Abstract
Co-doped Ce3+, Cr3+ and Pr3+ yttrium–aluminium–gallium garnet powders of various sizes were obtained by co-precipitation method. The microstructure and morphology were investigated by XRPD, TEM and gas porosimetry. The luminescence properties were studied by excitation and emission spectra, quantum yield and decay times. Thermoluminescence measurements were performed to evaluate the activation energy, traps redistribution and frequency factor. Limitation in the energy transfer between dopant ions in the small particles, traps depth and surface defects were considered and investigated as responsible for the quenching of persistent luminescence. The phosphors annealed at 1100 °C show the optimal persistent luminescence and nano-particle size.
Similar content being viewed by others
Introduction
Persistent luminescence (PersL) nanophosphors activated by doping with lanthanide and transition metal ions have recently gained much attention due to their promising applications in the fields of bioimaging1,2, optical nano-thermometry3, solar-blind glowing tags in bright daylight4, safety displays5,6, etc. Many research groups around the world are strongly motivated to develop new hosts for such phosphors and new synthetic routes5,6,7,8,9. Appropriately doped yttrium–aluminum-gallium garnet (Y3Al2Ga3O12, YAGG) is known as one of the new host matrices for persistent phosphors, which can show continuous PersL lasting from minutes to even hours after ceasing the excitation source8,10,11,12. The most recent studies are devoted to the nanophosphors emitting in red and near-infrared (NIR) regions2,13,14,15,16. Phosphors with such properties may be obtained by co-doping materials with Cr3+ and other trivalent ions, but still the intensity and duration of PersL in the red region cannot compete with green and blue ones14,15,17,18. Polycrystalline YAGG co-doped with Cr3+ and Ce3+ (YAGG:Ce3+,Cr3+) has been intensively studied by the group of Tanabe19,20,21, and it was proposed for the acquisition of long PersL of Ce3+ at room temperature. The development of this system by adding a third dopant to obtain red and NIR PersL is of current interest13,14,15,17,18. In fact, the addition of some lanthanide ions has been already studied in order to obtain their PersL due to energy transfer (ET) from Ce3+ and Cr3+ 11,22. Special attention has been put to the investigation of the mechanism involved in the PersL, in particular the electron and hole traps creation7,21,23,24,25,26. Moreover, even if systematic studies have been carried out for microsized samples prepared by solid-state reactions, the size effect on the PersL is not well known in nano and sub-micron sized particles1,5. For this reason, the synthesis of enough high quality doped-YAGG nanophosphors is still a challenge and the current subject of research. Most of the investigated doped-YAGG nanophosphors are obtained by combustion and Pechini methods14,27,28. Only few authors used a so-called wet-chemical methods such as co-precipitation, hydro- solvo-thermal or sol–gel with the aim of controlling the phase purity, to tailor the particles size and agglomeration degree15,28,29,30.
The aim of this work is to correlate microstructure and morphology of YAGG:Ce3+,Cr3+,Pr3+ nanophosphors with their luminescence properties. YAGG nanophosphors were synthesized by co-precipitation and then annealed at various temperatures. The undoped YAGG and singly doped with Ce3+, Cr3+ or with Pr3+ were synthesized as references at the same experimental conditions in order to investigate the impact of co-doping and the effect of thermal treatment on the phase stability of crystallization products, their microstructure, particles size and agglomeration. The set of co-doping ions was chosen to verify their applicability as persistent phosphors and to obtain already proved efficient energy downshifting from ultraviolet (UV) and blue light to red and NIR regions13,14,15,17,18. In this system, Ce3+ ions should absorb UV and blue light and then transfer the absorbed energy to Cr3+ and Pr3+ ions leading to red and NIR emission. The efficient ET should be able to enhance the effectiveness of luminescent solar concentrators by exploiting the UV/blue conversion of solar energy to the maximum of the spectral absorption of the photovoltaic substrate.
Experimental part
Materials
Y(NO3)3·6H2O (Aldrich, 99.8%), Al(NO3)3·9H2O (Aldrich, 98%), Ga(NO3)3·9H2O (Aldrich, 99.9%), Pr6O11 (Sigma–Aldrich, 99.99%), Ce(NO3)3·6H2O (Aldrich, 99.99%), Cr(NO3)3·9H2O (Fluka, 97%), ammonia solution (Aldrich, 28%) and nitric acid (Aldrich, 90%) were used as starting materials. All solutions were prepared using chemicals as received and adding deionized water (conductivity 1.5 μS/m).
Preparation of samples
Undoped (YAGG), singly doped (YAGG:Ce3+, YAGG:Cr3+, YAGG:Pr3+) and triply doped (YAGG:Ce3+,Cr3+,Pr3+) nanophosphors were prepared by the co-precipitation method following the procedure previously used for synthesis of YAG nanopowders31,32,33,34,35. First, nitrates were dissolved in deionized water and Pr6O11 was dissolved in dilute nitric acid in order to obtain praseodymium nitrate. For the synthesis of undoped Y3Al2Ga3O12 powders, yttrium, aluminum and gallium nitrates were mixed to obtain Y:Al:Ga atomic ratio equal to 3:2:3. The concentration of Al(NO3)3 was 0.5 mol L−1.
Nitrates were mixed to obtain the following atomic ratios: (Y + Ce):Al:Ga, (Y + Pr):Al:Ga, Y:(Al + Cr):Ga and (Y + Ce + Pr):(Al + Cr):Ga equal to 3:2:3 and the Y2.994Ce0.006Al2Ga3O12, Y2.9925Pr0.0075Al2Ga3O12, Y3Al1.9875Cr0.0125Ga3O12, and Y2.9865Ce0.006Pr0.0075Al1.9875Cr0.0125Ga3O12 formula. In the obtained samples, Ce3+ and Pr3+ ions substituted Y3+ ions with concentration 0.20 and 0.25 at.% in respect to Y3+ ions, and Cr3+ substituted Al3+ ions with concentration 0.63 at.% in respect to Al3+ ions.
The hydroxides were precipitated by dropwise addition of 0.5 mol L−1 ammonia solution until a pH equal to 8 was reached. The colloidal solution was thus maintained at pH 8 for 2 h. The white gelatinous precipitate was filtered and washed with deionized water to remove residual ammonia and nitrate ions. Finally, it was washed with ethanol and then was dried at 70 °C in the oven until it reached a constant weight. In order to obtain a crystalline powder, the dried powders were annealed at 900, 1000, 1100 and 1200 °C for 1 h in air with a synthesis yield of 80%. After the thermal treatment, undoped YAGG as well as YAGG:Cr3+, YAGG:Pr3+ powders were white, while the doped YAGG:Ce3+ , and YAGG:Ce3+,Cr3+, Pr3+ were green and light green tint, respectively.
