Abstract
High quality silver (Ag) decorated CeO2 nanoparticles were prepared by a facile one-step chemical method. The samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), High resolution transmission electron microscopy (HR-TEM), fourier transform infrared spectrometer (FT-IR), electron paramagnetic resonance (EPR), X-ray photoelectron spectroscopy (XPS), UV–Visible absorption (UV–Vis), photoluminescence (PL) and thermogravimetric analysis. The decoration of Ag on CeO2 surface was confirmed by XRD, EPR and HR-TEM analysis. Harmful textile pollutant Rose Bengal dye was degraded under sunlight using the novel Ag decorated CeO2 catalyst. It was found that great enhancement of the degradation efficiency for Ag/CeO2 compared to pure CeO2, it can be ascribed mainly due to decrease in its band gap and charge carrier recombination rate. The Ag/CeO2 sample exhibited an efficient photocatalytic characteristic for degrading RB under visible light irradiation with a high degradation rate of 96% after 3 h. With the help of various characterizations, a possible degradation mechanism has been proposed which shows the effect of generation of oxygen vacancies owing to the decoration of Ag on the CeO2 surface.
Similar content being viewed by others
Introduction
Metal oxide nanoparticles have been considered in more consideration due its attractive applications including energy conversion, storage, solar fuel, photocatalytic and medical. Particularly, these metal oxides have been successfully used for wastewater management and water splitting. They have additionally been used for a wide scope of chemical redox reactions, for example, the mineralization of natural contaminations in wastewater1,2. The preparation of semiconductor metal oxide nanoparticles with different shapes and sizes has been of interest for application in cutting edge oxidation procedures3,4. Various transition metal oxides such as TiO2, NiO, CuO, ZnO and BiVO4 have been have been widely examined as photocatalysts for photocatalytic hydrogen production and color removal from the textile wastewater5,6,7,8,9,10. Apart from the transition metal oxides, rare earth oxides have been paid more attention due to its interesting electronic, optical and catalytic properties. As one of the most significant earth oxides, ceria (CeO2) has fascinated in more consideration for its promising application in photocatalytic dye degradation, solid oxide fuel cells, electrochemical sensor, ultraviolet filter, supercapacitor, solar cells and optical materials11,12. CeO2 is a n-type semiconductor metal oxide, it has a few properties like TiO2, for example, chemical inactivity, cheap, photo stability and non-toxicity13,14,15,16,17.
For the past one decade, color removal of organic textile dyes using suitable catalyst is an emerging target for wastewater management18,19,20. Besides, rose bengal (RB) dyes are widely used in textile, plastic, printing and cosmetic industries. But RB dyes are highly soluble in water, which commonly pollutes water and highly toxic to the living organisms21,22. Therefore, it is highly essential the removal of toxic dye from water. Photocatalyst is one of a most common cost-effective method for decompose textile-dyes from water using efficient nanocatalyst. To improve creation of active species in CeO2, various modifications were made. Among the modifications, incorporation or decoration of metal ions like silver (Ag) is an attractive method which enhanced the optical and catalytic properties of metal oxides. Such enhancement of photocatalytic behavior was obtained because of several factors such as tuning size, improved surface to volume ratio, morphology, band gap and various types of defects23. Dawoud et al.24 observed that Ag doped ZrO2 nanoparticles are effectively degraded the RB dye in visible light irradiation because of the surface area, band gap and porosity. Similar degradation effect has been observed by Ziashahbi et al.25 using Ag decorated ZnO hybrid nanostructures against methylene blue under visible light.
In this study, pure and Ag decorated CeO2 nanoparticles have been synthesized and characterized using experimental techniques. Crystallography, microstructure, optical and magnetic properties of the nanoparticles were systematically analysed. Photocatalytic behaviour of the pure CeO2 and Ag decorated CeO2 nanoparticles were evaluated by photodegradation of RB dye under visible-light irradiation.
Materials and methods
Synthesis of pure CeO2 and Ag decorated CeO2 nanoparticles
All chemicals were used without further purification with analytical grade reagent. To synthesis of Ag doped CeO2 nanoparticles, 4.3 g (0.2 M) of Ce (NO3)3·6H2O in 50 mL of deionized water and 50 ml of 0.5 g polyvinylpyrrolidone (PVP; MW:40,000) were mixed under stirring at 80 °C. Then, 1 M of sodium hydroxide (NaOH) was added drop by drop into the above solution. After 1 h, 0.2 M of 1.7 g Ag(NO3) in 50 mL deionized water was added into the above solution. Subsequently, that the white color solution was transformed into dark brown color solution indicates the decoration of Ag on the surface CeO2 nanoparticles. After 2 h stirring, the obtained brown color colloidal was purified by washing with deionized water and acetone with several times to remove impurities. The resultant powder samples were dried in an oven at 120 °C for 4 h. Following the same procedure, pure CeO2 nanoparticles was synthesized without adding the silver nitrate. The growth mechanism of Ag decorated CeO2 is demonstrated in Fig. 1a.
