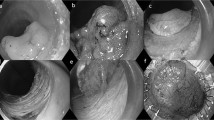
Abstract
Submucosal deep invasion of gastric cancer (T1b2; depth of submucosal invasion ≥ 500 μm) is a risk factor for lymph node metastasis and, thus, is one of the criteria for curative treatment. Our aim was to evaluate the specific influence of endoscopic submucosal dissection (ESD) on the prognosis of patients with T1b2 gastric cancer. This was a retrospective analysis of 248 consecutive patients, with 252 pT1b2 gastric cancer lesions, who underwent ESD prior to additional surgery (Group A, n = 101) or surgery only (Group B, n = 147). After propensity score-matching (for sex, age, tumor diameter and gross type), we compared pathological characteristics between the 2 groups and the prognosis over a follow-up period ≥ 60 months. Compared to Group B, patients in Group A were older, with a higher proportion of men. The proportion of depressed and undifferentiated type tumors was greater in Group B than A, with larger tumor size and depth of submucosal invasion as well. There was no incidence of local recurrence, but distant metastasis was identified in 5% of cases in Group A and 3% in Group B. After propensity score-matching, there were no difference in the 5-year overall survival rate between Group A and B (87.5% vs. 91.2%, respectively), nor in the 5-year disease-specific survival rate (96.3% vs. 96.4%, respectively). ESD prior to surgery for T1b2 gastric cancer did not adversely affect clinical outcomes after additional surgery.
Similar content being viewed by others
Introduction
In Japan, gastric cancer is one of the most common cancers and the third most common cause of cancer-related death. Endoscopic submucosal dissection (ESD) was developed in the late 1990s and has been widely used for early gastric cancer (EGC) worldwide. ESD allows en bloc resection of even large EGCs and precise histologic assessment of the resected specimen, while being a less invasive treatment than surgical resection. The current Japanese Gastric Cancer Treatment Guidelines (version 5) for ESD were published by the Japanese Gastric Cancer Association (JGC) in 20181. The new guidelines maintain the expanded criteria for ESD that were included in the previous guidelines: tumor size ≤ 30 mm; differentiated-type cancer; absence of vessel and lymphovascular involvement; and submucosal invasion < 500 μm. Additional surgery is also recommended in the new guidelines for gastric cancers with deep submucosal (SM) invasion (T1b2; depth of submucosal invasion ≥ 500 µm) identified in the pathological evaluation after ESD, due to the risk of lymph node (LN) metastasis.
The risk of LN metastases with gastric cancer with SM invasion < 500 µm (T1b1) ranges from 10.2–22.9%2,3,4,5,6,7, with the risk of LN metastases for gastric cancer with T1b2 invasion ranging between 10.6–26.8%8,9,10,11. The outcomes and prognosis of ESD for T1b1 gastric cancers have previously been reported12,13, with the usefulness and validity of ESD for T1b1 gastric cancers and EGC with ulceration having been demonstrated14,15,16. However, we identified only one study which examined the prognosis for patients with T1b2 gastric cancers who underwent ESD prior to additional surgery compared to those with T1b2 gastric cancers treated by surgical resection alone17. The evaluation of the usefulness and validity of ESD prior to additional surgery for T1b2 gastric cancers is currently limited by the significant differences in the clinical background of patients in the ESD and non-ESD groups in the study by Ojima et al.17, owing to wide range of indications for endoscopic treatment in the current guidelines. To address this limitation, we used propensity score-matching to evaluate the specific influence of ESD performed prior to additional surgery on the prognosis of patients with T1b2 gastric cancer.