Characterization techniques
X-ray powder diffraction (XRPD) patterns were acquired by a PANalytical X’Pert pro X-ray powder diffractometer at 40 kV and 30 mA in the 2θ range of 10°–80° (2θ step: 0.02626°) using nickel-filtered Cu Kα1 radiation. The phase identification was performed using the X’pert HighScore Software. Phase composition, cell parameters, crystallite sizes and microstrain of the phosphors were evaluated by using the Rietveld method36 and the MAUD software37. N2 adsorption–desorption isotherms were registered at 77° K using a Quantachrome NOVA 2200 Multi-Station High Speed Gas Sorption Analyzer. Samples were outgassed at 200 °C for 2 h in the degas station. Adsorbed nitrogen volumes were normalized to the standard temperature and pressure. The specific surface area (SBET) was calculated according to the standard B.E.T. method in the relative absorption pressure (P/P0) range from 0.045 to 0.25038. The average particle size (DBET) of the phosphors was calculated based on the SBET39. Transmission electron microscope (TEM) micrographs of nanophosphors annealed at 900 and 1200 °C were acquired using a JEM-2100 (JEOL, Japan) Electron Microscope operating at a 200 kV accelerating voltage. Each powder was homogeneously dispersed in isopropanol by sonication for 2 min. A drop of the suspension was deposited on a lacey-carbon grid of 300 meshes. After complete solvent evaporation, the grid was introduced into the TEM chamber for analysis. TEM micrographs of nanophosphors annealed at 1000 and 1100 °C were acquired using a high resolution scanning/transmission electron (S/TEM) microscope (Thermo Scientific Talos F200S) equipped with energy dispersive X-ray spectroscopy (EDS) and operating at 200 kV accelerating voltage. Samples were homogeneously dispersed in bi-distilled water (Millipore) by sonication for two minutes. A drop of each suspension was deposited on a copper grid of 200 mesh coated with a transparent polymer (Formvar/carbon) and then dried. Subsequently, specimens were carbonated (Carbon Coater—Balzers CED-010) for TEM investigations. Photoluminescence emission (PL) and excitation (PLE) spectra were measured using the FLS980 Fluorescence Spectrometer from Edinburgh Instruments equipped with 450 W Xenon lamp as an excitation source. Excitation arm was supplied with holographic grating of 1800 lines/mm, blazed at 300 nm, while the emission arm was supplied with ruled grating, 1800 lines/mm blazed at 500 nm. The R928P side window photomultiplier tube from Hamamatsu was used as a detector. The scanning range was from 250 to 680 nm for the PLE spectrum and 460 to 820 nm for PL spectrum with spectral resolution of 0.1 nm. The lifetime measurements were carried out using a femtosecond laser setup composed of a Coherent Libra-S all-in-one ultrafast oscillator and regenerative amplifier laser system, with pulse duration less than 100 fs at 1 kHz repetition rate, a Coherent OPerA-Solo optical parametric amplifier and a Hamamatsu C5677 streak camera with time resolution of 14 ps. Quantum efficiency of luminescence was measured using the same equipment as PL and PLE equipped with integrating sphere with diameter 30 cm. Measurement was performed for the pellets with a diameter of 5 mm and mass 1.5 mg prepared using press (pressure: 15 kN) from all synthesized powders. The PersL spectra of the YAGG nanophosphors were measured at room temperature after 450 nm LED irradiation for 5 min using Si CCD spectrometer (USB2000+, Ocean Optics), integration time 1 s. The detected spectra intensity was calibrated to a standard halogen lamp. The PersL decay curves and thermoluminescence (TL) curves were measured with a lexsyg research—fully automated TL/OSL Reader (Freiberg Instruments). The signal was collected with a R13456 PMT (Hamamatsu Photonics) monitoring the global emission from the whole spectral response (from 185 to 980 nm) with an integration (channel) time of 0.1 s. The TL glow curves were collected from room temperature to 300 °C with heating rate 2, 1, 0.75, 0.5 and 0.25 °C s−1. Blue Laser Diode PL 450B (λmax = 450 nm, FWHM = 2 nm) by Osram and Varian VF-50J/S RTG tube with tungsten core and copper case as an X-ray radiation source were used as irradiation sources. The excitation intensity of the LD was 1 mW/cm2 at sample position. The voltage and amperage for the X-Ray source were 15 kV and 0.1 mA, respectively. The persistent phosphors and ethanol were homogeneously mixed by using ultrasonics to obtain slurry. The homogeneous slurry was dripped on a metal holder and then the metal holder was heated to 100 °C and kept for 10 min to get rid of the solvent and form a thin film. For each phosphor annealed at different temperatures, approximately the same mass of sample was placed on the metal holder to obtain the film with almost the same thickness and realize the same PersL measurement condition. The samples thus prepared were placed in a TL instrument and the TL glow curves and PersL decay curves were collected. Finally, the TL and PersL intensities were normalized according to the mass of the sample.
Results and discussion
Microstructural and morphological investigation
The XRPD patterns of YAGG nanophosphors co-doped with Ce3+, Cr3+, Pr3+ are reported in Fig. 1. The XRPD patterns of undoped YAGG as well as YAGG:Ce3+, YAGG:Cr3+, YAGG:Pr3+ phosphors are shown in the Figures S1, S2 of the Supporting Information (S.I.).
All XRPD patterns are constituted by single garnet phase31 and are in good agreement with the XRPD pattern of YAGG (PDF#75-0555). The patterns of all phosphors obtained at lower annealing temperatures have broader and less intense peaks due to the lower crystallinity and smaller crystallite sizes. All diffraction peaks became narrower with increasing temperature, which indicates higher crystallinity of the powder in terms of particle size increase or lattice disorder (strain relaxation effect), which could also contribute to the peak broadening. The diffraction patterns were simulated by the Rietveld refinement analysis. For each sample, the agreement between experiment and model was evaluated by Rb (4–6%) and the curve of residues. XRPD patterns together with the best fits used for further calculations and the residual plots are shown in the Figures S3–S14 of S.I.. The cell parameter of the garnet phase, the crystallite size and the lattice microstrain are reported in Table 1.
The cell parameter (a) of undoped YAG is 12.008 Å, while those of YGG where all Al3+ ions are substituted with gallium is 12.27 Å40. The obtained values of cell parameter for the YAGG lattice are due to the difference in ionic radii between Ga3+ and Al3+ ions (Ga3+: 47 pm in tetrahedral site, 62 pm in octahedral site; Al3+: 39 pm in tetrahedral site, 53.5 pm in octahedral site)41 and thus are in agreement with the hypothesis of formation of YAGG phase having Y:Al:Ga atomic ratio equal to 3:2:3. The cubic cell parameter (a) decreases with increasing the annealing temperature was observed even for YAG powders doped with erbium or europium31,42 and this behaviour was attributed to the removal of vacancies or impurities42,43,44,45,46. It could also depend on a homogeneous disorder introduced into the lattice during the amorphous to crystalline transformation and on an imperfect local stoichiometry. At the same time, the annealing at higher temperature allows a microstrain decrease, indicating a release of this disorder with further densification.