Characterization
Structural information of the pure and doped CeO2 was studied by powder X-ray diffraction (XRD) pattern using Rigaku miniFlex IIC diffractometer with Cu Kα radiation (λ = 1.54060 Å). Morphology of the powder samples and microstructural information were performed using scanning electron microscopy (SEM, TESCAN, VEGASEM) and HR‒TEM (JEOL 3010) microscopy. Functional groups of molecules presented in the surface of the samples were investigated by FT-IR using an AVATOR 360 spectrometer. Chemical state of the compounds studied using X-ray photoelectron spectroscopy with an Omicron Nanotechnology The chemical state in the nanocomposites was investigated by X-ray photoelectron spectrometer (XPS) using an ESCA + Omicron XPS system with a Mg-Kα source and photon energy of 1486.7 eV. Free radical information of the samples was studied by Electron paramagnetic spectroscopy (EPR) using Bruker EMX Plus Electron spectrometer. Optical properties of the samples were investigated using Varian Carry 5000 scan UV–Vis double beam spectrometer. Thermal stability and phase transition of the sample was evaluated by thermogravimetric and differential thermal analysis (TG–DTA) using DST Q600 20 thermometer with heating rate of 10°/min.
Photocatalytic study
Photodegradation of rose bengal dye was studied using pure CeO2 and Ag decorated CeO2 nanoparticles in aqueous medium under sun light. For the photocatalytic reaction, 20 mg of photocatalyst was dispersed into 50 mL rose bengal dye solution, the concentration of dye was used as 0.2 g/L. The resultant solution was stirred in dark place for 1 h before the light irradiation. Then the solution was irradiated for 3 h under sun light. To evaluate degradation performance, the irradiated solution takes out in certain time interval and it was centrifuged at 4000 rpm for 20 min. Finally, the degradation efficiency of the reacted solution was determined using UV–Vis absorption spectroscopy. The degradation study was performed under 1180 watts per square meter light intensity. The light intensity was measured using the flux meter.
Results and discussion
Structural properties of CeO2 and Ag-CeO2 nanoparticles
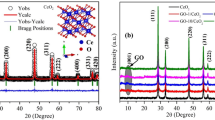
The XRD patterns of pure and Ag decorated CeO2 nanoparticles are shown in Fig. 1b. The pure nanoparticles show well–defined cubic structure of CeO2 (Fig. 1b) with the characteristic plane of (1 1 1) orientation. The obtained result is well matched with standard JCPDS data (file no. 34-0394). In the case of Ag incorporated CeO2 nanoparticles, crystalline Ag peaks are obtained along CeO2 peaks with the primary characteristic of Ag (1 1 1) plane. The attained Ag peaks are well matched with cubic structure of Ag (JCPDS file no. 87-0717). Moreover, there is no obvious peak shifts observed in CeO2 pattern by addition of Ag which reveals that Ag is presented on surface rather occupied in the interstitial or vacancy sites of CeO2. The estimated average crystallite size of pure CeO2 nanoparticle is 8.1 ± 1 nm and the size decreased to 6.5 ± 1 nm for the Ag incorporated CeO2 nanoparticles. The decrease in crystallite size is clearly evident from the peak broadening as shown in Fig. 1b. Besides the size of Ag crystallites found to be ~ 7.9 nm. The obtained predominant Ag peaks are revealed that equally distributed on the CeO2 surface. Moreover, it can be noticed that the calculated lattice parameter (~ 5.414 Å) of as-synthesized CeO2 nanoparticle is slightly higher than that of bulk CeO2 (~ 5.411 Å). For Ag incorporated samples, a higher value of CeO2 lattice parameter (~ 5.553 Å) is obtained which may be due to higher surface defects and decreasing crystallite size.
