack of a haustorium, the fungus is able to extract nutrients from the interior of its host21.
Such experiments have not been performed on Rickia species, but the possibility that nutrients may pass from the host into the basis of the fungus cannot be excluded. For this genus, or at least R. gigas, there may, however, be an alternative way to obtain nutrients: the small opening in the circular wall by which the thallus is attached to the host may allow nutrients from the surface of the millipede or from the environment to seep into the foot of the fungus. However, further experiments are needed in order to evaluate this hypothesis. Moreover, we should not exclude a potential role of primary and secondary appendages in Laboulbeniales nutrition, as we still do not understand exactly their functional role on the fungus life cycle11.
The predominant position of the Laboulbeniales on the host might be related to the absence or presence of a haustorium. Thus, the haustoriate species of the genus Arthrorhynchus are most frequently encountered in large numbers on the arthrodial membranes of the host’s abdomen, although some thalli are found on legs2,22. At the arthrodial membranes the cuticle is more flexible and therefore might be easier to penetrate by a parasite. Furthermore, most tissues providing/storing nutrition (e.g., fat body) are located within the abdomen. In contrast, non-haustoriate fungi as are often located on more stiff and sclerotized body-parts like the genus Rickia on the legs or body-rings of millipedes7,20,23 or the genus Laboulbenia on the elytra of beetles21,24. A reason for this might be that the non-haustoriate forms, which are only superficially attached to the host need a more or less smooth surface for adherence and can easily become detached from a flexible surface, which is movable in itself, like the arthrodial membrane, while the haustoriate forms are firmly anchored within the hosts’ cuticle.
Whereas the vast majority of the more than 2000 described species of Laboulbeniales show no sign of host penetration, haustoria have been reported from some other genera18, including Trenomyces parasitizing bird lice25,26, Hesperomyces growing on coccinellid beetles and Herpomyces on cockroaches (formerly a Laboulbeniales and now in the order Herpomycetales10), with pernicious consequences on the hosts’ fitness18,27. Micro-CT studies on these genera could help to understand the host penetration. In order to fully understand how Laboulbeniales obtain nourishment, although other approaches are, also needed—for the time being it remains a mystery how the non-haustoriate Laboulbeniales sustain themselves.
Methods
Specimens used
All specimens were obtained from the collection of the Natural History Museum of Denmark. Arthrorhynchus nycteribiae (Peyr.) Thaxt. on Penicillidia conspicua Speiser, 1901, a male bat fly, infected on the dorsal part of the abdomen, 21.07.2018, Igrejinha de Soídos, Algarve, Portugal, L. Rodrigues & S. Reboleira leg. (ref. SR-Nyct85); Rickia gigas Santam., Enghoff & Reboleira on Tropostreptus hamatus (Demange, 1977), a male millipede heavily infected on the legs, Udzungwa Mountains Natural Park, Sanje Chini camp, 598 m, 17-20.01.2014, Thomas Pape leg.
Specimens were initially examined under a binocular stereomicroscope Leica M165C, and measurements were made with the software Leica Application Suite V4.12. Fungal thalli were dissected and mounted on temporary slides in glycerine for morphological taxonomic study, following the standard methodology8, under light microscopy in a Leica DM2500 microscope with Differential Interference Contrast (DIC).
Micro-computed tomography (µCT)
Specimens for micro-computed tomography (µCT) were transferred to 100% ethanol, stained for 24 h in 3% Iodine-solution and washed subsequently with 100% ethanol. These specimens were then critical point dried (CPD) with a Leica EM CPD 300, mounted in a pipette tip and scanned using a SKYSCAN 1272 (Bruker microCT) with the following scanning parameters: source voltage = 25 kV, source current = 130 μA, exposure = 4000 ms, rotation of 360° in rotational steps of 0.2°, frame averaging = 7, random movement = 15, filter = none, pixel size = 0.8 µm. Post-alignment, ring-artefact reduction, beam-hardening correction and reconstruction was performed in NRecon 1.7.1.6 (Bruker microCT). The image stack was modified using Fiji ImageJ 1.50e28 (https://www.imagej.net). Volume rendering and measurements were performed in Drishti Version 2.6.329 (https://www.github.com/nci/drishti), and segmentation of the thallus and the haustorium was done in ITKSnap 3.8.030 (http://www.itksnap.org), within the host the lumen supposedly created by the haustorium was segmented. Smoothing, rendering and animation was performed in MeshLab 1.3.331 (https://www.meshlab.net) and Blender 2.77 (Blender Foundation; https://www.blender.org). Figures were prepared in GIMP 2.10.6 (GIMP Development Team; https://www.gimp.org) and Inkscape 1.0 (Inkscape Developers; https://inkscape.org). The generated µCT-data is deposited on Zenodo under doi: 10.5281/zenodo.4737626.
