Abstract
Few studies have been conducted on group B Streptococcus (GBS) in Vietnam. We determined the GBS colonization and antimicrobial resistance vaginal-rectal profile of 3863 Vietnamese pregnant women over 5 years. Maternal GBS colonization was characterized by antibiotic susceptibility. Overall, the GBS colonization rate was 8.02% (95% CI: 7.20–8.94%). Compared to sampling ≥ 35 weeks of gestation, the GBS colonization rate was statistically higher (p = 0.004) with sampling < 35 weeks. Among 272 antimicrobial susceptibility testing isolates, all were susceptible to ampicillin, penicillin, ceftriaxone, cefotaxime, vancomycin, and quinupristin/dalfopristin. Resistance was highest for tetracycline (89.66%), followed by erythromycin (76.23%) and clindamycin (58.21%). Multidrug resistance and resistance to ≥ 6 different antibiotics were 60.66% and 8.82%, respectively. Resistance to clindamycin but not erythromycin (L phenotype) was 2.2%. The clindamycin resistance rate was significantly increased (p = 0.005) during the study period. These data demonstrate a low rate of maternal GBS colonization. The high rate of erythromycin, clindamycin, and multidrug resistance to GBS that can be transmitted to neonates is an important risk factor to consider. β-lactams continue to be appropriate for first-line treatment and prophylaxis in the study area. Ongoing monitoring should be considered in the future.
Similar content being viewed by others
Introduction
Group B Streptococcus (GBS) causes severe early-onset infection in newborns. GBS is a Gram-positive, catalase-negative coccus found in pairs and chains on Gram staining1. GBS causes neonatal mortality and significant morbidity, including meningitis and sepsis2. Maternal GBS colonization relates to preterm birth, pregnancy loss3, and stillbirths4. GBS colonization among pregnant women has an average of 18%, ranging from 11 to 35%, totalling 21.7 million in 195 countries5. Maternal GBS colonization can be transmitted to neonates before or during birth6. The maternal GBS colonization rates vary geographically5. The Centers for Disease Control and Prevention (CDC) recommends screening for GBS colonization in all pregnant women to prevent newborn infection7. GBS monitoring provides information for decision-making, planning, prevention, and control strategies8,9,10.
Antibiotics are a vital resource to treat bacterial infections. Thus, antibiotic-resistant bacteria are a public health concern. The reduced susceptibility rate to penicillin and ampicillin, the first-choice drugs for the prevention and treatment of maternal and neonate GBS infections, is increasing11,12,13,14. Erythromycin and clindamycin are the second-choice drugs, particularly in cases of penicillin allergy7. However, resistance to such antibiotics has increased in recent years, raising concerns about their use as alternatives11,13,14. Resistance to other classes of antibiotics continues to increase, making it necessary to monitor antimicrobial resistance globally15. Resistance data could (1) provide evidence to improve clinical practice; (2) help to develop antimicrobial-prescribing guidelines; and (3) aid prospective interventions (such as antimicrobial prophylaxis)16.
Maternal GBS colonization and antibiotic resistance profiles vary geographically8. Few studies have been conducted on GBS in Vietnam. To date, only one study reported that the maternal GBS colonization rate in a province of Vietnam was 9.2%17. To contribute to regional data on maternal GBS colonization and antibiotic resistance profiles, an observational study of Vietnamese pregnant women was conducted at a hospital in Hanoi, Vietnam. These findings may provide information to guide disease prevention practices in developing countries. In Vietnam, the infrequent use of intrapartum antibiotic prophylaxis (IAP) and the underestimation of neonatal morbidity and mortality are of concern.
Results
GBS colonization and maternal GBS colonization characteristics
Table 1 provides the baseline characteristics of maternal GBS colonization. A total of 3,863 pregnant women participated in this study. The rate of GBS colonization was 8.02% (95% CI: 7.20–8.94%). The rates of GBS colonization of pregnant women at ≥ 35 weeks and < 35 weeks of gestational age were 3.34% (95% CI: 2.82–3.95%) and 4.66% (95% CI: 4.04–5.37%), respectively. The rate of maternal GBS colonization sampled at < 35 weeks was significantly higher than that sampled at ≥ 35 weeks gestation (p = 0.004).