Microstructural analysis of pure and Ag incorporated CeO2 nanoparticles
Microstructural characteristics of pure and Ag decorated CeO2 nanoparticles are examined using SEM technique with different magnifications and the results are presented in Fig. 2a–d. It can be clearly seen that the obtained particle sizes are obviously in nanoscale range with agglomeration. Besides, pure CeO2 nanoparticles are spherical in shape (Fig. 2a,b), whereas, the Ag decorated nanoparticles are relatively increasing size and defined grain boundaries as seen in Fig. 2c,d. This microstructure result implies that the crystalline Ag might be surrounded over the CeO2 particles. The formation of two distinct crystalline phases (Ag and CeO2) is evident from the XRD results. To further investigate this microstructure, the Ag decorated sample is investigated using HR-TEM analysis. Figure 3a–d shows the different magnification images of Ag decorated nanoparticles, it showed uniform size and shape with homogenous distribution. The higher magnification results clearly have shown the crystallites with size ranges between 6 and 8 nm. The estimated d spacing between the lattice fringes about 0.326 nm and 0.233 nm corresponding to the respective (1 1 1) plane of cubic structure of Ag and CeO2, respectively. The estimated grain size and structure in SAED patterns (insert Fig. 3d) are in good agreement with the XRD results. The distinguish fringes in SAED pattern is indicates we crystalline nature of the sample.
FT-IR study
Figure 4a illustrates the FT-IR spectra to analyse the adsorption species on the surfaces of as-synthesized CeO2 and Ag decorated CeO2 nanoparticles. The major absorption band observed at 460 cm−1 is due to the deformation mode of Ce–O bond. The other peaks obtained at 1389 cm−1 corresponding to the O–H bending vibrations and the peak at 1010 cm−1 due to the stretching vibrations of Ce–O. It can be clearly noticed that the Ag decorated CeO2 samples shows additional peak at 800 cm−1 which could be due to the formation of Ag–O stretching vibrations (non-bridging). The peak around 1600 cm−1 can be attributed to the symmetric bending of H2O and the peak around 2350 cm−1 divulges the stretching vibrations of C-O adsorbed from the atmospheric CO224. The minor peak at 3400 cm−1 could be observed owing to the stretching vibration of O–H group.
EPR studies
Electron paramagnetic resonance (EPR) spectroscopy is a more sensitive technique to analysis about doping process or surface effect, particularly evaluate electronic configurations with unpaired spins. Figure 4b shows EPR spectra for pure and Ag decorated CeO2 nanoparticles. The obtained result showed presence of paramagnetic species. Perceptible the signal showed between 3300 and 3600 G for Ag incorporated CeO2 demonstrates Ce3+ defect states. The obvious spectral changes (Fig. 4b) are clearly showing possibility of defects enhancement by decoration of Ag with CeO2 surface. To pure CeO2, it is possible to develop ∙OH free radical through Ce3+/Ce4+ redox cycle by activation of the water molecules on the oxygen vacancy site. The developed oxygen vacancy can boost degradation efficiency. In the case of Ag decorated CeO2, the photocatalytic activity could further increase by electron acceptor (Ag2+ ↔ Ag0) and/or hole donor (Ag2+ ↔ Ag+) along with concentration of ceria redox and oxygen vacancies26. The strong EPR signals are all attributed to the O2− species on the CeO2 surface. The obtained highest photocatalytic activity caused by the highest surface oxygen vacancy concentration, as well as existence of lattice oxygen species and lattice defects formed with the participation of both silver and ceria. The multiple spectral lines in the EPR spectrum for Ag decorated CeO2 sample shows possibility of the formation of acceptor and donor during light irradiation. Hence, the obtained EPR revealed that the Ag decorated CeO2 nanoparticles can be performing as a good photocatalyst due to creation of more free radicals.
Thermal study
The thermal analysis of Ag decorated CeO2 nanoparticles are carried out up to 1000 °C and the resultant curves are shown in Fig. 4c. The TG results indicate the total weight loss ~ 28% up to 1000 °C. It can be clearly seen that the continuous weight loss has occurred up to ~ 550 °C. With further increasing the temperature up to 900 °C, drastic weight loss observed indicates the complete dehydration. But much higher temperature (> 900 °C), the weight loss started gradual increase in trend as shown in Fig. 4c. This is due to the temperature reached the melting point of Ag (~ 960 °C) followed by the silver decomposition takes place. The DTA plot illustrates broad peak appeared up to 250 °C related to the decomposition and elimination of water and other adsorbed molecules. In addition, the strong exothermic peaks between 250 and 550 °C are attributed to the oxidation and crystallization takes place in the nanoparticles. It has been reported that the crystallization of Ag takes place at the temperature from 200 to 300 °C27. From the TG and DTA results, it can be established that the Ag decorated CeO2 nanoparticles are highly thermal stable upto 250 °C. To confirm the stability of the catalyst, XRD spectrum was recorded after 5 cycles of photodegradation experiment. Figure 4d shows XRD spectrum of Ag decorated CeO2 nanoparticles after cycling test. It clearly shows the diffraction planes in the Fig. 4d are identical with before cycling sample (Fig. 1b), it indicates the sample is more stable even after 5 cycling tests.