The reticular disorder, described by the microstrain ε, varies from 2.3 × 10–3 for the 900 °C, to 1.5 × 10–3 for the samples obtained at 1000, 1100 and 1200 °C. Knowing that the microstrain is the root mean square of the variations in the lattice parameters across the sample, a higher value means that the distance of the relevant crystal planes is not identical, possibly due to the presence of defects and stress in the lattice. Analogously to what is already well known for long and high temperature treatments, the YAGG crystallites average size increases with temperature increasing.
The cell parameter (a) of the doped samples is higher than that of undoped samples prepared at the same temperature. The cell parameter (a), as well as the crystalline size, is higher in the case of the triply doped system in agreement with the partial substitution of Y3+ ions by Ce3+ and Pr3+ and of Al3+ ions replaced by Cr3+. The ionic radius of Y3+ is 102 pm, while the ionic radius of Ce3+ and Pr3+ are 114 pm and 113 pm, respectively; the ionic radius of Al3+ in the B-site is 53.5 pm, lower than the ionic radius of Cr3+, 61.5 pm41. The increase of crystalline size indicates that doping affects the system at different length scales through different mechanisms: (1) the presence of dopants in the colloidal hydroxides could reduce the energy required for the particle formation, acting as a nucleation site47. A lower interfacial surface energy could increase the nucleation rates, which could be compensated by a decreased thermodynamic driving force48. (2) The decreased number of nucleation sites can result in a greater number of bigger particles. (3) The interactions between the dopants and surface/grain boundaries affect the surface energy/grain boundary energy, thus, leading the stabilization/destabilisation of the surfaces/grain boundaries, thus, under the same preparation conditions, in presence of dopant more surfaces/grain boundaries can be created; (4) the presence of defects could create a charge imbalance, which can be compensated for by the creation of oxygen vacancies. This in turns prompts a larger oxygen ion motion and thus increased grain growth and bigger particle size. Understanding the mechanism needs further study. Since it is not within the scope of this paper, we did not pursue an investigation.
In all cases, as observed in the undoped samples, the size increases with the temperature increase. In presence of dopants, together with the reason already discussed for undoped samples, the microstrain decrease could also indicate a segregation of dopant ions to the surface of garnet domains42.
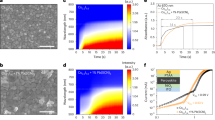
All the nitrogen adsorption–desorption isotherms are type IV according to IUPAC terminology. In all sets of samples, the SBET decreases with the temperature of treatment (Table 1), indicating the increase of particle size, which, compared with the DXRPD, is an indication of the low grade of agglomeration39,49,50. Thus, the size of aggregates evaluated by the DBET value particles for the powder annealed at 900 °C is still in the same order of magnitude as the average individual DXRPD. While above this annealing temperature, the size of aggregates is ~ 3 times larger, which confirms, that creating the solid bridges between the particles starts above these temperatures. Considering that the DXRPD increase and the microstrain decrease lead to the particle growth size, and considering the obtained values of DBET, the TEM micrographs of YAGG:Ce3+,Cr3+,Pr3+ samples were acquired. Three TEM micrographs with different magnifications of YAGG:Ce3+,Cr3+,Pr3+ samples annealed at different temperatures are reported in Fig. 2. From TEM micrographs it can be deduced that particles treated at 900 °C have slightly irregular shape, sizes around 50 nm (confirming DXRPD diameters in primary nanoparticles) and are agglomerated (confirming the DBET values of secondary nanoparticles). The particle sizes as well as agglomeration degree increase with increasing temperature. The higher temperature facilitates the rapid arrangement of the crystalline structure and subsequent coalescence of the chemical species to form particle agglomeration. This is more evident in the sample annealed at 1200 °C, where a very big agglomeration and coalescence of primary particles occurred. The contrasted bands consist of pairs of Fresnel fringes (black/white or white/black) that occur when primary nanoparticles favourably overlap. These fringes extended to the entire nanoparticle, thus revealing the interfaces with neighbouring nanoparticles. We can assess the presence of numerous nano-crystalline domains extended to the entire nanoparticle volume.
The EDS spectrum of one single grain of sample treated at 1100 °C, was collected obtaining the formula Y3.09Al2Ga2.72O10.36. This is very close to the theoretical one: Y3Al2Ga3O12, after elaboration on Raw EDS spectrum setting the Carbon as "deconvolution only" and using the Schreiber–Wims model for the ionization cross-section correction51 This finding is a validation of the methodology of the synthesis to guarantee the expected stoichiometry. The Raw EDS data are reported in the Figure S15 of the S.I..
Optical properties
PL and PLE spectra were analysed in order to investigate the optical properties of doped samples. The PL spectra of YAGG:Ce3+ and YAGG:Ce3+,Cr3+,Pr3+, annealed at different temperatures, were detected upon excitation at 450 nm (Fig. 3). The increase of the luminescence intensity of YAGG:Ce3+ and of YAGG:Ce3+,Cr3+,Pr3+ nanophosphors with increasing annealing temperature was observed and is associated with the particle size increase45,46. The phosphors with large particle size have low specific surface area, and thus have less surface defects52. The same tendency was observed for PLE spectra that were detected for each ion (S.I. Fig. S16–S19). As we can see in Fig. 3, the best sample in terms of PL properties, is the one annealed at 1200 °C, even if PersL intensity for this sample is lower than the PersL for sample annealed at 1100 °C (Fig. 7d). In addition, the particle size of the sample annealed at 1200 °C is no longer nanometric but sub-micron size (337 nm, see Table 1), being ~ 3 times larger than the sample annealed at 1100 °C (127 nm). For some practical applications such as bio-imaging, the nano-sized phosphors show greater potential than the micro-sized phosphors1,5. The YAGG:Ce3+,Cr3+,Pr3+ nanophosphor annealed at 1100 °C shows good PL properties, and the particle size is around 100 nm. Comprehensively considering the particle size and luminescence intensity, 1100 °C is the optimal annealing temperature. The next discussion is focused on this sample, comparing its spectroscopic properties to the singly doped with Ce3+ sample. Also, the PersL decay curves (S.I. Fig. S23), registered for the samples annealed at 1100 and 1200 °C, show the similar emission intensities and durations.