Maternal GBS colonization was tested in women between 18 and 54 years old (mean 30.49 ± 6.44 years). The preterm birth and stillbirth rates were 1.94% and 3.87%, respectively. Statistically significant differences were found between the two gestational age groups with respect to preterm birth (p = 0.043), stillbirth (p = 0.036), primiparity, in vitro fertilization and gestational age (p < 0.001). However, the mean age of pregnant women in the ≥ 35 weeks gestation group and < 35 weeks gestation group was not significantly different (p = 0.15). The same result was found for the second parity (p = 0.329), the third parity (p = 1), the fourth parity (p = 0.53) and the 5th parity (p = 0.42).
Most of the women with maternal GBS colonization were self-employed (103, 33.23%), followed by office workers (77, 24.84%) and teachers (62, 20%). No statistically significant differences were found in the occupation between the two groups by gestational age (p > 0.05).
Antimicrobial resistance
Table 2 provides the antibiotic-resistant rate of maternal GBS colonization. Among the 310 GBS colonization cases, 272 were tested for antibiotic susceptibility (AST), accounting for 87.74% (95% CI: 83.44–91.08%). All isolates were sensitive to ampicillin, penicillin, ceftriaxone, cefotaxime, vancomycin, and quinupristin/dalfopristin. Resistance to tetracycline was the highest at 89.66% (234/261), followed by erythromycin at 76.23% (202/265), clindamycin at 58.21% (156/268), chloramphenicol at 52.38% (22/42), and levofloxacin at 28.46% (74/260). No statistically significant differences were found for antibiotic resistance rates between the two maternal GBS colonization groups (p > 0.05).
Multidrug resistance (MDR) was found in 60.66% (165/272), and 8.82% (24/272) of isolates were resistant to six to seven antibiotics tested. We found a statistically significant difference in the MDR rate between the two maternal GBS colonization groups (p = 0.023).
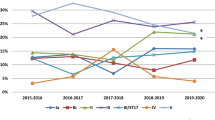
Clindamycin-resistant but erythromycin-sensitive (L phenotype) was found in 2.2% (6/272). Clindamycin resistance significantly increased from 41.3% of isolates in 2016 to 65.69% in 2019 (p for trend = 0.005). Erythromycin resistance decreased from 84.78% of isolates in 2016 to 82.18% in 2019, but the difference was not statistically significant (p for trend = 0.709) (Fig. 1).
Clindamycin and erythromycin resistance rates among Group B Streptococcus isolates by year. *Clindamycin (n = 102) and erythromycin (n = 101); in 2020, due to the COVID-19 pandemic in Vietnam, only 12 GBS strains (< 30) were collected; thus, the clindamycin and erythromycin resistance rates were not calculated.
Discussion
To date, the rates of GBS colonization and antibiotic resistance in many countries, including Vietnam, are not known. Due to the potential for mother-to-child transmission, screening for GBS in pregnant women is necessary. These data are essential for future GBS interventions. The rate of maternal GBS colonization in this study was 8.02%, which was comparable with India (7.8%)18, Korea (9.09%)8, and Nghe An, Vietnam (9.2%)17. Our result is high compared to the reported rate of maternal SGB colonization in China (3.5%)19 and Japan (5.7%)20. However, it was low compared to Serbia (15.6%)21, Brazil (17.2%)22, and Indonesia (30%)23. The difference in the rate of GBS colonization can be explained by differences in the region, ethnicity, socioeconomic status, methodological questions, sampling site, laboratory culture techniques, mother’s age, and gestational age6,8,24. International standardized sampling for maternal GBS colonization has not been established6. The use of standard sampling, detection, and interpreting procedures may contribute to differences in the GBS incidence. We recommend that GBS screening protocols be considered preventative strategies for pregnant Vietnamese women.
In a systematic review and meta-analysis, Russell et al. reported that among studies reporting the GBS colonization prevalence, 82 (26%) were sampled at delivery, and 94 (30%) were sampled from women < 35 weeks of gestation6. In a systematic review carried out recently, Abbasalizadeh et al. reported that the prevalence of GBS in pregnant women sampled before the 35th week was greater than that in women sampled after the 35th week25. In this context, we separated Vietnamese maternal GBS colonization into two groups, i.e., ≥ 35 versus < 35 weeks of gestation to investigate the differences in the GBS colonization and antibiotic resistance rates between them.