X-ray photoelectron spectroscopy (XPS)
Chemical bonding nature of Ag decorated CeO2 nanoparticles is analysed using XPS technique. The survey spectrum in Fig. 5a indicates the sample is mainly composed of Ce, O and Ag with the respective binding energies of 880–920 eV (Ce 3d), ~ 530 eV (O 1s) and ~ 360 eV (Ag 3d). Figure 5b divulges the high-resolution Ce 3d spectra consist of spin–orbit doublets Ce 3d5/2 (~ 882 eV) and Ce 3d3/2 (917 eV). These binding energies are well-assigned to the predominant Ce4+ and Ce3+ oxidation states of CeO228. The Ce 3d region has well-separated spin–orbit splitting about 18.6 eV. Besides, it can be seen that the satellite peaks (ν1, ν2, μ1 and μ2) are observed along with Ce 3d5/2 and Ce 3d3/2 peaks in Fig. 5b related to the energy-gain (shake-down) process29. On the other hand, signature of metallic Ag peaks is observed in the Ag 3d core-level spectra as shown in Fig. 5c. The peaks at 368.2 eV (Ag 3d5/2) and 374.3 eV (Ag 3d3/2) are metallic Ag (Ag0) and well-separated spin–orbit coupling of ~ 6 eV. The obtained results are well-agreement with the previous report30. The high intensity of Ag 3d peaks due to the presence of higher Ag concentration (> 10 wt%) in the nanoparticles which corroborates with the XRD results. Besides, the satellite peaks around the lower binding energy sides of Ag 3d5/2 (~ 365.1 eV and ~ 366.8 eV) and Ag 3d3/2 (~ 372.1 eV and ~ 373.2 eV) are attributed to the Ag+ oxidation states. The deconvoluted O1s (Fig. 5d) spectra indicates the three major binding energies 527.8 eV (OIII), ~ 530 eV (OII) and ~ 532.5 eV (OI) are associated to the lattice oxygen ions (Olat) and surface adsorbed oxygen ions (Oads). The signature of surface adsorbed oxygen could be observed due to the oxygen vacancies in the crystal structure resulting from the enrichment of Ag ions29,30. In addition, C 1S peak (Fig. 5(e)) is observed corresponding to the binding energies of ~ 281.5 eV (C–C), ~ 285.1 eV (C–O) and ~ 288.9 eV (C=O) indicates the presence of organic carbon in the nanoparticles. Interestingly, no hydroxide peaks are detected in the Ag decorated CeO2 nanoparticles.
Figure 6a–c demonstrates the UV–visible and florescence spectra of pure CeO2 and Ag decorated CeO2. A strong absorption peak obtained around 320 nm for the pure CeO2 nanoparticles, and this peak is shifted towards higher wavenumber at 430 nm for the Ag decorated samples, as shown in Fig. 6a. This peak variation depicts the defects in the form of oxygen vacancies induced by the higher amount of Ag ions around the CeO2 particles. Tauc plot (Fig. 6b) illustrates the variation in band gap energy of the nanoparticles. The band gap of pure CeO2 is found to be 3.1 eV and the band gap increased to 2.8 eV for the Ag decorated CeO2 nanoparticles. The photoluminescence emission spectra of pure CeO2 and Ag decorated CeO2 nanoparticles are shown in Fig. 6c using an excitation wavelength of 365 nm. For pure CeO2 nanoparticles, the spectrum showed an intense emission peak in blue region 425 nm corresponding to the surface related defects and the other minor shoulder peaks at 460 nm and 480 nm are attributed to the dislocation or oxygen vacancies. In addition, emission at 535 nm could be anticipated from the oxygen vacancies associated defects31. In the case of Ag decorated CeO2 nanoparticles, the emission were observed in red region centred at 615 nm with much lower intensity as compared to the pure CeO2 nanoparticles. This red region emission are expected from the higher surface defects by the incorporation of more Ag metal ions as already revealed in microstructural analysis. Depending on the concentration of surface defects, the recombination process of electrons and holes are delayed which results lower intensity than pure CeO2. It has been reported that the delayed recombination process facilitates the large number of photo-generated electrons and holes during photochemical reactions32. Such behaviour is beneficial for the enhanced photocatalytic activity of Ag decorated CeO2 nanoparticles.
Photocatalytic studies
The photocatalytic properties studied for the prepared pure CeO2 and Ag decorated CeO2 samples, the degradation of Rose Bengal (RB) used as a model dye by irradiation of visible sun light. Especially, the activity of prepared photocatalysts were depends on important factors like crystal structure, size of catalytic particle morphology and composition concentration. The efficiency of photocatalytic degradation premeditated by the decolourisation of model pollutant was determined by UV–Visible spectra.