The PL spectra of YAGG:Ce3+ and YAGG:Ce3+,Cr3+,Pr3+, annealed at 1100 °C, were collected upon excitation at 450 nm (Fig. 4). The typical broadband Ce3+ emission (centred at 520 nm) due to the 5d → 4f transition for YAGG:Ce3+ and YAGG:Ce3+,Cr3+,Pr3+ nanophosphors was observed. For the triply doped YAGG:Ce3+,Cr3+,Pr3+ sample, in addition to the Ce3+ emission, the broad band with maximum around 709 nm (Cr3+:4T2g → 4A2g transition) and the strong narrow lines due to 4f → 4f transitions in Pr3+:1D2 → 3H4 (606 nm) and 3P0 → 3F2 (634 nm) were detected. The weak sharp lines at 485, 560 and 616 nm due to f–f transitions in Pr3+:3P0 → 3H4; 3H5 and 3H6, respectively53 were also observed.
Comparing the PL spectra (Fig. 4) of YAGG:Ce3+ and YAGG:Ce3+, Cr3+, Pr3+, it was found that the intensity of Ce3+ emission decreased by more than 30% for the triply doped sample. This difference can be connected to re-absorption of Ce3+ emission by other doping ions. As result, two parallel processes can be observed together: ET and re-absorption by Cr3+.
The PLE spectra were collected at 520 (5a), 606 (5b) and 709 nm (5c), for Ce3+, Pr3+ and Cr3+ emission, respectively (Fig. 5). PLE spectra for each excitation, as f(T), were reported in the Figures S16–S19 of the S.I. In all spectra, broad bands centred at 350 and 435 nm were recorded, while the band at 278 nm appeared only for the co-doped sample. For the excitation spectra of Pr3+ (at 606 nm) and Cr3+ (at 709 nm) the sharp bands near 450 nm are due to 4f → 4f transition of Pr3+. The presence of Pr3+ bands on the Cr3+ PLE spectra indicates the presence of ET from Pr3+ to Cr3+ ions. In the PLE spectra of Cr3+ (at 709 nm) a broad band at 617 nm (Cr3+:4A2g → 4T2g transition) was also observed.
In the PLE spectra of Ce3+, acquired at 520 nm, the two bands centred at 350 and 435 nm are attributed to the transition from 4f to 5d levels, split by the crystal field. Broad band at 278 nm, which appeared for samples YAGG:Ce3+,Cr3+,Pr3+ and which is absent for the YAGG:Ce3+, connected with Pr3+:4f → 5d transitions11 as well as with Cr3+:4A2g → 4T1g(4P) transition54,55, which overlap.
In the PLE spectra of Pr3+, acquired at 606 nm, the broad band recorded at 278 nm corresponds to Pr3+:4f → 5d transitions and sharp bands near 450 nm correspond to Pr3+:4f → 4f (3H4 → 3PJ) transitions. The narrow peaks (Pr3+:4f → 4f) are in superposition with the broad band centred at 435 nm coming from Ce3+ and the existence of Ce3+ bands in Pr3+ PLE spectra can indicate the ET from Ce3+ to Pr3+ ions11.
The PLE spectra of Cr3+ can be ascribed to the Cr3+ ion absorption corresponding to spin-allowed transitions:4A2g → 4T2g (maximum at 617 nm) and 4A2g → 4T1g (maximum at 445 nm)54,55. It should also be noted that a broad band with maximum at 445 nm can be a superposition of two bands: Cr3+:4A2g → 4T1g transition and another spin-allowed Ce3+ 4f → 5d transition. The effect of overlapping two separate bands is most pronounced at lower annealing temperatures, because the distance between them is greatest. As the annealing temperature increases (and also the crystallite size), the crystal field strength changes and the Cr3+:4A2g → 4T1g transition band shifts to the blue region56 and, for a temperature of 1200 °C, completely overlaps with the Ce3+ 4f → 5d transition band. The band centred at 350 nm from Ce3+ may also indicate the ET from Ce3+ to Cr3+21. The band located at 278 nm (as we noted for Ce3+ excitation spectra) may be a result of superposition of Cr3+:4A2g → 4T1g(4P)55 and Pr3+:4f → 5d transitions53. Additionally, the series of sharp bands at around 450 nm which refer to Pr3+:4f → 4f (3H4 → 3PJ) transitions are also presented11,53.
The emission decay time (DT) of the YAGG:Ce3+ and the YAGG:Ce3+,Cr3+,Pr3+ samples were measured for the emission of Ce3+ at 520 nm under the laser excitation at 450 nm. The data acquired for the samples annealed at 1100 °C are reported in Fig. 6. The DT for the co-doped samples are not the mono exponential ones. The main factor that can influence the obtained data are the appearance of afterglow for the co-doped crystal, which significantly increases the background. The effective DT57 was calculated to compare the obtained curve shapes and further calculate the ET coefficient. Based on the DT, the ET efficiencies from Ce3+ to Pr3+ and Cr3+ were calculated for each annealing temperature (see S.I. Fig. S20). The DT decrease with increasing annealing temperatures could be explained by an increase of grain size of nanophosphors45.
The effective DT of the YAGG:Ce3+ and YAGG:Ce3+,Cr3+,Pr3+ nanophosphors are equal at 69 and 52 ns, respectively. The value of DT decreases for the phosphors co-doped with Cr3+ and Pr3+ is caused by ET from excited states of Ce3+ to Cr3+ and/or Pr3+. The efficiency of ET from Ce3+ to Pr3+ and Cr3+ is equal to 24%. This is close to value for YAGG:Ce3+,Cr3+ with similar concentrations of Ce3+ and Cr3+ 58.
The QY are equal to 47% and 27%, respectively for singly and triply doped nanophosphors. The QY decreasing in the latter case may result from trapping energy. For this reason, the PersL properties of the YAGG:Ce3+,Cr3+,Pr3+ and of YAGG:Ce3+ nanophosphors were also investigated.
Persistent luminescence properties
The PersL spectra of the YAGG:Ce3+ and YAGG:Ce3+,Cr3+,Pr3+ nanophosphors after blue light (450 nm) irradiation were recorded as a function of the nanophosphors’ size (annealing temperature), and are presented in Fig. 7. It can be seen that the PL and PersL spectra of both nanophosphors show similar shape bands. Both spectra are composed of two broad bands from Ce3+ and Cr3+ and sharp lines from Pr3+. The main broad band centred at 520 nm is ascribed to the 5d → 4f electronic transition of Ce3+, the broad band centred at around 709 nm is due to the 4T2g → 4A2g transition of Cr3+. Finally, the sharp line at 606 nm is ascribed to the 1D2 → 3H4 transition of Pr3+. Only the intensity ratio between associated Ce3+ and Cr3+ bands are changing analogously as we previously observed for YAGG:Ce3+,Cr3+59. The PersL intensity (Fig. 7d) increases with increasing annealing temperature up to 1100 °C. A further increase in the annealing temperature to 1200 °C causes a PersL decrease, probably due to defect elimination, which may work as traps and lead to PersL.