Methods
Study group
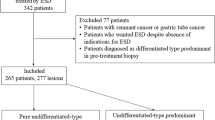
We retrospectively identified 311 consecutive patients with T1b2 gastric cancers who underwent ESD prior to additional surgery or surgery alone, between February 2002 and February 2017, at the Hiroshima University Hospital. Among patients who underwent ESD, those who did not meet the curative criteria of the JGC Guidelines were advised to undergo additional surgery. Of the 311 patients identified, 63 patients were treated using ESD only due to various reasons (refusal of surgery, comorbidity burden and/or advanced age) and were excluded. The remaining 248 patients (with 254 T1b2 gastric cancer lesions) were included in the analysis, 101 of whom underwent ESD prior to additional surgery (Group A) and the other 147 treated by surgical resection alone (Group B). In Group A, 78 patients (77%) enrolled in this study were diagnosed as M or SM1 cancer preoperatively, and the other patients were preoperatively diagnosed with SM2 cancer but they refused to undergo initial surgical resection due to old age, comorbidities, and/or activity of daily living status. According to the Japanese classification of gastric cancer, SM1 cancer was defined as a cancer which was preoperatively diagnosed as the depth of submucosal invasion < 500 µm, and SM2 cancer was defined as a cancer which was preoperatively diagnosed as the depth of submucosal invasion ≥ 500 µm. We first compared the clinicopathological features between Group A and B, and the clinical outcomes of ESD (operative time, en bloc resection, vertical margin, and complications) in Group A. En bloc resection was defined as resection in a single piece. Secondly, one-to one propensity score-matching was used to control for potential confounders for patients in Group A. After matching, 80 patients were identified in each group. Finally, we analyzed pathological characteristics between the 2 groups and the prognosis of patients over a follow-up period ≥ 60 months (Fig. 1).
Written informed consent was obtained from all patients prior to the treatment, and the study design was approved by the Ethics Committee of Hiroshima University Hospital. (No. E-1682).
The data collection and all experiments were performed in accordance with the relevant guidelines and regulations.
ESD procedure
ESD was performed using a single-channel endoscope (H260 or H260Z, Q260J; Olympus Optical Co., Ltd., Tokyo, Japan, or EG-450RD5; Fujifilm Medical, Tokyo, Japan) or a two-channel scope (GIF-2TQ260M, Olympus, or EG-450D5; Fujifilm Medical). First, marking dots were placed on the normal mucosa, at approximately 5 mm from the tumor margin, to provide a safe margin. Second, after the local injection of a 10% glycerin solution and/or 4% sodium hyaluronate into the submucosa of the gastric wall, the mucosa around the lesion was incised circumferentially using an IT-knife or IT-knife2 (Olympus Medical Systems Co., Ltd., Tokyo, Japan). Third, dissection of the submucosal layer was performed using an IT-knife / IT-knife2 (Olympus Medical Systems Co., Ltd., Tokyo, Japan) or SB Knife-GX / SB Knife-Jr (Sumitomo Bakelite Co., Ltd., Tokyo, Japan). Finally, all vessels exposed to the ulceration after ESD were coagulated using hemostatic forceps (FD-410LR; Olympus Medical Systems Co., Ltd., Tokyo, Japan). Second-look endoscopy was consistently performed on the day after ESD. Once hemostasis of the vessels on the ulceration after ESD was confirmed, the patient was permitted to eat a light meal in the evening or the following day.
Histopathological evaluation
The histopathological examination was based on the Japanese classification of gastric cancer. The specimens resected by ESD or those that were surgically resected were fixed with formalin and then sliced at 2-mm and 5-mm apart, respectively. The sections were stained with hematoxylin–eosin and then analyzed in detail. The histopathological type, tumor diameter, depth of submucosal invasion, lateral and vertical margins, and lymphovascular invasion were assessed for each slice. Immunohistochemical staining with antibodies against podoplanin (D2–40) was employed to distinguish small blood vessels from lymphoid capillaries to determine the presence of lymphatic invasion. Venous invasion was determined using Elastica van Gieson staining. The histopathological types were as follows: differentiated (well or moderately differentiated tubular adenocarcinoma or papillary adenocarcinoma) and undifferentiated (poorly differentiated tubular adenocarcinoma, mucinous adenocarcinoma, or signet ring cell carcinoma).
Surveillance program after ESD and surgery
In both Group A and B, follow-up gastroduodenal endoscopy, laboratory measurements, including tumor markers, and chest and abdominal computed tomography were conducted annually after the index procedure. Recurrence was diagnosed based on imaging studies and histopathological findings.
Measured variables
The following clinicopathological variables were evaluated for each group: sex, age, tumor location, tumor diameter, gross type, main histopathological type, and depth of SM invasion. These variables were compared between the two groups before and after propensity score-matching. The 5-year overall survival (OS) and 5-year disease-specific survival (DSS) rates for each group after propensity score-matching were assessed as long-term outcomes. The OS rate was defined as the percentage of patients who survived for a certain period after treatment. The DSS rate was defined as the percentage of patients who had not died of gastric cancer for a certain period after the index treatment.