Figure 7a,b represents the UV–Visible spectra of RB dye degradation using CeO2 and Ag decorated CeO2 catalyst with irradiation time interval of 180 min under sun light. The absorption of dye molecule is gradually reduced with intensifying irradiation time of degradation due to deprivation of chromophore to form a transitional product. The degradation efficiency was calculated by,
where C0 and C are the initial and final concentration of aqueous dye solution (20 mg/L). The degradation study was carryout with different catalysts such as CeO2 and Ag decorated CeO2 catalyst. The degradation efficiency was calculated using the Eq. (1). The obtained degradation efficiency is presented in Fig. 7c.
(a,b) UV–Vis spectra of the CeO2 and Ag decorated CeO2 catalyst dispersed in Rose bengal dye dispersed solution with light irradiation of different time interval, respectively. (c,d) (C/C0) versus time interval and Plot of − ln(C/C0) versus time interval, respectively. (e) Photocatalytic degradation of rose bengal at different catalytic loads (RB—20 ppm pH-8). (f) Effect of PH on 50 mL of 20 ppm with 20 mg Ag decorated CeO2 catalyst.
The CeO2 catalyst achieved the degradation efficiency of about 74% against RB under the irradiation of visible sun light for 180 min. However, the Ag decorated CeO2 catalyst, the degradation was conducted by the same process interestingly the degradation rate was dramatically increased. The maximum efficiency obtained by the catalyst is 96% (Ag decorated CeO2) is significantly higher efficiency when compared with CeO2 due to the energy level of the Ag acts as trapper and promote the higher and easier reactive oxygen species generation. Particularly, the photo generated electron in the conduction band is effectively separated and then migrated with the energy levels of the Ag leads to reduction process. Simultaneously, in the valence band holes catalyst leads to that holes that reacts with H2O molecules that leads to the formation hydroxyl ions then promotes to hydroxyl radicals under the irradiation of visible sun light. Plasmonic effect is an another major reason to enhancing the photocatalytic performance of Ag decorated CeO2 under visible light33,34,35.
The Lagergren rate equation is widely used rate equation for the degradation of adsorbate from aqueous solution. The Lagergren first-order model can be represented as
where K was kinetic constant and the kinetic rate constants are studied by plotting—ln(C/C0) with the irradiation time (min), which is depicted in Fig. 7d. The results obtained from the pseudo first-order kinetic model beside with the experimental k and R2 values are presented in Table 1.
In Fig. 7d, the kinetic constants (k) calculated from the photocatalytic degradation of CeO2 and Ag decorated CeO2 catalysts were 0.0178, and 0.0370 min−1, respectively. The kinetic constants showed the Ag decorated CeO2 catalyst had superior performance for RB dye photocatalytic degradation under the sun light. The effect of catalyst loads of Ag decorated CeO2 nanoparticles for photocatalytic degradation was calculated 50 mL of 20 ppm dye solution at pH 8. As we know that the amount catalyst influences the degradation rate. Especially in this catalytic dosage enhance the ROS generation and increase the overall efficiency but after certain concentration the turbidity of the solution increases due to inter collisions between the particles, it becomes block the penetration of radiation. So overall efficiency again decreased. Figure 7e is clearly indicating the optimum dose value of 0.8 g/L is showed higher efficiency of photocatalytic degradation.
The pH of the solution also affects the photo degradation efficiency. The effect of pH on the photocatalytic degradation of dye was investigated by 50 mL of the dye solution with 10 mg of Ag decorated CeO2 nanoparticles and the pH value was varied from 5 to 10 by adding of 0.1 N NaOH and HCl. The surface charge of catalyst is altered imparts a change in the overall efficiency. Under the lower pH value, the anionic dye in their protonated form and catalyst also owns positive charge due to adsorption of H+ ions. So, in the acidic medium, the dye molecule repel the from the catalyst and shows lower degradation rate. As seen in the Fig. 7f, when the pH is increased from 5 to 10, initially increased the degradation rate upto pH 8, after that it was decreased. At the higher pH solution, the dye molecule and catalyst interaction increased resulting increasing degradation efficiency. Further increasing the pH value more than 8, the surface of catalyst becomes negatively charged and it promote the repulsion of dye molecule due to that reduced the overall efficiency36,37. Table 2 shows comparison of CeO2 and Ag doped CeO2 of present work with previously reported photocatalysts.