The photographs and corresponding PL and PersL spectra of singly and triply doped YAGG nanophosphors, acquired under excitation at 450 nm, at 1 and 10 s after ceasing irradiation source are presented in Fig. 7. The PL CIE coordinates of YAGG:Ce3+,Cr3+,Pr3+ annealed at 1100 °C were calculated. The results are (0.34, 0.54). Additionally, the CIE coordinates of PersL with delay time 1 and 10 s were also calculated. The results are 1 s: (0.28, 0.50) and 10 s: (0.28, 0.52). The CIE diagram was added in the Supplementary Information (Fig. S21). The YAGG:Ce3+,Cr3+,Pr3+ shows a yellow green component PL in the CIE coordinates. Compared to the PL, the PersL (recorded both after at 1 and 10 s) slightly shifts to green. The reason is that the red or the NIR PersL of Cr3+ and Pr3+ is caused by the energy transfer from Ce3+. The energy transfer is less efficient than the excitation direct from the laser diode. The red emission from the YAGG:Ce3+,Cr3+,Pr3+ cannot be observed by naked eye, because the red emission from Cr3+ and Pr3+ is weaker than the green emission from Ce3+, and the maximum of spectral sensitivity of the human eye is in the green colour region.
One of the side effects of co-doping YAGG:Ce3+ nanophosphors with Cr3+ and Pr3+ ions is extending the duration of the PersL. Comparing photographs taken immediately after ceasing the excitation source and after 10 s, it is possible to observe that YAGG:Ce3+ has a very intense PL but after removing excitation source, emission quickly disappears. On the other hand, YAGG:Ce3+,Cr3+,Pr3+ nanophosphors have weaker PL (compared to corresponding YAGG:Ce3+) but the intensity of the signal remains almost unchanged after the removal of the excitation source (Fig. 8). The PersL decay curve was recorded in the visible region (from 340 to 850 nm) for each sample (Fig. 8; SI Fig. S22–S23) after irradiation with LD 450 nm (1 mW cm−2) for 5 min, and the observed signal intensity was corrected by mass. As a reference signal, the PersL decay curve from the undoped YAGG was recorded at the same condition. This approach was possible because the measurements were performed under the same conditions for similar samples. Unfortunately, because of limits of equipment used for TL measurements, we cannot directly compare the duration time of PersL for obtained nanophosphors with other existing analogues, as it was proposed and described e.g. by Smet et al.60. As it is well evidenced by the obtained curve, the PersL is longer than 1 h (according to the obtained data). The green emission of YAGG:Ce3+,Cr3+,Pr3+ is easy to be recognized up to 30 min in a dark room with the naked eyes.
Thermoluminescence
Glow curves of singly doped YAGG:Ce3+, YAGG:Cr3+ and YAGG:Pr3+ and triply doped YAGG:Ce3+,Cr3+,Pr3+ after irradiation by blue LD (@450 nm) and X-ray source were recorded and analysed to determine shape factors (μ) and order of kinetics (b) by using the methods of analysis based on the shape of the glow curve61,62. Each curve was detected after the sample was illuminated for 5 min, and the heating rate was 1 °C s−1 (heating rate β—0.25, 0.50, 0.75, 1.0 and 2.0 °C s−1 were also used for calculation of E and s by heating rate method61). As it is evident (Fig. 9 and other annealing temperatures in the S.I.), all curves show a broad band that can be the result of the quasi-continuous trap distribution producing a broad band centred at around 335 K. From TL curves of singly doped nanophosphors annealed at 900 °C, we can distinguish a series of separated traps with different trap depths. As the annealing temperature increases, the specific surface area and the number of surface defects decreases (as O–H groups on the surface63,64). As a consequence, the number of shallow traps decreases. For higher annealing temperatures, the defects mainly coming from the partial substitution of Y3+ ions by Ce3+ and Pr3+ and of Al3+ ions replaced by Cr3+ begin to act as traps. At the same time, it has to be noted that for annealing temperature at 1200 °C, the shape of the line in the high temperature side of the TL peak approaches the curve with second order. As can be seen from Table 1, ε for annealed nanoparticles at 1100 °C and 1200 °C is unchanged, which means that a further increase in the size does not reduce the lattice microstrain, while the cell parameter is further decreased. This means that the ordering of the ions within the crystal lattice improves and, as a result, the intensity of PL further increases reaching the maximum for the sample annealed at 1200 °C. Moreover, the luminescence intensity increases and its QY is an indication of the elimination of surface defects, which is clearly seen from the above data. The disappearance of deeper traps thus reduces the PersL duration. That is, the resulting sample has much brighter initial (first 10 s) emission but shorter duration. The addition of Cr3+ forms stable med-depth traps which, as can be seen from the TL curves of single-doped Cr3+, remain unchanged for all annealing temperatures (Fig. 10; S.I. Table S1). As we can see, the TL curves of samples singly doped with Ce3+ after X-ray irradiation have a different shape on the high-temperature side compared to the curves after LD (@450 nm) irradiation. This may be due to the fact that, by using the high energy X-rays, we fill deeper traps with maximum around 400 K. For the blue light irradiation, no signal from Cr3+ and Pr3+ singly doped samples was detected, because the energy of blue light is insufficient to prompt the electrons of Cr3+ and Pr3+ to the conduction band. On the other hand, for the Ce ion-doped sample, the movement of electrons from the excited 5d level to the electron traps passes through the conduction band. After the X-ray irradiation, TL curves of YAGG:Cr3+ annealed at different temperatures show similar shape and position of the maximum, while some differences have been observed for YAGG:Ce3+ and YAGG:Pr3+. The TL curves shift to higher temperature for YAGG:Ce3+, while the curves of YAGG:Pr3+ shift to lower temperature.