Statistical analysis
The chi-squared test and Fisher's exact tests were used to assess the association between various categorical variables and for intergroup comparisons of clinicopathologic characteristics. The survival period was defined as the period from the date of the index procedure (ESD or surgery) to the most recent date of confirming that the patient was alive or the date of the patient's death. The OS and DSS rates were estimated using the Kaplan–Meier method. Propensity scores were calculated using a logistic regression model with the variables of sex, age, tumor diameter, and gross type. After the propensity scores were estimated, one-to-one matching was performed using the nearest-neighbor method with a caliper set at 0.2. Further, p values < 0.05 was considered significant. All analyses were performed using JMP pro 14 software (SAS Institute, Cary, NC, USA).
Results
Clinicopathological features of T1b2 gastric cancers before propensity score-matching
The baseline characteristics for Group A and Group B, before propensity score-matching, are reported in Table 1, with significant differences summarized follows. Group A had a higher proportion of men than Group B (79% (80/101) vs. 63% (93/147), p < 0.01), and patients were also older in Group A than B (69.4 ± 10.1 years vs. 67.0 ± 11.5 years, respectively; p < 0.05). In Group A, 34% (34/101) of patients were over the age of 75 years, and 14% (14/101) over the age of 89 years. A higher proportion of patients in group B than A had tumors located in the middle third of the stomach (31% (32/102) vs. 57% (86/152); p < 0.01). Likewise, the proportion of depressed type tumors and undifferentiated type tumors was also greater in group B than A: depressed tumors, 65% (66/102) versus 78% (119/152), p < 0.05; and undifferentiated tumors, 21% (21/102) versus 49% (74/152), p < 0.01. The mean tumor diameter was larger in Group B than A (24.9 ± 18.0 mm (8-60 mm) vs. 32.6 ± 22.5 mm (7-150 mm), p < 0.01), as was the depth of SM invasion (1348 ± 841 µm (500–4250 µm) vs. 1855 ± 1087 µm (500–6000 µm), p < 0.01). The mean follow-up periods were 69.8 ± 39.8 months (1–174 month) in Group A and 75.7 ± 40.7 months (0–176 months) in Group B (p = 0.241). With regard to comorbidities in Group A, 8 patients (8%) had heart disease, 5 patients (5%) had cerebrovascular disease, and 8 patients (8%) had been treated for other types of cancer. LN metastasis was identified in 16 patients (16%) in Group A and 25 (16%) in Group B (p = 0.81).
Clinical outcomes of ESD for T1b2 gastric cancers
The mean operative time was 105 ± 85 (range, 15–570) min. The en bloc resection rate was 91% (93/102), with 15% (15/102) of lesions having a positive vertical margin. In all cases, submucosal fibrosis was recognized in the resected specimen. Other characteristics of tumors in Group A were as follows (Table 2): 5% lesions (5/102) were located in the upper third of stomach and 1% (1/102) in the remnant stomach; and 4% (4/102) were undifferentiated type tumors. Bleeding occurred in 7% (7/102) lesions, with perforation in 5% (5/102). All cases could be treated conservatively, without additional surgery. Nine patients with 9 lesions in Group A (9%, 9/102) showed residual cancer in specimens resected by surgery after ESD and 16 patients with 16 lesions in Group A (16%, 16/102) showed lymph node metastasis in specimens resected by surgery after ESD.