Stability test of the CeO2 and Ag decorated CeO2 catalyst is showed as 68.5% and 90%for RB dye after 5 cycles as shown in Fig. 8a. Furthermore, the presence of Ag accelerates the charge separation and reactive oxygen species (ROS) generation. The study of scavenger test is helpful to understand the mechanism of the photodegradation of dyes over active photocatalyst. Hence, the h+, OH−, and .O2− are eliminated by adding EDTA (h+ scavenger), methanol (·OH scavenger), and p-BQ (·O2− scavenger) into reaction solution. Figure 8b depicts the efficiency of with and without scavengers studies. The results reveal the influence of super oxide radicals because, addition of Benzoquinone shows less efficiency. The photocatalytic mechanism of CeO2 and Ag decorated CeO2 is shown in Fig. 8c. The mechanism of MB degradation is described as follows,
Conclusions
In summary, the pure and Ag/CeO2 nanoparticles were synthesized and characterized the structural, morphological and optical properties using various characterization techniques. The presence of Ag on CeO2 surface was confirmed by XRD, XPS and EPR spectroscopy. The UV absorption peak of pure and Ag decorated CeO2 was found at 320 nm and 430 nm and corresponding band gap calculated as 3.1 eV and 2.8 eV, respectively. The enhanced catalytic performance of Ag/CeO2 composites strongly depends on the preparation method that determines the homogeneous morphology and equally distribution of Ag on the ceria surface. Moreover, the obtained highest photocatalytic activity has proposed due to the presence of the formation of optimum level of Ce3+, oxygen vacancies and plasmonic effect. The obtained remarkable degradation efficiency for Ag decorated CeO2 nanoparticles promotes its utilization in environmental remediation. We believe that this work would have a considerable impact on the future development of efficient plasmonic metal–semiconductor photocatalysts.
References
Yoshimura, J., Ebina, Y., Kondo, J., Domen, K. & Tanaka, A. Visible light-induced photocatalytic behavior of a layered perovskite-type rubidium lead niobate, RbPb2Nb3O10. J. Phys. Chem. 97, 1970 (1993).
Bamwenda, G. R., Sayama, K. & Arakawa, H. The effect of selected reaction parameters on the photoproduction of oxygen and hydrogen from a WO3–Fe2+–Fe3+ aqueous suspension. J. Photochem. Photobiol. A 122, 175 (1999).
Rajeshwar, K. et al. Heterogeneous photocatalytic treatment of organic dyes in air and aqueous media. J. Photochem. Photobiol. C 9, 171 (2008).
Ikram, M. et al. Dye degradation performance, bactericidal behavior and molecular docking analysis of Cu-doped TiO2 nanoparticles. RSC Adv. 10, 24215–24233 (2020).
Su, J., Guo, L., Bao, N. & Grimes, C. A. Nanostructured WO3/BiVO4 heterojunction films for efficient photoelectrochemical water splitting. Nano Lett. 11, 1928–1933 (2011).
Sabzehmeidani, M. M., Karimi, H. & Ghaedi, M. Enhanced visible light-active CeO2/CuO/Ag2CrO4 ternary heterostructures based on CeO2/CuO nanofiber heterojunctions for the simultaneous degradation of a binary mixture of dyes. New J. Chem. 44, 5033–5048 (2020).
Chen, H. M. et al. Quantum dot monolayer sensitized ZnO nanowire-array photoelectrodes: True efficiency for water splitting. Angew. Chem. Int. Ed. 122, 6102–6105 (2010).
Manibalan, G. et al. High electrochemical performance and enhanced electrocatalytic behavior of a hydrothermally synthesized highly crystalline heterostructure CeO2@NiO nanocomposite. Inorg. Chem. 58, 13843–13861 (2019).
Manibalan, G., Murugadoss, G., Thangamuthu, R., Kumar, M. R. & Kumar, R. M. Facile synthesis of CeO2-SnO2 nanocomposite for electrochemical determination of L-cysteine. J. Alloys Comp. 792, 1150–1161 (2019).
Ogunniran, K. O., Murugadoss, G., Thangamuthu, R. & Nishanthi, S. T. Nanostructured CeO2/FeO3/Mn-rGO composite as anode material in Li-ion battery. J. Alloys Comp. 786, 873–883 (2019).
Kempaiah, D. M., Yin, S. & Sato, T. A facile and quick solvothermal synthesis of 3D microflower CeO2 and Gd:CeO2 under subcritical and supercritical conditions for catalytic applications. CrystEngCommun 13, 741–746 (2011).
Shajahan, S., Arumugam, P., Rajendran, R. & Munusamy, A. P. Optimization and detailed stability study on Pb doped ceria nanocubes for enhanced photodegradation of several anionic and cationic organic pollutants. Arab. J. Chem. 13, 1309–1322 (2020).
Wang, Y., Liu, T. & Liu, J. Synergistically boosted degradation of organic dyes by CeO2 nanoparticles with fluoride at low pH. ACS Appl. Nano Mater. 3, 842–849 (2019).