Our results showed that the maternal GBS colonization rate sampled < 35 weeks (4.66%) was higher than that of the ≥ 35 weeks of gestation sampled (3.34%) and the difference was statistically significant (p = 0.004). This result is consistent with some studies6,26 but inconsistent with others27. This variation may be due to differences in demographics, sexual activity during pregnancy, douching habits, and the transient nature of colonization26,28. These factors may alter the vaginal microbiota or disturb the vaginal mucosa to promote colonization of GBS. These findings require confirmation in the future.
Penicillin and ampicillin are the first-choice drugs for the prevention and treatment of maternal GBS infections7; however, reduced sensitivity to penicillin and ampicillin is becoming increasingly common11,12,13,14. In this study, all GBSs were susceptible to penicillin, ampicillin, and vancomycin. Thus, these antibiotics would be appropriate for the prophylaxis and treatment of maternal GBS colonization in this study area. Macrolides and lincosamides share similar binding sites, so resistance to one class often crosses the others29. Thus, GBS resistance to clindamycin but not to erythromycin (L phenotype) is rare. However, the L phenotype has been documented2. In this study, the rate of the L phenotype was 2.2%, which was higher than that in a previous study (0.31%)2. This finding suggests that laboratories should consider examining the L phenotype. To our knowledge, this is the first report on the L phenotype among GBS strains of pregnant Vietnamese women.
Erythromycin and clindamycin are the second-choice drugs for colonization of GBS in women allergic to penicillin7. However, the antibiotic resistance rate remains high and has increased in recent years. Resistance to other classes of antibiotics (fluoroquinolone and tetracycline) continues to increase, which will cause clinical problems. If patients are allergic to penicillin and the second-choice drugs are not effective, the antibiotic of last resort, vancomycin, is used. However, resistance to vancomycin within GBS has been reported15. The rate of resistance to vancomycin in GBS is low, possibly due to the lack of testing for vancomycin susceptibility. In addition, resistance to vancomycin may increase, and this possibility is a concern. Therefore, vancomycin resistance in GBS requires monitoring.
In this study, 76.23% and 58.21% of GBS strains were resistant to erythromycin and clindamycin, respectively. These resistance rates were higher than that of previous studies, e.g., 26.7% and 22.1%21, 36.8%, and 7.7%30, 50.7%, and 38.4%31, indicating that more pregnant women in Vietnam are at risk. High rates of erythromycin and clindamycin resistance threaten the health care of patients, limiting the options to treat mothers and newborns who are colonized with GBS. Further steps are thus required to protect the mother and newborn. These data suggest that clindamycin and erythromycin may be ineffective in treating GBS infections in Vietnamese pregnant women. Therefore, using clindamycin and erythromycin as empirical antimicrobial therapy may be prudent. The high rate of resistance to clindamycin and erythromycin is alarming and requires further investigation.
In pregnant women with penicillin allergies, erythromycin and clindamycin resistance should be tested, and only clindamycin should be reported32. The results from this study demonstrate that clindamycin resistance has increased over time (p for trend = 0.005), which is consistent with a previous study33. Therefore, the choice of an alternative prophylactic antibiotic for patients with penicillin allergies should be based on antibiotic resistance profiles in each region.
The GBS resistance to different antibiotic classes in this study is worrying and indicates that alternative treatment strategies are warranted sooner than later. The antibiotic resistance rates were tetracycline (89.66%), chloramphenicol (52.38%), and levofloxacin (28.46%). The resistance to tetracycline, chloramphenicol, and levofloxacin was high compared with previous studies34,35,36. Monitoring the levels of antibiotic resistance is therefore important. High rates of multidrug resistance (60.66%) and 8.82% resistance to 6–7 antimicrobials tested suggest that antibiotic susceptibility should be determined. The high rate of MDR in GBS is alarming and justifies further investigations.