Prognosis after treatment, after propensity score-matching
Figure 2 showed the receiver operating characteristic (ROC) curve for goodness of fit in this propensity score model. ROC curve was calculated by fitting a logistic regression model, using following clinically relevant variables; sex, age, tumor diameter, and gross type. The area under the curve was 0.635. After propensity score-matching, there was no significant difference in any of the variables between Group A and B (Table 3): sex, age, tumor location, tumor diameter, gross type, main histopathological type, depth of invasion. There was no local recurrence in either Group A or B. However, distant metastasis occurred in 5% (5/102) of cases in Group A and 3% (4/147) in Group B. The details of the cases with distant metastasis are reported in Table 4. Of note, among patients with distant metastasis, lymphovascular and/or vessel invasion was observed in 4 of the 5 patients in Group A and 3 of the 4 patients in Group B. All cases with distant metastasis within 5-years after treatment had the lymphatic vessel invasion and/ or vascular invasion. One of 5 patients with distant metastasis in Group A had no lymphovascular invasion, with distant metastasis observed at 79 months after the index treatment. The mean period to distant metastasis after treatment was 29 ± 29 (range, 7–98) months. Two of the patients with distant metastasis in Group A were alive at 98 and 27 months, respectively, with one of the patients with distant metastasis in Group B still alive at 71 months after treatment. The other patients with distant metastasis in Group A and B died of gastric cancer. Among the 3 patients with distant metastasis who were alive, LN metastasis was detected at 36 months after surgical resection in 1 patient, who subsequently underwent chemotherapy treatment, using Tegafur, Gimeracil and Oteracil Potassium, for a period of 34 months. The other 2 patients received supportive care. There was no significant difference in the rate of recurrence and the rate of death of gastric cancer between the two groups.
After propensity score-matching, there were no difference between Group A and B in terms of the 5-year OS rate (87.5% vs. 91.2%, respectively,) and 5-year DSS rates (96.3% vs. 96.4%) after treatment of T1b2 gastric, over a mean follow-up of 87 ± 36 months (Figs. 3).
This figure showed Kaplan–Meier curves for the prognosis of the two groups. After propensity score-matching, there were no significant differences in 5-year OS rates and 5-year DSS rates after treatment of gastric cancer with submucosal deep invasion between two groups. OS; overall survival rate, DSS; disease specific survival rate.
Discussion
According to the JGC Association Treatment Guideline (version 5), there are several criteria for curative resection using ESD1, including the depth of invasion, tumor diameter, and ulceration. The combination of these criteria determines the conditions for curative resection because of the risk of LN metastasis. T1b2 gastric cancers, defined by a depth of mucosal invasion ≥ 500 µm alone, were excluded from the curative criteria for ESD, with additional surgery being recommended, with LN dissection, as LN metastasis negatively impacts the prognosis of patients with gastric cancer.
Previously, we reported on the outcomes and prognosis of ESD treatment for T1b1 gastric cancers (depth of SM invasion < 500 µm) and undifferentiated-type gastric cancers12,13,18. To the best of our knowledge, our study is the first to have evaluated the effect of preceding ESD prior to additional surgery on the prognosis of patients with T1b2 gastric cancer, after propensity score-matching.
In our study group, 101 patients, with 102 T1b2 gastric cancers, underwent proceeding ESD prior to additional surgery. There were 2 main reasons why we performed ESD prior to additional surgery in patients with T1b2 gastric cancer. The first is refusal of patients, and/or their family, to proceed with surgical resection as a first course of treatment due to age, comorbidities, and/or activity of daily living status19. It has previously been reported that 34–37% of patients with gastric cancer who were over the age of 80 years died of other comorbidities20,21. It has also been previously reported that the incidence of complication with ESD was not significantly difference between elderly and non-elderly patients22. Furthermore, compared to surgical resection, ESD facilitated preservation of the whole stomach, provided a better quality of life than did surgical resection, and provided a better quality of life for patients23. Based on this evidence, we did regard our decision to proceed with ESD resection of T1b2 gastric cancers as a feasible alternative to surgical resection in these cases, due to their advanced age and level of comorbidity. We performed conventional endoscopy, endoscopic ultrasonography (EUS), and chromoendoscopy for all gastric cancers, with the exception of obvious advanced cases of gastric cancer. A meta-analysis reported on the high sensitivity and specificity of accurate prediction of the depth of invasion of gastric cancer by EUS24. However, the pre-operative diagnosis of tumor invasion by conventional endoscopy and/or EUS does not have the same level of diagnostic performance, with a rate of accurate diagnosis of tumor invasion depth ranging between 62–81% for convention endoscopy and 67–85% for EUS25,26,27,28. Moreover, several studies have reported on the difficulty in performing accurate diagnosis for the lesions in the cardia of the stomach, as well as lesions with ulceration or perifocal inflammatory, and large lesions (> 30 mm)24,29,30,31. In our study, 78 lesions (77%) that were diagnosed as M or SM1 cancer were resect by ESD and finally diagnosed as T1b2 carcinoma by pathological examination.