Based on the estimation of trap depth for materials exhibiting PersL, the optimal value of trap depth may be in the range 0.58–1.13 eV, while the s value lies in the range 108–1014 s−1. In particular, at room temperature (around 300 K) and activation energy around 0.71 eV (same as we have for triply doped samples) for long term PersL s should be around 1010, while for s equal 108, the best PersL will be at about 350 K65. An additional factor that positively affects the duration of afterglow is its order. The TL peak must follow second-order kinetics. For values of E less than the specified one, together with the decrease of the parameter s and the order of the curve, the recombination process will take place quickly. As a result, the PersL duration will be shorter. As we can see from Table S1, μ is in the range between 0.51 and 0.72, which indicates that it is a second (μ = 0.52) or more order TL peak, responsible for overlapping at least two or more traps with shallow and deep depth resulting in the long PersL. The E of singly doped YAGG:Ce3+, YAGG:Cr3+and YAGG:Pr3+ and triply doped YAGG:Ce3+,Cr3+,Pr3+ nanophosphors is found around 0.71 eV (with exception around 0.5 eV for the sample singly doped with Ce3+ ions) which is in the range for which one can expect a long PersL at room temperature65. As for the s factor, it acquires optimal values in relation to E for samples prepared at 1100 °C65. Consequently, the bright initial emission for samples prepared at higher temperature (1200 °C) and without co-dopants (at least Cr3+) was observed after the removal of the irradiation source, but the duration was significantly shorter than the samples annealed at 1100 °C and co-doped with Cr3+. It means that, at almost the same activation energy for samples prepared at 1100 °C and 1200 °C, we register a more homogeneous distribution of traps (the order of the TL peak follows the first-order) and the probability of electron release increases with its subsequent recombination. The latter may be, due to the fact, that for the samples prepared at 1200 °C Ce3+ ions are distributed more evenly on the crystal lattice and the probability of its finding near the trap increases.
Conclusions
This study shows the correlation occurring between the microstructure, the particle size and PersL properties. It was confirmed that the optical properties of YAGG:Ce3+,Cr3+,Pr3+ nanophosphors are significantly affected by the annealing temperature. By showing a lower degree of agglomeration and a smaller size of nanoparticles, samples annealed at 1100 °C show at the same time a very effective ET from Ce3+ to Cr3+ and Pr3+ compared to samples annealed at 1200 °C. On the other hand, smaller crystallites obtained below 1100 °C have more surface defects which can lead to luminescence quenching. The same trend was observed for the PersL where the duration time of emission of samples annealed at 1100 °C was much longer than the samples annealed at lower temperatures. It should be noted, however, that for samples annealed at 1200 °C PersL duration and intensity was almost the same as the recorded samples annealed at 1100 °C. The observed changes in ET from Ce3+ to Cr3+ and Pr3+ can be correlated with the difference of the lattice parameter. Lattice parameter in turn affects the crystal field strength and the Cr3+:4A2g → 4T1g transition band completely overlaps with the Ce3+:4f → 5d transition band for annealing temperature 1200 °C. Additionally, a higher annealing temperature and ordered surface structure does not lead to a significant improvement in both activation energy E and frequency factor s (E = 0.73 eV, s = 1.18 × 1010 s−1 for 1200 °C vs E = 0.76 eV, s = 2.38 × 1010 s−1 for 1100 °C—after X-ray irradiation; E = 0.71 eV, s = 5.55 × 109 s−1 for 1200 °C vs E = 0.71 eV, s = 5.56 × 109 s−1 1100 °C—after blue LD irradiation). However, for the samples annealed at 1200 °C, the high-temperature shoulder disappears on the TL curves, compared to samples annealed at 1100 °C. Thus, based on the activation energy in relation to the s factor, we proved that we are able to point-out the material characterized by the best PersL properties even without measurements of the absolute intensity (as it is in our case for comparison of the samples obtained at 1100 and 1200 °C, indicating that PersL for the sample annealed at 1100 °C is better at RT).
Summarizing, the co-precipitation can be considered as a low-cost method for the production of triply doped tailored PersL nanophosphors, which may be used for the fabrication of optical ceramics, polymeric composites and silica-based thin films. It can be concluded that higher annealing temperature in the tested range did not improve the optical properties of nanophosphors. Therefore, the YAGG nanophosphor annealed at 1100 °C showing an acceptable degree of particle agglomeration may already be suitable for practical applications. The reported stable emissions from Cr3+ can have a significant potential application in biological imaging technology in the first biological window. The emission from Pr3+ indicates the nanophosphors have potential to work as luminescent concentrators in band-matched solar cells, although the efficiency of PersL nanophosphors must be still further optimised in particular by controlling their synthesis and thermal treatment conditions.
References
Jaque, D. et al. Inorganic nanoparticles for optical bioimaging. Adv. Opt. Photon. 8, 1–103 (2016).
Singh, S. K. Red and near infrared persistent luminescence nano-probes for bioimaging and targeting applications. RSC Adv. 4, 58674–58698 (2014).
Piñol, R. et al. Real-time intracellular temperature imaging using lanthanide-bearing polymeric micelles. Nano Lett. 15, 33 (2020).
Wang, X., Chen, Y., Liu, F. & Pan, Z. Solar-blind ultraviolet-C persistent luminescence phosphors. Nat. Commun. 11, 1–8 (2020).
Xu, J. & Tanabe, S. Persistent luminescence instead of phosphorescence: History, mechanism, and perspective. J. Lumin. 205, 581–620 (2019).
Li, Y., Gecevicius, M. & Qiu, J. Long persistent phosphors—from fundamentals to applications. Chem. Soc. Rev. 45, 2090–2136 (2016).
Hölsä, J. Persistent luminescence beats the afterglow: 400 years of persistent luminescence. Electrochem. Soc. Interface 18, 42–45 (2009).
Mori, M. et al. Scintillation and optical properties of Ce-doped YAGG transparent ceramics. J. Rare Earths 34, 763–768 (2016).
Kim, J., Lee, C. K. & Kim, Y. J. Low temperature synthesis of Lu3Al5-xGaxO12:Ce3+, Cr3+ powders using a sol-gel combustion process and its persistent luminescence properties. Opt. Mater. 104, 109944 (2020).
Fu, S. et al. Effect of Al/Ga substitution on the structural and luminescence properties of Y3(Al1-xGax)5O12: Ce3+ phosphors. Opt. Mater. 75, 619–625 (2018).
Wang, B. et al. Ce3+/Pr3+:YAGG: A long persistent phosphor activated by blue-light. J. Am. Ceram. Soc. 97, 2539–2545 (2014).
Chen, X. et al. Microstructure evolution in two-step-sintering process toward transparent Ce:(Y, Gd)3(Ga, Al)5O12 scintillation ceramics. J. Alloys Compd. 846, 156377 (2020).
Xu, J., Murata, D., Ueda, J. & Tanabe, S. Near-infrared long persistent luminescence of Er3+ in garnet for the third bio-imaging window. J. Mater. Chem. C 4, 11096–11103 (2016).
Dai, Z. et al. Optical studies of Y3(Al, Ga)5O12:Ce3+, Cr3+, Nd3+ nano-phosphors obtained by the Pechini method. J. Rare Earths 37, 1132–1136 (2019).