Due to the lack of resources, there are several limitations to this research. First, the serotype distribution of isolates was not determined. As such, data on the serotype distribution of GBS in Vietnam remains insufficient for vaccine development. Second, although GBS genotypic analysis is useful for AST verification, it could not be conducted due to limited resources. As the GBS strains were not cryopreserved, retrospective genotypic analyses were impossible to perform. However, the rate of antibiotic resistance highlights the present problem in Vietnam and raises important questions for access to molecular methods in low- and middle-income countries. Third, the frequency of mother-to-newborn transmission of GBS was not estimated, and risk factors for both outcomes were not identified. Only detailed information on maternal GBS colonization was collected; thus, the baseline characteristics of mothers who were not colonized with GBS were not fully elucidated. We carried out convenience sampling in one hospital, so the results may not represent all pregnant women in Vietnam.
Despite these limitations, this is the first study in Vietnam over 5 years to show the maternal colonization and antibiotic resistance profiles of GBS. A larger multisite study on neonatal transmission, serotype distribution, and molecular characterization of GBS is required.
Conclusion
The GBS colonization rate in this study was low and within the range of other global studies. The resistance to erythromycin and clindamycin and the multidrug resistance rate were high. Clindamycin resistance increased significantly throughout the study period. Penicillin and ampicillin are the drugs of choice for preventing and treating GBS-related maternal infections in the study area. Ongoing monitoring of newborn transmission, serotype distribution, and molecular characterization of GBS should be considered in the future.
Materials and methods
Ethics
This study was approved by the Institutional Review Board of Vietnam Military Medical University, Hanoi, Vietnam (VMMU-IEC-AMR-03–20151605-V3). All methods were carried out in compliance with the Declaration of Helsinki. Written informed consent was obtained from all study participants.
Study area
This study was conducted at the National Hospital of Obstetrics and Gynaecology (NHOG) in Hanoi, Vietnam, between January 2016 and December 2020. NHOG is the largest reference hospital in northern Vietnam, with approximately 1500 beds. The hospital provides primary and tertiary care to almost all pregnant women in northern Vietnam and treats pregnant women with associated diseases.
Study design
This observational study was conducted from January 2016 to December 2020 at NHOG, Vietnam. Pregnant women who attended the National Hospital of Obstetrics and Gynaecology (NHOG) in Hanoi, Vietnam, were enrolled. Pregnant women were included in the study if they had not used antibiotics in the last 3 weeks and provided informed consent.
Specimen collection, handling, and transport
Vaginal-rectal swabs were collected from pregnant women before vaginal examinations and as described previously24. Briefly, a single flocked swab (BD Diagnostics, Korea) was used to collect the vaginal-rectal specimens. First, the swab was inserted ~ 2 cm without the use of a speculum into the vagina, and then the same swab was inserted ~ 1 cm through the anal sphincter. After specimen collection, the swabs were immediately inserted into Amies transport medium (Thermo Scientific™, Singapore) and transported to the Medical Microbiology Department at NHOG within 4 h.
GBS identification
The samples were processed as recommended by the American Society for Microbiology’s guidelines24. Briefly, after incubating the swabs in Todd-Hewitt broth (Thermo Scientific™, Singapore) aerobically at 37 °C for 18–24 h, 10 μl of each broth was subcultured on Columbia agar plates with 5% sheep blood (Oxoid, Singapore). The plates were incubated for 24 h at 37 °C in 5% CO2. If GBS was not detected, the blood agar plate was incubated and examined after 48 h. All suspected GBS appeared as either beta-haemolytic or nonhemolytic, and Gram-positive cocci and catalase-negative cocci were taken for the CAMP (Christie–Atkins–Munch-Peterson) test. Streptococcus pyogenes ATCC 19615, Streptococcus agalactiae ATCC12386, and Staphylococcus aureus ATCC 25923 (Thermo Scientific™, Singapore) were the controls. All colonies that yielded a positive CAMP were considered GBS. Positive CAMP test results were confirmed using the Streptex™ Latex Agglutination test (Thermo Scientific™, Singapore) according to the manufacturer’s instructions. This latex agglutination test provides a complete solution for the isolation and differentiation of Lancefield Groups A, B, C, D, F, and G.
Antimicrobial susceptibility testing
The antimicrobial resistance of GBS was determined through the disk diffusion method (Kirby-Bauer), Vitek 2 using the AST-ST03 card (bioMérieux, France), or E-test (bioMérieux, France). The clinical isolates were tested for susceptibility to 11 different antibiotics, including ampicillin 10 µg, penicillin 10 units, cefotaxime 30 µg, ceftriaxone 30 µg, vancomycin 30 µg, quinupristin/dalfopristin 15 µg, tetracycline 30 µg, erythromycin 15 µg, clindamycin 2 µg, chloramphenicol 30 µg, and levofloxacin 5 µg (Oxoid, Singapore). Streptococcus pneumoniae ATCC 49619 was the control.