Previous studies reported a rate of perforation during ESD procedure of 1.2–6.1%32,33,34,35. The risk factors for perforation include the location of the lesion in the stomach, tumor size, elevated macroscopic type, old age, submucosal fibrosis, and depth of invasion35. Of note, depth of invasion was reported as a risk factor for perforation for lesions with invasion of the muscularis mucosa. For lesions with submucosal invasion, the rate of perforations did not significantly differ between EGCs with mucosal invasion and those with submucosal invasion. In fact, in our study, intra-operative perforation occurred in 5 cases (5%, 5/102), with all 5 cases successfully treated using a conservative approach. Our results, therefore, indicate that ESD can be safely performed, even for T1b2 gastric cancer.
With regard to long term outcomes in Group A, the recurrence with distant metastasis occurred in 5 cases (5%, 5/102). Of these five cases with distant metastasis, 1 case had a positive vertical margin in ESD specimen (4%, 1/23) and 1 case had perforation during ESD (20%, 1/5).
LN metastasis is a risk factor for cancer recurrence. In our study, LN metastasis was identified in 16 patients (16%) in Group A and 25 (16%) in Group B (p = 0.81). This is consistent with the findings of previous studies that reported a rate of LN metastasis of 10–17% after surgical resection of T1b2 gastric cancer36,37,38,39,40. A depth of mucosal invasion ≥ 500 μm is, itself, a risk factor for LN metastasis. Several studies have reported the risk factors for LN metastasis to be a positive vertical margin, submucosal invasion, lymphovascular invasion, venous invasion, and undifferentiated-type cancers12,40,41,42,43. The 2018 JGC Treatment Guidelines adopted the eCura as risk scoring system for LN metastasis1. The eCura system included 5 clinicopathologic factors, with 3 points for lymphatic invasion and 1 point each for tumor size > 30 mm, positive vertical margin, venous invasion, and A depth of mucosal invasion ≥ 500 μm. A total score 0–1 is indicative of a low risk for LN metastasis, with a score of 2–4 indicative of an intermediate risk, and a score of 5–7 of a high risk. In Group A, 2 cases of LN metastasis were in the low risk group (4%: 2/49), with 4 cases being in the intermediate risk group (14%: 4/28), and 10 in the high-risk group (42%: 10/24). Finally, the overall survival rate and disease-specific survival rate after propensity score-matching was not significantly different between Group A and B. The results of this study suggest that the strategy of initial ESD may be acceptable prior to the additional surgical resection for SM2 gastric cancer preoperatively.
The limitations of our study need to be acknowledged. First, the results were obtained from a retrospective assessment based on the medical records of patients undergoing gastric ESD at a single cancer center in Japan. As such, a selection bias cannot be denied. We did perform a propensity score-matching analysis to minimize differences between the two groups. Second, the sample size after propensity score-matching was relatively small as the study was conducted in a single center and there were large differences in the background characteristics between the two groups. Thus, a prospective multicenter study over a period of 5 years is required to more precisely evaluate the clinical outcomes of ESD in patients with T1b2 gastric cancer. Third, the cut intervals of the specimens resected by ESD and surgery are different (2 mm vs. 5 mm). This may affect the histopathological examination. Fourth, in this study, many lesions enrolled in Group A were diagnosed as M or SM1 cancer preoperatively. Ideally, the inclusion in group A is considered to be limited to the patients with SM2 who rejected initial standard gastrectomy.
In conclusion, ESD for patients with T1b2 gastric cancer does not adversely impact clinical outcomes after additional surgery, so long as an appropriate vertical margin can be obtained during resection.
Abbreviations
- ESD:
-
Endoscopic submucosal dissection
- EGC:
-
Early gastric cancer
- JGC:
-
Japanese Gastric Cancer Association
- SM:
-
Submucosal
- LN:
-
Lymph node
- OS:
-
Overall survival
- DSS:
-
Disease-specific survival
- ROC curve:
-
Receiver operating characteristic curve
References
Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2018 Ver. 5 (Kanehara & CO. Ltd., Tokyo, 2018).
Choi, J. Y. et al. Non-curative endoscopic resection does not always lead to grave outcomes in submucosal invasive early gastric cancer. Surg Endosc. 29, 1842–1849 (2015).
