Abstract
A cuffless blood pressure (BP) device (TestBP) using pulse transit time is in clinical use, but leads to higher BP values compared to a cuff-based 24 h-BP reference device (RefBP). We evaluated the impact of a recent software update on BP results and TestBP’s ability to differentiate between normo- and hypertension. 71 individuals had TestBP (Somnotouch-NIBP) and RefBP measurements simultaneously performed on either arm. TestBP results with software version V1.5 were compared to V1.4 and RefBP. Mean 24 h (± SD) BP for the RefBP, TestBP-V1.4 and TestBP-V1.5 were systolic 134.0 (± 17.3), 140.8 (± 20) and 139.1 (± 20) mmHg, and diastolic 79.3 (± 11.7), 85.8 (± 14.1) and 83.5 (± 13.0) mmHg, respectively (p-values < 0.001). TestBP-V1.5 area under the curve (95% confidence interval) versus RefBP for hypertension detection was 0.92 (0.86; 0.99), 0.94 (0.88; 0.99) and 0.77 (0.66; 0.88) for systolic and 0.92 (0.86; 0.99), 0.92 (0.85; 0.99) and 0.84 (0.74; 0.94) for diastolic 24 h, awake and asleep BP respectively. TestBP-V1.5 detected elevated systolic/diastolic mean 24 h-BP with a 95%/90% sensitivity and 65%/70% specificity. Highest Youden’s Index was systolic 133 (sensitivity 95%/specificity 80%) and diastolic 87 mmHg (sensitivity 81%/specificity 98%). The update improved the agreement to RefBP. TestBP was excellent for detecting 24 h and awake hypertensive BP values but not for asleep BP values.
Similar content being viewed by others
Introduction
Hypertension is a global healthcare burden and the most important modifiable risk factor for cardiovascular disease and stroke1. Detecting and managing arterial hypertension is a daily task in medicine. However, much controversy exists on how blood pressure (BP) should be measured and how hypertension should be diagnosed and monitored. Different guidelines recommend non-invasive oscillometric, cuff-based, 24 h ambulatory BP pressure monitoring for the accurate diagnosis of arterial hypertension using validated devices2,3. In general, patients are not keen on this BP measurement (BPM) method, finding the repeated inflations of the cuff, especially at night, uncomfortable and disruptive. The stimulation caused by the inflations, during sleep, could also cause the readings to be inaccurate due to sympathetic arousal, pressure on anatomical structures, and measurement artefacts caused by movement4,5.
Since 2015, a novel cuffless BP device using pulse transit time (PTT) for the beat-to-beat calculation of BP values has been in clinical use. The device was validated according to the ESH International Protocol Revision 2010 for the Validation of Blood Pressure Measuring Devices in Adults (ESH IP 2010)6. After initial calibration with a standard cuff-based measurement, this standard validation technique is carried out over an approximate 25-min period in a non-stimulating environment. This setting does not represent the usual circumstances with which the device is used, namely over a 24 h period with normal activities.
In our previous study, we compared the cuffless device (TestBP) to a standard cuff-based device (RefBP). The novelty of this study was that the measurements took place over a 24 h period with both devices simultaneously worn by the participant, capturing BPM during daily activities and sleep. A significant difference between the two devices was detected. In particular, the cuffless device revealed systematically higher systolic and diastolic values, especially during the asleep phase. This precluded that the values of the devices were not directly interchangeable in a clinical context and needs to be taken into account when implementing the cuffless device in a clinical setting7.
In 2018, the TestBP device manufacturer (Somnomedics GmbH, Randersacker Germany) updated the software algorithm for better BP calculation. With the present analysis, we aimed to investigate the impact of this software update on device accuracy compared to the RefBP. Furthermore, we evaluated diagnostic accuracy for the detection of hypertensive BP values to give clinical guidance and possibly derive device specific cut-off values against the gold standard (RefBP).
Experimental section
The study protocol complies with the Declaration of Helsinki, was approved by the local ethics committee, Ethikkommission Nordwest- und Zentralschweiz (Ethics Commission Northwest and Central Switzerland), (EKNZ 2017-00323), registered (NCT 03054688) and externally monitored. Informed consent was obtained from all participants.
