Abstract
Individual differences in personality are associated with variation in healthy aging. Health behaviours are often cited as the likely explanation for this association; however, an underlying biological mechanism may also exist. Accelerated leukocyte telomere shortening is implicated in multiple age-related diseases and is associated with chronic activation of the hypothalamus–pituitary–adrenal (HPA) axis, providing a link between stress-related personality differences and adverse health outcomes. However, the effects of the HPA axis are tissue specific. Thus, leukocyte telomere length may not accurately reflect telomere length in disease-relevant tissues. Here, we examined the correlation between stress reactivity and telomere length in heart and brain tissue in young (6–9 month) and aging (18 month) zebrafish. Stress reactivity was assessed by tank diving and through gene expression. Telomere length was assessed using quantitative PCR. We show that aging zebrafish have shorter telomeres in both heart and brain. Telomere length was inversely related to stress reactivity in heart but not brain of aging individuals. These data support the hypotheses that an anxious predisposition contributes to accelerated telomere shortening in heart tissue, which may have important implications for our understanding of age-related heart disease, and that stress reactivity contributes to age-related telomere shortening in a tissue-specific manner.
Similar content being viewed by others
Introduction
Multiple epidemiological studies report associations between personality traits such as dispositional optimism, anxiety or depression and (un)healthy aging in both cross-sectional and longitudinal designs1,2. However, the majority of studies of personality and aging have relied on self-report measures of symptoms, pain or physical functioning in old age. This means that much of the correlation between personality and healthy aging may not be causal, but instead could be explained by the reporting biases of individuals. Recognising this limitation, some studies have focused on objective measures of aging including mortality, cardiovascular disease, immune function, or cancer. While these studies support the role of personality in healthy aging2, rather than measuring aging per se, many of these objective measures relate to symptoms of diseases or disorders with their own specific genetic and environmental risk factors.
In an attempt to obtain a more objective measure of the impact of personality on aging, more recent research has examined the link between personality and telomere length3,4,5,6. Telomeres, tandem repeat guanine-rich DNA sequences, are specialised protective caps located at the ends of eukaryotic chromosomes. Crucial for the maintenance of genomic stability, telomeres protect against the attrition of genetic material, shortening with each cell division in most somatic tissues due to incomplete chromosome replication7. Over time, progressively shortened telomeres may lead to cell-cycle arrest8, apoptosis9 or senescence10. As such, telomere length may be considered as both a ‘mitotic clock’, reflecting cellular aging, and a mechanism through which age-related disease occurs11,12. Using leukocyte telomere length as a biomarker of cellular aging, several studies have reported a significant correlation between personality traits and telomere length3,4,5,6.
Whilst personality influences several behaviours that may contribute to accelerated cellular aging, for example, exercise, smoking, and dietary habits13,14, personality differences, such as optimism or depressive tendency, are also associated with differences in stress perception and physiological stress response15,16. Psychological stress leads to activation of the hypothalamus–pituitary–adrenal (HPA) axis, the body’s physiological stress response system. Research has demonstrated a link between physiological stress response and cellular aging, with telomere shortening associated with chronic activation of the HPA axis17, as well as with pro-inflammatory cytokines generated during stress related disorders such as major depression18,19. Consistent with the hypothesis that individuals with an increased tendency to perceive a given situation as stressful are likely to have a higher lifetime of perceived stress, greater lifetime activation of the HPA axis and age-related disease, the likelihood of onset and progression of age-related diseases is associated with an anxious personality20,21,22 and HPA axis dysfunction23,24. It is therefore thought that individual differences in stress reactivity (individual differences in tendency to perceive and respond to a given situation as stressful) may contribute to telomere shortening, i.e. accelerated aging, and, in turn, age-related disease25,26,27,28.
However, many clinical studies pointing to a relationship between telomere length and either personality or age-related disease assess telomere length from blood samples, reporting leukocyte telomere length as a proxy measure for cellular aging3,4,5,6,29,30,31. The true meaning of such research relies upon the fundamental assumption that changes in telomere length are robust across tissues, and that leukocyte telomere length faithfully reflects tissues relevant to disease, such as the heart and brain. However, the HPA axis stress response has been shown to elicit its effects in a tissue-specific manner32,33. Thus, leukocyte telomere length may not accurately reflect the relationship between stress reactivity and telomere length in disease-relevant tissues.