Scanning electron microscopy (SEM)
Specimens for SEM were transferred to 100% ethanol, critical point-dried in a Tousimis Autosamdri 815 Series A critical point dryer, mounted on an aluminium stub, coated with platinum/palladium and studied under a JEOL JSM-6335F scanning electron microscope.
References
Sulivan, D. Hyperparasitism. in Encyclopedia of Insects. 486–488. (Academic Press, 2009)
Jensen, K. M. et al. Hyperparasitism in caves: Bats, bat flies and ectoparasitic fungus interaction. J. Invertebr. Pathol. 166, 107206. https://doi.org/10.1016/j.jip.2019.107206 (2019)
Haelewaters, D. et al. Parasites of parasites of bats: Laboulbeniales (Fungi: Ascomycota) on bat flies (Diptera: Nycteribiidae) in central Europe. Parasit. Vectors. 10(1), 96. https://doi.org/10.1186/s13071-017-2022-y (2017).
Haelewaters, D., Page, R. & Pfliegler, W. P. Laboulbeniales hyperparasites (Fungi, Ascomycota) of bat flies: Independent origins and host associations. Ecol. Evol. 8(16), 8396–8418. https://doi.org/10.1002/ece3.4359 (2018).
Haelewaters, D. et al. Studies of Laboulbeniales on Myrmica ants (IV): Host-related diversity and thallus distribution patterns of Rickia wasmannii. Parasite https://doi.org/10.1051/parasite/2019028 (2019).
Santamaria, S., Enghoff, H., Gruber, J. & Reboleira, A. S. P. S. First Laboulbeniales from harvestmen: The new genus Opilionomyces. Phytotaxa 305(4), 285–292. https://doi.org/10.11646/phytotaxa.305.4.4 (2017).
Santamaria, S., Enghoff, H. & Reboleira, A. S. P. S. New species of Troglomyces and Diplopodomyces (Laboulbeniales, Ascomycota) from millipedes (Diplopoda). Eur. J. Taxon. 429, 1–20. https://doi.org/10.5852/ejt.2018.429 (2018).
Reboleira, A. S. P. S., Enghoff, H. & Santamaria, S. Novelty upon novelty visualized by rotational scanning electron micrographs (rSEM): Laboulbeniales on the millipede order Chordeumatida. PLoS ONE 13(11), e0206900. https://doi.org/10.1371/journal.pone.0206900 (2018).
Goldmann, L. & Weir, A. Molecular phylogeny of the Laboulbeniomycetes (Ascomycota). Fungal Biol. 122, 87–100. https://doi.org/10.1016/j.funbio.2017.11.004 (2018).
Haelewaters, D., Pfliegler, W., Gorczak, M. & Pfister, D. Birth of an order: Comprehensive molecular phylogenetic study excludes Herpomyces (Fungi, Laboulbeniomycetes) from Laboulbeniales. Mol. Phylogenet. Evol. 133, 286–301. https://doi.org/10.1016/j.ympev.2019.01.007 (2019).
Haelewaters, D., Blackwell, M. & Pfister, D. H. Laboulbeniomycetes: Intimate fungal associates of arthropods. Annu. Rev. Entomol. 66, 257–276. https://doi.org/10.1146/annurev-ento-013020-013553 (2021).
Blackwell, M., Haelewaters, D. & Pfister, D. H. Laboulbeniomycetes: Evolution, natural history, and Thaxter’s final word. Mycologia 112(6), 1192–1202. https://doi.org/10.1080/00275514.2020.1718442 (2020).
Sundberg, H., Kruys, Å., Bergsten, J. & Ekman, S. Position specificity in the genus Coreomyces (Laboulbeniomycetes, Ascomycota). Fungal Syst. Evol. 1(1), 217–228. https://doi.org/10.3114/fuse.2018.01.09 (2018).
Szentiványi, T. et al. Laboulbeniales (Fungi: Ascomycota) infection of bat flies (Diptera: Nycteribiidae) from Miniopterus schreibersii across Europe. Parasit. Vectors 11(1), 395. https://doi.org/10.1186/s13071-018-2921-6 (2018).
Szentiványi, T. et al. Climatic effects on the distribution of ant- and bat fly-associated fungal ectoparasites (Ascomycota, Laboulbeniales). Fungal Ecol. 39, 371–379. https://doi.org/10.1016/j.funeco.2019.03.003 (2019).
Csősz, S. et al. Ectoparasitic fungi Rickia wasmannii infection is associated with smaller body size in Myrmica ants. Sci. Rep. 11, 14355. https://doi.org/10.1038/s41598-021-93583-0 (2021).