Device details
Somnotouch-NIBP (Somnomedics GmbH, Randersacker, Germany) is a cuffless, non-invasive system which estimates BP based on the PTT technique (TestBP). Allowing for continuous beat-to-beat BP monitoring. Finger photoplethysmography, three ECG leads and a watch-like-device with integrated actigraph are the hardware of the device. Transit time of a pulse wave from the corresponding ECG R-wave to the finger photoplethysmography signal is measured6. Systolic and diastolic BP levels are calculated using non-linear modelling incorporating changes of the PTT and its relation to BP. The measurements are derived after an initial single-cuff based calibration measurement on the contralateral upper arm8. Increasing pulse wave propagation results in shorter PTT and is associated with higher BP values. Logically, decreasing pulse wave propagation and longer PTT is linked to lower BP values. Arterial wall stiffness and tension influence pulse wave propagation and PTT, both of which effect BP9,10. In this study the reference device (RefBP) was the cuff-based Spacelabs 90217A (Spacelabs Healthcare Inc., USA) 24 h BP monitor11. In July 2018, Somnomedics completed an update of the software. This update of the Domino light software version 1.4 led to the current version 1.5 (02/07/2018).
Study center
In April 2015, the cuffless device was introduced at the Medical Outpatient and Hypertension Clinic at the University Hospital Basel. The study team was trained to use the TestBP correctly and at the initiation of the study, the centre had two years of experience and had performed > 500 measurements. Experienced cardiologists (TB, AV) read all TestBP and RefBP measurements.
Enrolment
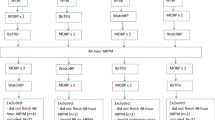
Enrolment took place at the Medical Outpatient and Hypertension Clinic at the University Hospital Basel between May and December 2017. The target sample consisted of consecutively recruited participants with an indication for a 24 h BPM as well as healthy volunteers. Distribution over pre-defined BP ranges and pre-specified recruitment numbers were defined prior to the initiation of the study as reported previously7. This included at least 15 participants in low (< 135 mmHg), intermediate (≥ 135 and < 150 mmHg) and high (≥ 150 mmHg) mean-awake systolic BP categories measured by the RefBP. BP categorisation for 24 h mean BP currently does not exist therefore the definition of low, intermediate and high categories was espoused from the National Institute of Health and Care Excellence clinical guidelines12,13. In addition to the BP category requirements, at least 15 male and 15 female subjects needed to be included. The ability to give informed consent and age ≥ 25 years as proposed by the ESH-IP 2010 were main inclusion criteria12. Exclusion criteria consisted of age < 25 years, a systolic BP difference > 10 mmHg between both upper extremities, atrial fibrillation at the time of enrolment and other medical reasons prohibiting BPM on the upper extremities. For evaluation of inter-arm differences, the following procedure took place: After 5 min of rest, the inter-arm BP difference was assessed sequentially with a single measurement on the right and left upper arm, in a sitting, upright position with legs uncrossed and back supported using the validated, cuff based, oscillometric Omron HBP-1300 device14,15.
Active recruitment took place until all recruitment categorizes were fulfilled.
Measurement procedure
Both devices were mounted on the participant in a sitting, upright position with legs uncrossed and back supported. An appropriately sized cuff was placed on the right arm and connected to the RefBP. The TestBP was placed on the left forearm and connected to the photoplethysmograph on the left index finger and the ECG electrodes according to manufacturer’s instructions. The first manually triggered RefBP measurement was taken after 5 min of rest. This measurement was used as a calibration measurement for the TestBP. The RefBP was programmed for measurements every 20 min from 08:00 to 22:00 and every 30 min during the remaining period. Simultaneously, the TestBP recorded beat-to-beat PTT according to the manufacturer’s instructions and standard programming. Participants were given questionnaires to individually record their activities and sleep schedules.
After completion of the 24 h measurement period, the RefBP measurements were analysed using its own standard software (Spacelabs Healthcare Inc, USA). A minimum of 27 BP values were required to deem the RefBP measurement valid. Otherwise, the data set was excluded16. The information about time in bed that we obtained from the patient protocol defined the asleep phase. Mean systolic and diastolic 24 h, awake and asleep values were calculated. TestBP measurements were evaluated twice using the incorporated standard software (Domino Light; Somnomedics GmbH). The first analysis (TestBP-V1.4) used the former software version (Domino Light Version V1.4; Somnomedics GmbH) and the second analysis (TestBP-V1.5) used the updated software version (Domino Light V1.5; Somnomedics GmbH). Automatic detection of artefacts during asleep time (feature included in the Somnotouch Domino light software package) were implemented. Only data sets, with < 50% artefact time, were deemed as valid and included in the final analysis. TB and AV chose the ECG lead with the best signal quality.