Onogawa, S. et al. Expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in early gastric carcinoma: correlation with clinicopathological parameters. Cancer Lett. 226, 85–90 (2005).
Park, Y. D. et al. Factors related to lymph node metastasis and the feasibility of endoscopic mucosal resection for treating poorly differentiated adenocarcinoma of the stomach. Endoscopy 40, 7–10 (2008).
Hirasawa, T. et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer 12, 148–152 (2009).
Son, H. J. et al. Characteristics of submucosal gastric carcinoma with lymph node metastatic disease. Histopathology 46, 158–165 (2005).
Nonaka, S. et al. Clinical impact of a strategy involving endoscopic submucosal dissection for early gastric cancer: determining the optimal pathway. Gastric Cancer 14, 56–62 (2011).
Hoteya, S. et al. Clinicopathological outcomes of patients with early gastric cancer after non-curative endoscopic submucosal dissection. Digestion 93, 53–58 (2016).
Lee, I. S. et al. Suitability of endoscopic sub-mucosal dissection for treatment of submucosal gastric cancers. Br. J. Surg. 100, 668–673 (2013).
Kurihara, N. et al. Lymph node meta-stasis of early gastric cancer with submucosal invasion. Br. J. Surg. 85, 835–839 (1998).
Park, D. J. et al. Lymph node metastasis in early gastric cancer with submucosal invasion: feasibility of minimally invasive surgery. World J. Gastroenterol. 10, 3549–3552 (2004).
Sanomura, Y. et al. Clinical validity of endoscopic submucosal dissection for submucosal invasive gastric cancer: a single-center study. Gastric Cancer 15, 97–105 (2012).
Sanomura, Y. et al. Predicting the absence of lymph node metastasis of submucosal invasive gastric cancer: expansion of the criteria for curative endoscopic resection. Scand. J. Gastroenterol. 45, 1480–1487 (2010).
Oka, S. et al. Endoscopic submucosal dissection for residual/local recurrence of early gastric cancer after endoscopic mucosal resection. Endoscopy 38, 996–1000 (2006).
Higashimaya, M. et al. Endoscopic submucosal dissection for residual early gastric cancer after endoscopic submucosal dissection. Gastrointest. Endosc. 77, 298–302 (2013).
Higashimaya, M. et al. Outcome of endoscopic submucosal dissection for gastric neoplasm in relationship to endoscopic classification of submucosal fibrosis. Gastric Cancer 16, 404–410 (2013).
Ojima, T., Takifuji, K., Nakamura, M., Nakamori, M. & Yamaue, H. Feasibility of endoscopic submucosal dissection for submucosal-invasive gastric cancer and the predictors of residual or recurrent cancer. Surg. Laparosc. Endosc. Percutan. Tech. 26, 401–405 (2016).
Sanomura, Y. et al. Continued use of low-dose aspirin does not increase the risk of bleeding during or after endoscopic submucosal dissection for early gastric cancer. Gastric Cancer 17, 489–496 (2014).
Kim, E. R. et al. Effect of rescue surgery after non- curative endoscopic resection of early gastric cancer. Br. J. Surg. 102, 1394–1401 (2015).
Moriguchi, S., Maehara, Y., Korenaga, D., Sugimachi, K. & Nose, Y. Relationship between age and the time of surgery and prognosis after gastrectomy for gastric cancer. J. Surg. Oncol. 52, 119–123 (1993).
Kitamura, K. et al. Clinicopathological characteristics of gastric cancer in the elderly. Br. J. Cancer 73, 798–802 (1996).
Yamaguchi, H. et al. Impact of gastric endoscopic submucosal dissection in elderly patients: the latest single center large cohort study with a review of the literature. Medicine https://doi.org/10.1097/MD.0000000000014842 (2019).
Jeon, H. K. et al. Long-term outcome of endoscopic submucosal dissection is comparable to that of surgery for early gastric cancer: a propensity-matched analysis. Gastric Cancer 21, 133–143 (2018).
Lee, I. S. et al. Suitability of endoscopic submucosal dissection for treatment of submucosal gastric cancers. Br. J. Surg. 100, 668–673 (2013).