Ji, P., Zhang, J., Chen, F. & Anpo, M. Study of adsorption and degradation of acid orange 7 on the surface of CeO2 under visible light irradiation. Appl. Catal. B 85, 148 (2009).
Kominami, H., Tanaka, A. & Hashimoto, K. Mineralization of organic acids in aqueous suspensions of gold nanoparticles supported on cerium (IV) oxide powder under visible light irradiation. Chem. Commun. 46, 1287 (2010).
Manibalan, G., Murugadoss, G., Thangamuthu, R., Kumar, R. M. & Jayavel, R. Facile synthesis of heterostructure CeO2-TiO2 nanocomposites for enhanced electrochemical sensor and solar cell applications. J. Alloys Comp. 773, 449–461 (2019).
Murugadoss, G., Ma, J., Ning, X. & Kumar, M. R. Selective metal ions doped CeO2 nanoparticles for excellent photocatalytic activity under sun light and supercapacitor application. Inorg. Chem. Commun. 109, 107577 (2019).
Chouhan, N. et al. Photocatalytic CdSe QDs-decorated ZnO nanotubes: An effective photoelectrode for splitting water. Chem. Commun. 47, 3493–3495 (2011).
Primo, A., Marino, T., Corma, A., Molinari, R. & García, H. Efficient visible-light photocatalytic water splitting by minute amounts of gold supported on nanoparticulate CeO2 obtained by a biopolymer templating method. J. Am. Chem. Soc. 133, 6930–6933 (2011).
Lu, X.-H. et al. Monodisperse CeO2/CdS heterostructured spheres: One-pot synthesis and enhanced photocatalytic hydrogen activity. RSC Adv. 1, 1207–1210 (2011).
Lops, C. et al. Sonophotocatalytic degradation mechanisms of Rhodamine B dye via radicals generation by micro-and nano-particles of ZnO. Appl. Catal. B 243, 629–640 (2019).
Aarthi, T. & Madras, G. Photocatalytic degradation of rhodamine dyes with nano-TiO2. Ind. Eng. Chem. Res. 46, 7–14 (2007).
Seal, S. et al. Engineered defects in cerium oxides: Tuning chemical reactivity for biomedical, environmental, & energy applications. Nanoscale 12, 6879–6899 (2020).
Dawoud, T. M. S., Pavitra, V., Ahmed, P., Syed, A. & Nagaraju, G. Photocatalytic degradation of an organic dye using Ag doped ZrO2 nanoparticles: Milk powder facilitated eco-friendly synthesis. J. King Saud Univ. Sci. 32, 1872–1878 (2020).
Ziashahabi, A., Prato, M., Dang, Z., Poursalehi, R. & Naseri, N. The effect of silver oxidation on the photocatalytic activity of Ag/ZnO hybrid plasmonic/metal-oxide nanostructures under visible light and in the dark. Sci. Rep. 9, 11839 (2019).
Tang, T. et al. Photocatalytic removal of organic phosphate esters by TiO2: Effect of inorganic ions and humic acid. Chemosphere 206, 26–32 (2018).
Garner, W. E. & Reeves, L. W. The thermal decomposition of silver oxide. Trans. Faraday Soc. 50, 254–260 (1954).
Beche, E., Charvin, P., Perarnau, D., Abanades, S. & Flamant, G. Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (CexTiyOz). Surf. Interface Anal. 40, 264–267 (2008).
Chahal, S., Rani, N., Kumar, A. & Kumar, P. Electronic structure and photocatalytic activity of samarium doped cerium oxide nanoparticles for hazardous rose bengal dye degradation. Vacuum 172, 109075 (2020).
Wu, K., Zhou, L., Jia, C.-J., Sun, L.-D. & Yan, C.-H. Pt-embedded-CeO2 hollow spheres for enhancing CO oxidation performance. Mater. Chem. Front. 1, 1754–1763 (2017).
Wang, G., Mu, Q., Chen, T. & Wang, Y. Synthesis, characterization and photoluminescence of CeO2 nanoparticles by a facile method at room temperature. J. Alloys Compd. 493, 202–207 (2010).
Martha, S., Reddy, K. H., Biswal, N. & Parida, K. M. Facile synthesis of InGaZn mixed oxide nanorods for enhanced hydrogen production under visible light. Dalton Trans. 41, 14107–14116 (2012).
Kavinkumar, V. et al. Investigation of the structural, optical and crystallographic properties of Bi2WO6/Ag plasmonic hybrids and their photocatalytic and electron transfer characteristics. Dalton Trans. 48, 10235–10250 (2019).