Standardised questionnaires were used to evaluate personal and medical factors including intake of antihypertensive medication and biometrics.
Statistical analysis
Distribution of continuous variables was determined using skewness, kurtosis and visual inspection of the histogram. Continuous data were presented as means (± standard deviations) and compared using paired t-tests. Categorical variables were described as counts (percentages) and compared using chi square tests.
Mean absolute BP differences between the RefBP vs. TestBP-V1.4 and RefBP vs. TestBP-V1.5 were obtained by reversing all negative values to positive values before calculation. Intraclass correlation coefficients between RefBP and both software versions (TestBP-V1.4 and TestBP-V1.5) were calculated to evaluate the general reliability index of a test–retest of the two devices. We followed the guidelines described by Koo and Li, with values less than 0.5, between 0.5 and 0.75, between 0.75 and 0.9, and greater than 0.90 indicating poor, moderate, good, and excellent reliability, respectively17.
Accuracy tables and Bland–Altman plots were adapted based on the ESH-2010 protocol18.
Receiver operating characteristic (ROC) curves were constructed to assess the sensitivity and specificity of TestBP-V1.5 and to detect hypertension in comparison to RefBP.
The diagnostic performance of the TestBP-V1.5 was further analysed by calculating Youden’s index and defining highest value as optimal systolic and diastolic cut-off value for mean 24 h, awake, asleep BP for the TestBP compared to RefBP. Youden’s index (J) was calculated as (J) = sensitivity + specificity − 1.
Cut-off values for RefBP were based on the 2018 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension using definitions of hypertension by office and out-of-office BP levels3.
Positive predictive value (PPV) and negative predictive value (NPV) of the TestBP measurements were calculated with sensitivity, specificity and the corresponding prevalence of elevated BP according to the RefBP measurements in our cohort of participants.
This study was designed and performed without patient involvement. Participants and or patients were not invited to remark on the study design and were not consulted to change patient relevant outcomes or interpret the results. Patients were not asked to contribute to the writing or editing of this manuscript for readability or accuracy.
Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC) and SPSS Version 22 (IBM), a p-value of < 0.05 was pre-specified to indicate statistical significance.
Results
Baseline characteristics
Consecutive enrolment of 83 individuals took place from May to December 2017. Twelve participants were excluded due to insufficient recording times, poor recording quality, technical problems, and atrial fibrillation7. Finally, a total of 71 participants’ data were analysed. Table 1 shows baseline characteristics. Mean systolic and diastolic BP (SD) of the calibration measurements were 139.5 (21.9) and 85.8 (15.1) mmHg, respectively.
Comparison of the RefBP versus TestBP-V1.4 and TestBP-V1.5
Mean systolic and diastolic 24 h, awake and asleep values of RefBP, TestBP-V1.4 and TestBP-V1.5 are presented in Table 2 showing statistically significant differences in all comparisons—with TestBP-V1.5 being closer to RefBP than TestBP-V1.4 especially in asleep BP values.
Bland–Altman plots for comparison of the RefBP, TestBP-V1.4 and TestBP-V1.5
Bland–Altman plots comparing mean systolic, diastolic and asleep BP values of TestBP-V 1.4/V1.5 and RefBP are shown (Fig. 1), illustrating that the best agreements of the TestBP-V1.4 and V1.5 to RefBP were seen during the awake measurements. Of note, better agreements during asleep were measured with TestBP-V1.5. Overall BP values were systematically higher with both Test BP versions.
Bland Altman plots comparing systolic mean (A) 24 h, (B) awake and (C) asleep. BP values of TestBP-V1.4 /V1.5 and Ref BP and RefBP. Horizontal lines indicate mean differences Legend: Version 1.4—open red circle, Version 1.5—open green diamond, Version 1.4—red dotted line, and Version 1.5—green dashed line.
Intraclass correlation coefficients of RefBP and TestBP-V1.4 and TestBP-V1.5
The intraclass correlation coefficients (ICC) for mean systolic and diastolic 24 h, awake and asleep BP are shown in Table 3. The ICC show a good to excellent range of conformity with the exception of systolic and diastolic asleep values (moderate reliability)17. With TestBP-V1.5, ICC were especially higher for systolic and diastolic asleep values but did not improve after the update for systolic and diastolic awake values.