The GBS colonies from an overnight (18 to 20 h) sheep blood agar plate were suspended in 5 ml of sterile saline, and the turbidity was adjusted to 0.5 McFarland using the DensiCHEK Plus instrument (bioMérieux, France). The GBS suspension was streaked over the dried surface of 5% sheep blood Mueller–Hinton agar plates (bioMérieux, France) using a sterile swab. The antibiotic disks/or E-tests were placed on the plates and incubated for 20–24 h at 37 °C in 5% CO2. After incubation, the inhibition zone around the disks/or E-test was analysed. Vitek 2 susceptibility testing was performed according to the manufacturer’s instructions with the same bacterial suspension using the AST-ST03 card. The results were analysed and interpreted by AES 4.02 software. The isolates were considered susceptible, resistant, or intermediate according to the recommendations by the CLSI (Clinical and Laboratory Standards Institute)32. Multidrug resistance was defined as resistance to at least three antibiotics in at least three antimicrobial classes.
Data analysis
Maternal GBS colonization was calculated for the entire study population. All data were separated by gestational age group (35 vs. < 35 weeks). The results of antibiotic resistance indicated the percentage of isolates tested resistant to certain antimicrobials for each identified phenotype. The clindamycin and erythromycin resistance percentages each year were only calculated when the number of isolates was ≥ 30 to ensure a minimum level of precision in the calculation.
Statistical analysis
Statistical analyses were performed using R 4.0.537. The chi-square or Fisher's exact test was used to compare categorical variables; Fisher's exact test was used for variables with less than five in at least one cell. The chi-square test for trend was used to examine trends across the study period. For continuous variables, the Kolmogorov–Smirnov test or the Shapiro–Wilk test (depending on the data number) was used to test for normality; the Wilcoxon rank-sum test (two-tailed) was used for nonnormally distributed data; Student’s t-test (two-tailed) was used for normally distributed data. A p value below 0.05 was considered statistically significant.
Data availability
The data supporting the reported results are available on request.
References
Six, A. et al. Molecular characterization of nonhemolytic and nonpigmented group B Streptococci responsible for human invasive infections. J. Clin. Microbiol. 54, 75–82. https://doi.org/10.1128/JCM.02177-15 (2016).
Hawkins, P. A. et al. Cross-resistance to lincosamides, streptogramins A and pleuromutilins in Streptococcus agalactiae isolates from the USA. J. Antimicrob. Chemother. 72, 1886–1892. https://doi.org/10.1093/jac/dkx077 (2017).
van den Berg, A. W. V., Sprij, A. J., Dekker, F. W., Dorr, P. J. & Kanhai, H. H. Association between colonization with group B Streptococcus and preterm delivery: A systematic review. Acta Obstet. Gynecol. Scand. 88, 958–967. https://doi.org/10.1080/00016340903176800 (2009).
Seale, A. C. et al. Estimates of the burden of group B streptococcal disease worldwide for pregnant women, stillbirths, and children. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 65, S200–S219. https://doi.org/10.1093/cid/cix664 (2017).
Organization, W. H. Group B Streptococcus infection causes an estimated 150,000 preventable stillbirths and infant deaths every year. https://www.who.int (2017).
Russell, N. J. et al. Maternal colonization with group B Streptococcus and serotype distribution worldwide: Systematic review and meta-analyses. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am. 65, S100–S111. https://doi.org/10.1093/cid/cix658 (2017).
Verani, J. R. et al. Prevention of perinatal group B streptococcal disease—Revised guidelines from CDC, 2010. MMWR. Recomm. Rep.: Morb. Mortal. Wkly. Rep. Recomm. Rep. 59, 1–36 (2010).
Bobadilla, F. J., Novosak, M. G., Cortese, I. J., Delgado, O. D. & Laczeski, M. E. Prevalence, serotypes and virulence genes of Streptococcus agalactiae isolated from pregnant women with 35–37 weeks of gestation. BMC Infect. Dis. 21, 73. https://doi.org/10.1186/s12879-020-05603-5 (2021).