Kavinkumar, V., Jaihindh, D. P., Atul, V., Fu, Y.-P. & Jothivenkatachalam, K. Influence of cobalt substitution on the crystal structure, band edges and photocatalytic properties of hierarchical Bi2WO6 microspheres. New J. Chem. 43, 9170–9182 (2019).
Singh, J., Rathi, A., Rawat, M., Kumar, V. & Kim, K. H. The effect of manganese doping on structural, optical, and photocatalytic activity of zinc oxide nanoparticles. Compos. B Eng. 166, 361–370 (2019).
Vidya, C., Prabha, M. N. C. & Raj, M. A. L. A. Green mediated synthesis of zinc oxide nanoparticles for the photocatalytic degradation of Rose Bengal dye. Environ. Nanotechnol. Monit. Manage. 6, 134–138 (2016).
Malini, B. & Raj, G. A. G. C, N and S-doped TiO2-characterization and photocatalytic performance for rose bengal dye degradation under day light. J. Environ. Chem. Eng. 6, 5763–5770 (2018).
Leng, Q. et al. Building novel Ag/CeO2 heterostructure for enhancing photocatalytic activity. Mater. Res. Bull. 65, 266–272 (2015).
Wu, L. et al. Facile synthesis of Ag@CeO2 core–shell plasmonic photocatalysts with enhanced visible-light photocatalytic performance. J. Hazard. Mater. 300, 93–103 (2015).
Saravanakumar, K., Ramjan, M. M., Suresh, P. & Muthuraj, V. Fabrication of highly efficient visible light driven Ag/CeO2 photocatalyst for degradation of organic pollutants. J. Alloys Compd. 664, 149–160 (2016).
Zeleke, M. A. & Kuo, D. H. Synthesis and application of V2O5-CeO2 nanocomposite catalyst for enhanced degradation of methylene blue under visible light illumination. Chemosphere 235, 935–944 (2019).
Qi, E., Niu, B., Zhang, S. & Wang, J. Preparation of nanostructured Ag/CeO2 by microwave synthesis and its photocatalysis activity. Adv. Mater. Res. 624, 88–93 (2013).
Zhang, L. et al. Synthesis of novel CeO2–BiVO4/FAC composites with enhanced visible-light photocatalytic properties. J. Environ. Sci. 26, 1936–1942 (2014).
Khan, M. H. et al. Biogenic fabrication of Au@CeO2 nanocomposite with enhanced visible light activity. J. Phys. Chem. 118, 9477–9484 (2014).
Wang, Q. et al. Synthesis of monodisperse Bi2O3-modified CeO2 nanospheres with excellent photocatalytic activity under visible light. CrystEngComm 17, 671–677 (2015).
Sabari Arul, N., Mangalaraj, D. & In Han, J. Enhanced photocatalytic property of self-assembled Fe-doped CeO2 hierarchical nanostructures. Mater. Lett. 145, 189–192 (2015).
Deng, W., Chen, D., Hu, J. & Chen, L. A. A general and green approach to synthesize monodisperse ceria hollow spheres with enhanced photocatalytic activity. RSC Adv. 5, 80158–80169 (2015).
Author information
Authors and Affiliations
Contributions
G.M.—Design the work, prepared samples and written the first draft and further modifications; D.D.K.—Written characterization part; M.R.K.—Drawn pictures and support to experiment and result in discussion; N.V.—Support to photocatalyst experiment work; P.S.—Providing lab facility for photocatalyst and formation discussion. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Murugadoss, G., Kumar, D.D., Kumar, M.R. et al. Silver decorated CeO2 nanoparticles for rapid photocatalytic degradation of textile rose bengal dye. Sci Rep 11, 1080 (2021). https://doi.org/10.1038/s41598-020-79993-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79993-6
This article is cited by
-
Diatomaceous Biosilica Doped with Heteroepitaxially Growing Ag/AgCl/CeO2 Composite Nanoparticles: Synthesis, Characterisation and Antibacterial Application
Journal of Cluster Science (2024)
-
Green Synthesis of Platinum Nanoparticles Using Polymer Bio-reduction Approach and Their Photocatalytic Organic Dye Degradation
Journal of Polymers and the Environment (2024)
-
Artemisia Pallens Assisted Synthesis of CeO2–NiO Nanocomposite for the Removal of Hazardous Rose Bengal Dye and Voltammetric Sensing of Heavy Metals
Chemistry Africa (2024)
-
Sol–gel assisted growth of nanostructured NiS/CeO2 p-n heterojunctions for fast photooxidation of ciprofloxacin antibiotic under visible light
Applied Nanoscience (2023)
-
Chemical synthesis of pristine and tin-doped ZnO for rose bengal photocatalytic application
Journal of Materials Science: Materials in Electronics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.