Standard errors of the measurements between RefBP and TestBP-V1.4 and TestBP-V1.5
The greatest difference between RefBP and TestBP-V1.4 and TestBP-V1.5 was found in asleep systolic BP measurements. Standard error of measurements for TestBP-V1.4 for mean systolic and diastolic 24 h, awake and asleep BP values was 6.5, 5.7, and 11.4 mmHg and 5.1, 4.2 and 9.1 mmHg respectively. Standard error of measurements for TestBP-V1.5 for systolic and diastolic 24 h mean, awake and asleep BP values was 6.2, 5.7, and 10.1 mmHg and 4.4, 4.6 and 7.3 mmHg, respectively (Table 4).
Agreement of measurements between the RefBP vs. TestBP-V1.5
Overall, when comparing mean 24 h systolic BPM of RefBP and TestBP-V1.5 agreements ≤ 2, ≤ 5, ≤ 10, and ≤ 15 mmHg were 16.9%, 38%, 62%, 77.5% respectively. In the awake intermediate BP group (135–150 mmHg), the best agreements were seen. The asleep BP groups had the poorest agreements (Table 5).
Overall, in the diastolic measurements, we saw the following agreements for ≤ 2, ≤ 5, ≤ 10, and ≤ 15 mmHg, 21.1%, 50.7%, 74.7% and 95.8% respectively. Best agreements were seen in the awake low and intermediate BP groups and the poorest agreements in the asleep BP values (Table 5).
Diagnostic accuracy of TestBP-V1.5 for the detection of hypertension compared to gold standard diagnosis by RefBP
The area under the curve (AUC) (95% confidence interval) for the TestBP-V1.5 in detecting hypertension in comparison to the RefBP was 0.92 (0.86; 0.99), 0.94 (0.88; 0.99) and 0.77 (0.66; 0.88) for systolic 24 h, awake and asleep BP values and 0.92 (0.86; 0.99), 0.92 (0.85; 0.99) and 0.84 (0.74; 0.94) for diastolic 24 h, awake and asleep BP values, respectively (Fig. 2).
Cut-off values for the clinical interpretation of the TestBP-V1.5
For the detection of hypertension, TestBP-V1.5 characteristics for standard mean systolic and diastolic 24 h cut-off values and for highest Youden’s indices are shown in Table 6. TestBP-V1.5 had 95% sensitivity, 65% specificity, a PPV of 74% and a NPV of 92% when applying a standard systolic diagnostic cut-off value of 130 mmHg, and a 90% sensitivity, 70% specificity, a 70% PPV and a 90% NPV when applying standard diastolic cut-off values of 80 mmHg. Highest Youden’s Index for mean systolic and diastolic 24 h BP was at systolic 133 mmHg (95% sensitivity, 80% specificity, 83% PPV, and 93% NPV) and diastolic 87 mmHg (70% sensitivity, 93% specificity, 87% PPV, and 81% NPV) respectively. Additional cut-off values (representing ≥ 95% sensitivity or ≥ 95% specifity) corresponding to TestBP-V1.5 awake and asleep systolic and diastolic values are show in Supplementary Table S1.
Discussion
Over a century, non-invasive cuff-based BP measurement, using the initial approach of Scipione Riva Rocci and Nicolai Sergeivich Korotkoff remained more or less unchanged until the advances through the development of oscillometric devices in the last decades19. Recently, devices using different techniques such as PTT or pulse wave analysis have increased, challenging the clinician to understand these new measurements. Generally guidelines suggest the use of validated devices in clinical practice however some newer techniques fail validation when compared to standard measurements2. A caveat in the validation of BP devices may be that the ESH-IP 2010 protocol was developed to compare single device measurements to single gold standard measurements using a mercury sphygmomanometer under laboratory conditions. This is useful and adequate to validate standard cuff based BP devices, but may give a misleading security for 24 h BP measurement devices with alternative techniques and beat-to-beat BP determination18,20. Therefore in 2017 our group evaluated the accuracy of a ESH-IP 2010 validated cuffless PTT device (Test BP) against a cuff-based standard device (Ref BP) over 24 h under usual clinical condition7.
We showed a significant difference in all measurements (mean 24 h, awake, and asleep), with the most divergence seen during assessment of blood pressure during sleep. The TestBP revealed higher BP values than the RefBP and overall, the study brought attention to the fact that, the two devices which measure BP differently came out with significant differences in values7, Highlighting that results of cuff-less and cuff-based devices are not directly interchangeable in clinical practice.