Boiko, V., Markowska, M., Consentino, L., Saladino, M. L. & Hreniak, D. Effect of Ce3+ concentration on persistent luminescence of YAGG:Ce3+, Cr3+, Nd3+ nanophosphors obtained by the co-precipitation method. Opt. Mater. 107, 109956 (2020).
Viana, B. et al. Long term in vivo imaging with Cr3+ doped spinel nanoparticles exhibiting persistent luminescence. J. Lumin. 170, 879–887 (2016).
Wu, L. et al. Synthesis and optical properties of a Y3(Al/Ga)5O12:Ce3+, Cr3+, Nd3+ persistent luminescence nanophosphor: A promising near-infrared-II nanoprobe for biological applications. Nanoscale 12, 14180–14187 (2020).
Xu, J., Tanabe, S., Sontakke, A. D. & Ueda, J. Near-infrared multi-wavelengths long persistent luminescence of Nd3+ ion through persistent energy transfer in Ce3+, Cr3+ co-doped Y3Al2Ga3O12 for the first and second bio-imaging windows. Appl. Phys. Lett. 107, 081903 (2015).
Ueda, J., Kuroishi, K. & Tanabe, S. Bright persistent ceramic phosphors of Ce 3+-Cr 3+-codoped garnet able to store by blue light. Appl. Phys. Lett. 104, 101904 (2014).
Xu, J., Ueda, J., Kuroishi, K. & Tanabe, S. Fabrication of Ce3+–Cr3+ co-doped yttrium aluminium gallium garnet transparent ceramic phosphors with super long persistent luminescence. Scr. Mater. 102, 47–50 (2015).
Ueda, J., Dorenbos, P., Bos, A. J. J., Kuroishi, K. & Tanabe, S. Control of electron transfer between Ce3+ and Cr3+ in the Y3Al5−xGaxO12 host via conduction band engineering. J. Mater. Chem. C 3, 5642–5651 (2015).
Xu, J., Ueda, J. & Tanabe, S. Novel persistent phosphors of lanthanide–chromium co-doped yttrium aluminum gallium garnet: Design concept with vacuum referred binding energy diagram. J. Mater. Chem. C 4, 4380–4386 (2016).
Ueda, J. et al. Evidence of valence state change of Ce3+ and Cr3+ during UV charging process in Y3Al2Ga3O12 persistent phosphors. Opt. Mater. Express 7, 2471 (2017).
Hölsä, J. et al. Role of defect states in persistent luminescence materials. J. Alloy. Compd. 374, 56–59 (2004).
Wang, Y. et al. Hole trapping process and highly sensitive ratiometric thermometry over a wide temperature range in Pr3+-doped Na2La2Ti3O10 layered perovskite microcrystals. J. Phys. Chem. A 123, 4021–4033 (2019).
Van der Heggen, D. et al. The almost hidden role of deep traps when measuring afterglow and thermoluminescence of persistent phosphors. J. Lumin. 226, 117496 (2020).
Sengar, P. et al. β-Irradiated thermoluminescence response of nanocrystalline YAGG:Pr3+ for radiation dosimetry. Mater. Res. Bull. 90, 195–204 (2017).
Sengar, P. et al. Simultaneous paramagnetic and persistence-luminescence in GAGG:Ce, Pr nanoparticles synthesized by sol-gel for biomedical applications. J. Appl. Phys. 126, 083107 (2019).
Praveena, R. et al. Sol-gel synthesis and thermal stability of luminescence of Lu3Al5O12:Ce3+ nano-garnet. J. Alloys Compd. 509, 859–863 (2011).
Wang, L. et al. Preparation and photoluminescence properties of YAG:Ce3+ phosphors by a series of amines assisted co-precipitation method. J. Alloys Compd. 661, 148–154 (2016).
Saladino, M. L., Caponetti, E., Martino, D. C., Enzo, S. & Ibba, G. Effect of the dopant selection (Er, Eu, Nd or Ce) and its quantity on the formation of yttrium aluminum garnet nanopowders. Opt. Mater. 31, 261–267 (2008).
Caponetti, E., Saladino, M. L., Serra, F. & Enzo, S. Co-precipitation synthesis of Nd:YAG nano-powders: The effect of Nd dopant addition with thermal treatment. J. Mater. Sci. 42, 4418–4427 (2007).
Caponetti, E., Enzo, S., Lasio, B. & Saladino, M. L. Co-precipitation synthesis of neodymium-doped yttrium aluminium oxides nanopowders: Quantitative phase investigation as a function of joint isothermal treatment conditions and neodymium content. Opt. Mater. 29, 1240–1243 (2007).
Caponetti, E. et al. Luminescence Properties of neodymium-doped yttrium aluminium garnet obtained by the co-precipitation method combined with the mechanical process. Solid State Phenom. 106, 7–16 (2005).
Saladino, M. L., Nasillo, G., Chillura Martino, D. & Caponetti, E. Synthesis of Nd:YAG nanopowder using the citrate method with microwave irradiation. J. Alloys Compd. 491, 737–741 (2010).
Young, R. A. & Robert, A. Y. The Rietveld Method (International Union of Crystallograhy, Chester, 1993).
Lutterotti, L. & Gialanella, S. X-ray diffraction characterization of heavily deformed metallic specimens. Acta Mater. 46, 101–110 (1998).
Brunauer, S., Emmett, P. H. & Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938).
Caponetti, E., Martino, D. C., Saladino, M. L. & Leonelli, C. Preparation of Nd:YAG nanopowder in a confined environment. Langmuir 23, 3947–3952 (2007).
Jiang, L. et al. Preparation and luminescence properties of Y3−yAl5−xGaxO12:Ce3+y phosphors. J. Mater. Sci. Mater. Electron. 29, 9045–9051 (2018).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 32, 751–767 (1976).
Saladino, M. L., Caponetti, E. & Enzo, S. Effects of thermal treatment on the structure of Eu:YAG nanopowder. Solid State Phenom. 128, 107–114 (2007).
Armetta, F. et al. Synthesis of yttrium aluminum garnet nanoparticles in confined environment II: Role of the thermal treatment on the composition and microstructural evolution. J. Alloys Compd. 719, 264–270 (2017).
Hankare, P. P., Chate, P. A., Sathe, D. J., Chavan, P. A. & Bhuse, V. M. Effect of thermal annealing on properties of zinc selenide thin films deposited by chemical bath deposition. J. Mater. Sci. Mater. Electron. 20, 374–379 (2009).
Głuchowski, P., Małecka, M., Stręk, W., Ryba-Romanowski, W. & Solarz, P. Size effect in novel red efficient garnet nanophosphor. J. Phys. Chem. C 121, 25561–25567 (2017).