Perreau, M., Haelewaters, D. & Tafforeau, P. A parasitic coevolution since the Miocene revealed by phase-contrast synchrotron X-ray microtomography and the study of natural history collections. Sci. Rep. 11, 2672. https://doi.org/10.1038/s41598-020-79481-x (2021).
Tragust, S., Tartally, A., Espadaler, X. & Billen, J. Histopathology of Laboulbeniales (Ascomycota: Laboulbeniales): Ectoparasitic fungi on ants (Hymenoptera: Formicidae). Myrmecol. News 23, 81–89 (2016).
Enghoff, H. A new East African genus of spirostreptid millipedes (Diplopoda, Spirostreptida, Spirostreptidae), with notes on their fungal ectoparasite Rickia gigas. Zootaxa 4273(4), 501–530. https://doi.org/10.11646/zootaxa.4273.4.3 (2017).
Santamaria, S., Enghoff, H. & Reboleira, A. S. P. S. Hidden biodiversity revealed by collections-based research—Laboulbeniales in millipedes: Genus Rickia. Phytotaxa 243(2), 101–127. https://doi.org/10.11646/phytotaxa.243.2.1 (2016).
Scheloske, H. W. Beiträge zur Biologie, Ökologie und Systematik der Laboulbeniales (Ascomycetes) unter besonderer Berücksichtigung des Parasit-Wirt-Verhältnisses. Parasitol. Schriftenreihe 19, 1–176 (1969).
Blackwell, M. Incidence, host specificity, distribution and morphological variation in Arthrorhynchus nyctyeribiae and Arthrorhynchus eucampsipodae (Laboulbeniomycetes). Mycologia 72(1), 143–158. https://doi.org/10.2307/3759427 (1980).
Santamaria, S., Enghoff, H. & Reboleira, A. S. P. S. Laboulbeniales on millipedes: The genera Diplopodomyces and Troglomyces. Mycologia 106(5), 1027–1038. https://doi.org/10.3852/13-381 (2014).
Santamaria, S. New or interesting Laboulbeniales (Fungi, Ascomycota) from Spain, V. Nova Hedwigia 82(3–4), 349–363 (2006).
Meola, S. & Devaney, J. Parasitism of mallophaga by Trenomyces histophtorus. J. Invertebr. Pathol. 28, 195–201. https://doi.org/10.1016/0022-2011(76)90122-1 (1976).
Meola, S. & Tavares, I. I. Ultrastructure of the haustorium of Trenomyces histophthorus and adjacent host cells. J. Invertebr. Pathol. 40, 205–215 (1982).
Szentiványi, T. Effects of fungal infection on the survival of parasitic bat flies. Parasites Vectors 13(1), 1–8. https://doi.org/10.1186/s13071-020-3895-8 (2020).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. https://doi.org/10.1038/nmeth.2019 (2012).
Limaye, A. Drishti: A volume exploration and presentation tool. in Developments in X-Ray Tomography VIII. (Stock, S.R. Ed.). Proceedings of SPIE. Vol. 8506. 85060. https://doi.org/10.1117/12.935640 (2012).
Yushkevich, P. A. et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 31(3), 1116–1128. https://doi.org/10.1016/j.neuroimage.2006.01.015 (2006).
Cignoni, P. et al. Meshlab: An open-source mesh processing tool. in Eurographics Italian chapter conference. 129–136 (2008).
Acknowledgements
This work is supported by a research grant (15471) from the Villum Fonden, and by Portuguese National Funds through “Fundação para a Ciência e Tecnologia” (FCT) within the cE3c Unit funding UIDB/00329/2020. LM is funded within the scope of the project “Phylogeny of the Diplopoda: micro-CT scans, morphology and morphometry of all millipede orders”, German Research Foundation (DFG), Project WE 2479/4-1 by Prof. Dr. A. Blanke (University of Bonn) and Dr. Thomas Wesener (ZFMK). LM thanks Thomas Wesener (ZFMK) and Alexander Blanke (University of Bonn) for support and encouragement. We are grateful to Dr. Luísa Rodrigues from the Portuguese Institute for Nature Conservation and Forests (ICNF) for support in collecting nycteribiids under the National Bats Monitoring Program in Portugal. All the specimens from Portugal were collected under permits of the ICNF.
Author information
Authors and Affiliations
Contributions
All authors contributed to the interpretation of results and manuscript production. A.S.P.S.R. and H.E. conceived and coordinated the study, provided resources and wrote the first draft; A.S.P.S.R. performed SEM analysis and funding acquisition; L.M. performed µCT analysis; S.S. participated in the design of the study and provided critical revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Reboleira, A.S.P.S., Moritz, L., Santamaria, S. et al. Penetrative and non-penetrative interaction between Laboulbeniales fungi and their arthropod hosts. Sci Rep 11, 22170 (2021). https://doi.org/10.1038/s41598-021-01729-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-01729-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.