In July 2018, the TestBP device’s manufacturers updated the software algorithm. This created the need to reassess the data and evaluate the diagnostic accuracy of the TestBP device. Comparing the RefBP to the TestBP-V1.4 and TestBP-V1.5, we see improvements in the accuracy of measurements in relation to the RefBP especially for the asleep diastolic values with various modes of comparison. However, significantly higher measurements are still seen with the TestBP device, as was apparent before the update. Improvement in data output in terms of better agreement to RefBP was seen overall but especially with asleep values. Best agreement was in the high normal to grade 1-hypertension categories.
Therefore, the issue remains that measurements of the RefBP and the TestBP are not interchangeable. The fact that the device is on the market and already in clinical use, led to the further analysis of how the TestBP-V1.5 results could reliably discriminate between normotension and hypertension. In doing, a compass for clinical interpretation could be created with the aim of device specific cut-off values. Therefore, we used an alternative approach to analyze the data, implementing a technique used when determining the diagnostic accuracy of biomarkers against a gold standard diagnosis. In our case, the TestBP device was the biomarker and the RefBP was the gold standard to create the adjudicated diagnosis of hypertension according to standard cut-off values given in the 2018 ESC/ESH guidelines2. Our ROCs demonstrated that for systolic and diastolic mean 24 h and awake measurements, the TestBP-V1.5 has a high diagnostic accuracy with an AUC of ≥ 0.92–0.94 while the TestBP-V1.5 showed less ability to accurately detect systolic and diastolic hypertension during asleep measurements with an AUC of 0.77 and 0.84, respectively when compared to the RefBP. Using Youden’s index as the optimal device specific cut-off value to detect hypertension, we found cut-off values of 133/87 mmHg for 24 h mean, 136/91 mmHg awake and 136/83 mmHg asleep best for the distinction of hypertension and normotension.
With the awareness that the TestBP derives higher BP values than the RefBP, the calculated PPV, NPV, and Youden’s Index may help to guide clinicians on how to interpret the results of the TestBP. This is a vital step when incorporating new devices into practice—especially when usual validation protocols were limited due to the measurement technique.
Strengths and limitations
The strength of our study was that our data reflected a 24 h period, simultaneously created with two different devices. To date, this is the largest cohort comparing the TestBP device to a RefBP device simultaneously over a 24 h period in a routine clinical setting. Since this was an investigator-initiated trial with the aim of evaluating the accuracy of the PTT device in clinical practice, we had no insights into the technical changes applied by the manufacturer to this latest algorithm update. This software update is now available and in use by customers. Therefore our study adds very important information for these practitioners.
This study implemented the widely used Spacelabs 90217A 24 h BPM device as a clinically validated reference device11. All cuff-based BPM devices show differences when compared to the gold standard for device validation, which are comparisons to mercury sphygmomanometers, the reported mean differences and accuracy tables may differ slightly in a comparison to another cuff-based 24 h measurement device. Nevertheless, the Spacelabs device meets the Association for the Advancement of Medical Instrumentation’s standard for standing, sitting and supine measurements and was graded “A” for systolic and diastolic BP in a modified British Hypertension Society protocol.
As mentioned in the methods section of the paper we used 27 measurements as the minimum number for a valid RefBP measurement set. This criteria is in line with current recommendations in clinical settings. Although in some research settings stricter criteria are sometimes applied, we would like to emphasize that this study focused on the comparability of two devices in a typical clinical setting and even then that we maintained a very high quality control standard with an average of 60 measurements per participant (Table 1).
Another limitation is for the derivation of device specific cut-off values, more participants should be enrolled from various demographics (age, race, comorbidities, BP ranges). Therefore, the current data should be interpreted in the framework of a pilot evaluation.
Conclusions
We showed that the software update significantly improved the agreement of the tested cuff-less device (TestBP) in comparison to the cuff-based device (RefBP). This development emphasized that asleep values improved with the software update. In summary, we showed that TestBP-V1.5 correlates better to the RefBP than TestBP-V1.4. After the software update, the difference between the RefBP and the TestBP-V1.5 is less but remains statistically and clinically significant. When applying device specific cut-off points the TestBP device may be a reliable tool for the detection of systolic and diastolic hypertension for 24 h and awake BP values, but less so for asleep measurements.
References
Kearney, P. M. et al. Global burden of hypertension: Analysis of worldwide data. Lancet 365 (9455), 217–223 (2005).
Williams, B. et al. 2018) ESC/ESH Guidelines for the management of arterial hypertension. The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). G. Ital. Cardiol. (Rome) 19 (11), 3–73 (2018).
Williams, B. et al. 2018 Practice guidelines for the management of arterial hypertension of the European Society of hypertension (ESH) and the European Society of cardiology (ESC). Blood Press. 27 (6), 314–340 (2018).