Hreniak, D., Hölsä, J., Lastusaari, M. & Stręk, W. Effect of grain size and concentration of active ions on structural and optical behavior of Eu3+-doped Y3Al5O12 nanocrystallites. J. Lumin. 122–123, 91–94 (2007).
Thieme, K., Avramov, I. & Rüssel, C. The mechanism of deceleration of nucleation and crystal growth by the small addition of transition metals to lithium disilicate glasses. Sci. Rep. 6, 1–16 (2016).
Pal, M. et al. Influence of doping on crystal growth, structure and optical properties of nanocrystalline CaTiO3: A case study using small-angle neutron scattering. J. Appl. Crystallogr. 48, 836–843 (2015).
Li, J. et al. Co-precipitation synthesis route to yttrium aluminum garnet (YAG) transparent ceramics. J. Eur. Ceram. Soc. 32, 2971–2979 (2012).
Saito, N., Matsuda, S. I. & Ikegami, T. Fabrication of transparent yttria ceramics at low temperature using carbonate-derived powder. J. Am. Ceram. Soc. 81, 2023–2028 (1998).
Schreiber, T. P. & Wims, A. M. A quantitative X-ray microanalysis thin film method using K-, L-, and M-lines. Ultramicroscopy 6, 323–334 (1981).
Yan, X. H. et al. Preparation of YAG:Ce3+ phosphor by sol-gel low temperature combustion method and its luminescent properties. Trans. Nonferrous Met. Soc. China (English Ed.) 18, 648–653 (2008).
Cavalli, E. et al. YAG:Pr3+ transparent ceramics for applications in photonics: Synthesis and characterization. Mater. Res. Express 1, 45903 (2014).
Xu, J., Ueda, J., Zhuang, Y., Viana, B. & Tanabe, S. Y3Al5−xGaxO12:Cr3+: A novel red persistent phosphor with high brightness. Appl. Phys. Express 8, 042602 (2015).
Katayama, Y., Viana, B., Gourier, D., Xu, J. & Tanabe, S. Photostimulation induced persistent luminescence in Y3Al2Ga3O12:Cr3+. Opt. Mater. Express 6, 1405 (2016).
Głuchowski, P., Pazik, R., Hreniak, D. & Strek, W. Luminescence properties of Cr3+:Y3Al5O12 nanocrystals. J. Lumin. 129, 548–553 (2009).
Cortelletti, P. et al. Luminescence of Eu3+ activated CaF2 and SrF2 nanoparticles: Effect of the particle size and codoping with alkaline ions. Cryst. Growth Des. 18, 686–694 (2018).
Chen, D., Zhou, Y., Xu, W., Zhong, J. & Huang, P. Persistent and photo-stimulated luminescence in Ce3+/Cr3+ activated Y3Al2Ga3O12 phosphors and transparent phosphor-in-glass. J. Mater. Chem. C 4, 11457–11464 (2016).
Boiko, V. et al. Persistent luminescence from Y3Al2Ga3O12 doped with Ce3+ and Cr3+ after X-ray and blue light irradiation. J. Rare Earths 37, 1200–1205 (2019).
Van der Heggen, D., Joos, J., Rodríguez Burbano, D., Capobianco, J. & Smet, P. Counting the photons: Determining the absolute storage capacity of persistent phosphors. Materials 10, 867 (2017).
Pagonis, V., Kitis, G. & Furetta, C. Numerical and Practical Exercises in Thermoluminescence (Springer, New York, 2006). https://doi.org/10.1007/0-387-30090-2.
Chen, R. Glow curves with general order kinetics. J. Electrochem. Soc. 116, 1254 (1969).
Hari Krishna, R. et al. Effect of calcination temperature on structural, photoluminescence, and thermoluminescence properties of Y2O3:Eu3+ nanophosphor. J. Phys. Chem. C 117, 1915–1924 (2013).
Dai, Z. et al. Effect of annealing temperature on persistent luminescence of Y3Al2Ga3O12:Cr3+ co-doped with Ce3+ and Pr3+. Opt. Mater. https://doi.org/10.1016/j.optmat.2020.110522 (2020).
Magdalene Mashangva, M. & Nara Singh, T. B. S. Estimation of optimal trapping parameters relevant to persistent luminescence. Indian J. Pure Appl. Phys. 49, 583–589 (2011).
Acknowledgements
D.H. acknowledges the Polish National Science Centre for the OPUS 11 2016/21/B/ST5/02385 project. M.L.S. and D.H. thank the University of Palermo for supporting this research through the CORI2017 (Bando per la concessione di contributi per l’avvio e lo sviluppo di collaborazioni dell’Ateneo 2017—Azione D—CORI-2016-D-D15-180463). TEM experimental data of the samples annealed at 900 and 1200 °C were provided by ATeN Center—Università di Palermo (http://www.atencenter.com). Thanks are due to Dr. Massimo Tonelli and Dr. Mauro Zapparoli, CIGS, University of Modena and Reggio Emilia for their help in the TEM of the samples annealed at 1000 and 1100 °C. This work is also supported by the Project to carry out research or development activities and related tasks for the development of young researchers and doctoral candidates (INTiBS PAN) 2020 Edition.
Author information
Authors and Affiliations
Contributions
V.B. performed optical measurements (included persistent luminescence and thermoluminescence), interpreted and discussed obtained data, wrote the original draft and reviewed the manuscript; Z.D. synthesized the phosphors, performed the structure characterization, reviewed and submitted the manuscript; M.M. participated in the phosphor synthesis and performed optical measurements; C.L. and C.M. were responsible for the TEM investigation at the University of Modena and Reggio Emilia; F.A. was responsible for the acquisition and analysis of the N2 adsorption-desorption isotherms; F.U. synthesized the phosphors and discussed Rietveld refinement of XRPD patterns; G.N. was responsible for the TEM investigation at the University of Palermo; M.L.S. conceptualization, supervised the research and reviewed the manuscript; D.H. administrated and supervised the research, interpreted and discussed spectroscopic data, reviewed the manuscript. All authors discussed the work and reviewed the manuscript. All authors approved final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boiko, V., Dai, Z., Markowska, M. et al. Particle size-related limitations of persistent phosphors based on the doped Y3Al2Ga3O12 system. Sci Rep 11, 141 (2021). https://doi.org/10.1038/s41598-020-80335-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-80335-9
This article is cited by
-
Influence of Fe3+ ions on the crystallographic, optical and electrochemical performance of ZnS quantum dots
Indian Journal of Physics (2024)
-
Effect of Ag loading on praseodymium doped ceria catalyst for soot oxidation activity
Korean Journal of Chemical Engineering (2022)
-
Effect of co-dopant proportion on the structural, optical and magnetic properties of pristine NiO nanoparticles synthesized by Sol–gel method
Journal of Materials Science: Materials in Electronics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.