Hays, C. et al. Changing epidemiology of group B Streptococcus susceptibility to fluoroquinolones and aminoglycosides in France. Antimicrob. Agents Chemother. 60, 7424–7430. https://doi.org/10.1128/AAC.01374-16 (2016).
Vuillemin, X. et al. Invasive group B Streptococcus infections in non-pregnant adults: A retrospective study, France, 2007–2019. Clin. Microbiol. Infect.: Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 27(129), e121-129 e124. https://doi.org/10.1016/j.cmi.2020.09.037 (2021).
Puopolo, K. M. et al. Management of infants at risk for group B Streptococcal disease. Pediatrics https://doi.org/10.1542/peds.2019-1881 (2019).
Yang, L., Bao, F., Wu, Y. & Sun, L. Relationship of group B Streptococcus colonization in late pregnancy with perinatal outcomes. Zhejiang da xue xue bao. Yi xue ban = J. Zhejiang Univ. Med. Sci. 49, 389–396. https://doi.org/10.3785/j.issn.1008-9292.2020.06.05 (2020).
Genovese, C., D’Angeli, F., Di Salvatore, V., Tempera, G. & Nicolosi, D. Streptococcus agalactiae in pregnant women: Serotype and antimicrobial susceptibility patterns over five years in Eastern Sicily (Italy). Eur. J. Clin. Microbiol. Infect. Dis.: Off. Publ. Eur. Soc. Clin. Microbiol. 39, 2387–2396. https://doi.org/10.1007/s10096-020-03992-8 (2020).
Asghar, S., Khan, J. A., Mahmood, M. S. & Arshad, M. I. A cross-sectional study of group B Streptococcus-associated sepsis, coinfections, and antibiotic susceptibility profile in neonates in Pakistan. Adv. Neonatal Care: Off. J. Natl. Assoc. Neonatal Nurses 20, E59–E69. https://doi.org/10.1097/ANC.0000000000000701 (2020).
Hayes, K., O’Halloran, F. & Cotter, L. A review of antibiotic resistance in group B Streptococcus: The story so far. Crit. Rev. Microbiol. 46, 253–269. https://doi.org/10.1080/1040841X.2020.1758626 (2020).
Ji, W. et al. Disease burden and antimicrobial resistance of invasive group B Streptococcus among infants in China: A protocol for a national prospective observational study. BMC Infect. Dis. 17, 377. https://doi.org/10.1186/s12879-017-2475-9 (2017).
Hanh, T. Q. et al. Prevalence and capsular type distribution of group B Streptococcus isolated from vagina of pregnant women in Nghe An province, Vietnam. Iran. J. Microbiol. 12, 11–17 (2020).
Ashary, N., Singh, A., Chhabria, K. & Modi, D. Meta-analysis on prevalence of vaginal group B Streptococcus colonization and preterm births in India. J. Matern.-Fetal Neonatal Med.: Off. J. Eur. Assoc. Perinat. Med., Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. https://doi.org/10.1080/14767058.2020.1813705 (2020).
Ge, Y. et al. Prevalence of group B Streptococcus colonization in pregnant women in Jiangsu, East China. BMC Infect. Dis. 21, 492. https://doi.org/10.1186/s12879-021-06186-5 (2021).
Tano, S. et al. Relationship between vaginal group B Streptococcus colonization in the early stage of pregnancy and preterm birth: A retrospective cohort study. BMC Pregnancy Childbirth 21, 141. https://doi.org/10.1186/s12884-021-03624-9 (2021).
Kekic, D. et al. Trends in molecular characteristics and antimicrobial resistance of group B streptococci: A multicenter study in Serbia, 2015–2020. Sci. Rep. 11, 540. https://doi.org/10.1038/s41598-020-79354-3 (2021).
Santana, F. A. F. et al. Streptococcus agalactiae: Identification methods, antimicrobial susceptibility, and resistance genes in pregnant women. World J. Clin. Cases 8, 3988–3998. https://doi.org/10.12998/wjcc.v8.i18.3988 (2020).