Leary, A. C. & Murphy, M. B. Sleep disturbance during ambulatory blood pressure monitoring of hypertensive patients. Blood Press. Monit. 3 (1), 11–15 (1998).
Sheshadri, V., Tiwari, A. K., Nagappa, M. & Venkatraghavan, L. Accuracy in blood pressure monitoring: The effect of noninvasive blood pressure cuff inflation on intra-arterial blood pressure values. Anesth. Essays Res. 11 (1), 169–173 (2017).
Bilo, G. et al. Validation of the Somnotouch-NIBP noninvasive continuous blood pressure monitor according to the European Society of hypertension international protocol revision 2010. Blood Press. Monit. 20 (5), 291–294 (2015).
Krisai, P. et al. Accuracy of 24-hour ambulatory blood pressure monitoring by a novel cuffless device in clinical practice. Heart. https://doi.org/10.1136/heartjnl-2018-313592 (2018).
Gesche, H., Grosskurth, D., Kuchler, G. & Patzak, A. Continuous blood pressure measurement by using the pulse transit time: Comparison to a cuff-based method. Eur. J. Appl. Physiol. 112 (1), 309–315 (2012).
Schiffrin, E. L. Vascular stiffening and arterial compliance Implications for systolic blood pressure. Am. J. Hypertens. 17, 39–48 (2004).
Callaghan, F. J., Babbs, C. F., Bourland, J. D. & Geddes, L. A. The relationship between arterial pulse-wave velocity and pulse frequency at different pressures. J. Med. Eng. Technol. 8 (1), 15–18 (1984).
Baumgart, P. & Kamp, J. Accuracy of the SpaceLabs Medical 90217 ambulatory blood pressure monitor. Blood Press. Monit. 3 (5), 303–307 (1998).
Mancia, G. et al. 2013 ESH/ESC practice guidelines for the management of arterial hypertension. Blood Press. 23 (1), 3–16 (2014).
McCormack, T., Krause, T. & O’Flynn, N. Management of hypertension in adults in primary care: NICE guideline. Br. J. Gen. Pract. 62 (596), 163–164 (2012).
Meng, L. et al. Validation of Omron HBP-1300 professional blood pressure monitor based on auscultation in children and adults. BMC Cardiovasc. Disord. 16, 9 (2016).
Takahashi, H., Yokoi, T. & Yoshika, M. Validation of the OMRON HBP-1300 Upper Arm Blood Pressure Monitor, in Oscillometry Mode, for Clinic Use in a General Population, According to the European Society of Hypertension International Protocol Revision 2010 (DablEducational Trust Dublin, Dublin, 2014).
Parati, G. et al. European Society of hypertension practice guidelines for ambulatory blood pressure monitoring. J. Hypertens. 32 (7), 1359–1366 (2014).
Koo, T. K. & Li, M. Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 15 (2), 155–163 (2016).
O’Brien, E. et al. European Society of hypertension international protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press. Monit. 15, 23–38 (2010).
O’Brien, E. & Fitzgerald, D. The history of blood pressure measurement. J. Hum. Hypertens. 8, 73–84 (1994).
Padwal, R. Cuffless blood pressure measurement: How did accuracy become an afterthought? Am. J. Hypertens. 32, 807–809 (2019).
Funding
The study was fully funded by the Medical Outpatient and Hypertension Clinic, ESH Hypertension Centre of Excellence, University Hospital Basel, Basel, Switzerland. This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization: T.S., P.K. and T.B.; Methodology: T.B., A.M. and A.V.; Validation: T.B. and A.V.; Formal Analysis: T.S. and P.K. ; Resources: T.B. and M.M.; Data Curation: T.S., P.K., A.V., A.M. and T.B.; Writing: Original Draft Preparation, T.S.; Writing—Review & Editing, P.K., A.V., A.M., T.B. and M.M.; Supervision T.B., A.V. and M.M.; Project Administration, T.S., P.K. and T.B.; Funding Acquisition, T.B. and M.M.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author (s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Socrates, T., Krisai, P., Vischer, A.S. et al. Improved agreement and diagnostic accuracy of a cuffless 24-h blood pressure measurement device in clinical practice. Sci Rep 11, 1143 (2021). https://doi.org/10.1038/s41598-020-80905-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-80905-x
This article is cited by
-
A fusion approach to improve accuracy and estimate uncertainty in cuffless blood pressure monitoring
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.