Kurihara, N. et al. Lymph node metastasis of early gastric cancer with submucosal invasion. Br. J. Surg. 85, 835–839 (1998).
Choi, J. et al. Is endoscopic ultrasonography indispensable in patients with early gastric cancer prior to endoscopic resection?. Surg. Endosc. 24, 3177–3185 (2010).
Tsujii, Y. et al. Integrated diagnostic strategy for the invasion depth of early gastric cancer by conventional endoscopy and EUS. Gastrointest. Endosc. 82, 452–459 (2015).
Watari, J. et al. What types of early gastric cancer are indicated for endoscopic ultrasonography staging of invasion depth?. World J. Gastrointest. Endosc. 8, 558–567 (2016).
Yamamoto, S. et al. Evaluation of endoscopic ultrasound image quality is necessary in endosonographic assessment of early gastric cancer invasion depth. Gastroenterol. Res. Pract. https://doi.org/10.1155/2012/194530 (2012).
Park, J. S., Kim, H., Bang, B., Kwon, K. & Shin, Y. Accuracy of endoscopic ultrasonography for diagnosing ulcerative early gastric cancers. Medicine https://doi.org/10.1097/MD.0000000000003955 (2016).
Costa, J. M. et al. Accuracy of endoscopic ultrasound in gastric adenocarcinoma patient selection for neoadjuvant therapy. United Eur. Gastroenterol. J. 7, 278–286 (2019).
Mannen, K. et al. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: analysis of 478 lesions. J. Gastroenterol. 45, 30–36 (2010).
Chung, I. K. et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest. Endosc. 69, 1228–1235 (2009).
Imagawa, A. et al. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy 38, 987–990 (2006).
Yamamoto, Y. et al. Management of adverse events related to endoscopic resection of upper gastrointestinal neoplasms: review of the literature and recommendations from experts. Dig. Endosc. 31(Suppl 1), 4–20 (2019).
Ishii, S. et al. Predictive factors for lymph node metastasis in additional gastrectomy after endoscopic resection of cT1aN0 gastric cancer. Surg. Today. 46, 1031–1038 (2016).
Ito, H. et al. Long-term oncological outcomes of submucosal manipulation during non-curative endoscopic submucosal dissection for submucosal invasive gastric cancer: a multicenter retrospective study in Japan. Surg. Endosc. 32, 196–203 (2018).
Ryu, K. W. et al. Surgical indication for non-curative endoscopic resection in early gastric cancer. Ann. Surg. Oncol. 14, 3428–3834 (2007).
Ito, H. et al. Surgical outcomes and clinicopathological characteristics of patients who underwent potentially noncurative endoscopic resection for gastric cancer. A report of single-center experience. Gastroenterol. Res. Pract. https://doi.org/10.1155/2013/427405 (2013).
Yang, H. J. et al. Predictors of lymph node metastasis in patients with non-curative endoscopic resection of early gastric cancer. Surg. Endosc. 29, 1145–1155 (2015).
Suzuki, H. et al. Clinical outcomes of early gastric cancer patients after noncurative endoscopic submucosal dissection in a large consecutive patient series. Gastric Cancer 20, 679–689 (2017).
Yamada, T. et al. Risk factors for submucosal and lymphovascular invasion in gastric cancer looking indicative for endoscopic submucosal dissection. Gastric Cancer 17, 692–696 (2014).
Oka, S. et al. Clinical validity of the expanded criteria for endoscopic resection of undifferentiated-type early gastric cancer based on long-term outcomes. Surg. Endosc. 28, 639–647 (2014).
Author information
Authors and Affiliations
Contributions
Study Concept and Design: K.K., S.O., S.T. Data Acquisition: K.K, N.Y., K.H, T.K., T.B., K.A., F.S. Analysis and Interpretation of Data: K.K., S.O., S.T. Drafting of the Manuscript: K.K., S.O. Critical Revision of the Manuscript: S.O., S.T., K.C. Statistical Analysis: K.K., S.O., S.T. Study Supervision: K.C. Final Approval of the Manuscript: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuroki, K., Oka, S., Tanaka, S. et al. Preceding endoscopic submucosal dissection in submucosal invasive gastric cancer patients does not impact clinical outcomes. Sci Rep 11, 990 (2021). https://doi.org/10.1038/s41598-020-79696-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79696-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.