Safari, D. et al. Prevalence, serotype and antibiotic susceptibility of group B Streptococcus isolated from pregnant women in Jakarta, Indonesia. PLoS ONE 16, e0252328. https://doi.org/10.1371/journal.pone.0252328 (2021).
Filkins, L. et al. American society for microbiology provides 2020 guidelines for detection and identification of group B Streptococcus. J. Clin. Microbiol. https://doi.org/10.1128/JCM.01230-20 (2020).
Abbasalizadeh, F. et al. Prevalence of group B Streptococcus in Vagina and rectum of pregnant women of Islamic & non-Islamic countries: A systematic review and meta-analysis. Iran. J. Public Health 50, 888–899. https://doi.org/10.18502/ijph.v50i5.6106 (2021).
Kwatra, G. et al. Serotype-specific acquisition and loss of group B Streptococcus recto-vaginal colonization in late pregnancy. PLoS ONE 9, e98778. https://doi.org/10.1371/journal.pone.0098778 (2014).
Ferjani, A., Abdallah, H. B., Saida, N. B., Gozzi, C. & Boukadida, J. Vaginal colonization of the Streptococcus agalactiae in pregnant woman in Tunisia: Risk factors and susceptibility of isolates to antibiotics. Bull. Soc. Pathol. Exot. 99, 99–102 (2006).
Sakru, N. et al. Does vaginal douching affect the risk of vaginal infections in pregnant women?. Saudi Med. J. 27, 215–218 (2006).
Leclercq, R. Mechanisms of resistance to macrolides and lincosamides: Nature of the resistance elements and their clinical implications. Clin. Infect. Dis. 34, 482–492. https://doi.org/10.1086/324626 (2002).
Schindler, Y. et al. Group B Streptococcus serotypes associated with different clinical syndromes: Asymptomatic carriage in pregnant women, intrauterine fetal death, and early onset disease in the newborn. PLoS ONE 15, e0244450. https://doi.org/10.1371/journal.pone.0244450 (2020).
Back, E. E., O’Grady, E. J. & Back, J. D. High rates of perinatal group B Streptococcus clindamycin and erythromycin resistance in an upstate New York hospital. Antimicrob. Agents Chemother. 56, 739–742. https://doi.org/10.1128/AAC.05794-11 (2012).
Wayne, P. A. Clinical and Laboratory Standards Institute (Wayne, 2018).
Slotved, H. C. & Hoffmann, S. The epidemiology of invasive group B Streptococcus in Denmark from 2005 to 2018. Front. Public Health 8, 40. https://doi.org/10.3389/fpubh.2020.00040 (2020).
Emaneini, M. et al. High incidence of macrolide and tetracycline resistance among Streptococcus agalactiae strains isolated from clinical samples in Tehran, Iran. Maedica 9, 157–161 (2014).
Shabayek, S. & Abdalla, S. Macrolide- and tetracycline-resistance determinants of colonizing group B Streptococcus in women in Egypt. J. Med. Microbiol. 63, 1324–1327. https://doi.org/10.1099/jmm.0.077057-0 (2014).
Guo, H. et al. Antimicrobial resistance and molecular characterization of Streptococcus agalactiae from pregnant women in southern China. J. Infect. Dev. Ctries. 13, 802–809. https://doi.org/10.3855/jidc.11395 (2019).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2021). https://www.R-project.org/.
Acknowledgements
We thank all study subjects for their participation.
Funding
This research was funded by the Do Xuan Hop Grant of Vietnam Military Medical University.
Author information
Authors and Affiliations
Contributions
N.T.V. conceived of the presented idea. N.D.T., V.V.D., and H.A.S. developed the theory and performed the computations. N.T.D., H.V.T., H.A.S., and N.T.V. contributed to the interpretation of the results. N.T.V., H.V.T., and H.A.S. verified the analytical methods and supervised the findings of this work. N.T.V. wrote the manuscript. H.V.T., N.T.D., C.V.M., N.L.T., and N.T.B. contributed to the final version of the manuscript. N.D.T., V.V.D., and H.A.S. helped supervise the project. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Van Du, V., Dung, P.T., Toan, N.L. et al. Antimicrobial resistance in colonizing group B Streptococcus among pregnant women from a hospital in Vietnam. Sci Rep 11, 20845 (2021). https://doi.org/10.1038/s41598-021-00468-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-00468-3
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.