Here, we used a population of zebrafish and a pseudo-longitudinal design to test the hypothesis that individual differences in stress reactivity contribute to age-dependent telomere shortening in disease-relevant tissues. Zebrafish have emerged as a promising animal model to explore telomere dynamics due to important similarities with humans. These include high genomic conservation34, a hypothalamic-pituitary-interrenal (HPI) axis analogous to the human HPA axis35, age-dependent telomere shortening36,37,38, and, unlike mice, similar telomere lengths to humans39,40. Furthermore, zebrafish have been shown to have stable individual differences in anxiety-like behaviours and stress reactivity41. On the basis that zebrafish with a higher reactivity to a novel stressor are likely fish that have a higher lifetime of perceived stress, and therefore higher lifetime HPI activity, we predict that fish that show high levels of anxiety-like behaviour in a novel tank diving test will have shorter telomeres.
Materials and methods
Subjects
All in vivo experimental protocols in this study were reviewed and approved by the QMUL ethics committee (AWERB) following consultation of the ARRIVE guidelines (NC3Rs, UK) and conducted in accordance with the Animals (Scientific Procedures) Act, 1986 and Home Office Licenses. 256 adult Tuebingen (TU, wild type) zebrafish (Danio rerio), 134 males and 122 females, were reared to 6–9 months (133 subjects) or 18 months (123 subjects) of age. Fish were housed in a recirculating system (Techniplast, UK) with a light:dark cycle of 14:10 and a constant temperature of 28.4 °C. All subjects were fed twice daily with ZM-400 fry food (Zebrafish Management Ltd, Winchester, United Kingdom) in the morning and brine shrimp in the afternoon.
Study design
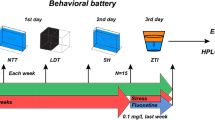
To test the hypothesis that stress reactivity contributes to age-related telomere shortening in a tissue-specific manner we identified individual differences in the zebrafish anxiety-like behavioural response in a population of adult zebrafish at 6–9 months (young) and 18 months (aging) of age using the novel tank diving assay. The novel tank diving assay is a zebrafish version of the rodent open field test and therefore assesses anxiety-like behavioural response to an immediate stressor; i.e. stress reactivity. Behaviourally determined stress reactivity was validated through the mRNA expression of HPI axis related genes. Correlations between behaviourally determined stress reactivity, HPI axis gene expression and tissue-specific telomere length in young and old, male and female zebrafish were determined.
Novel tank diving assay
The novel tank diving test was performed as described previously34. Briefly, after one hour of acclimation to the test room, zebrafish were individually placed in a 1.5-L trapezoid tank filled with system water (Fig. 1a). Their swimming behaviour was immediately recorded from the side using a DMK 21AF04 Firewire Monochrome camera for 5 min. Swimming activity, including the parameters of bottom dwelling, mobility state and distance covered, were tracked and analysed using EthoVision software (Noldus Information Technology, Wageningen, NL). Water was replaced between trials. Zebrafish were tested in two separate batches. For each batch the test was conducted over consecutive days between 10:00 and 13:00, with groups counter-balanced for age. On completion of the assay, subjects were single housed until assigned stress reactivity status and/or tissue harvesting. We performed a Tukey posthoc test on the bottom dwelling data pooled across age and sex to determine which time point represented the earliest ‘recovery’ from the stress. We constructed a betabinomial GLMM based on this pooled data with the proportion of time spent swimming on the bottom as the response variable, minute as the fixed effect, and batch number and individual ID as random effects. The determined ‘earliest recovery’ time point was used to select the individuals for molecular analysis.
Zebrafish selected for molecular analysis based on behaviourally determined stress reactivity. (a) Diagram of the novel tank assay: each fish is individually transferred from its home tank to a novel test tank. The time (seconds) spent in the bottom in each minute was used for analysis. (b) Entire population results for bottom dwelling by minute over the course of the novel tank test. (c) Population distribution of young (6–9 month) and aging (18 month) males and females based on their bottom dwelling in minute two (N = 256). (d) Bottom dwelling differences in minute two for the fish selected for molecular analysis (N = 64). Note that (d) corresponds to either extreme of the population distribution in (c). Error bars in (b) represent ± standard error mean (SEM). For (c) and (d) middle lines indicate median values and error bars represent interquartile range. YFL young female low stress reactivity, YFH young female high stress reactivity, YML young male low stress reactivity, YMH young male high stress reactivity, AFL aging female low stress reactivity, AFH aging female high stress reactivity, AML aging male low stress reactivity, AMH aging male high stress reactivity. ***p-value < 0.001.
Tissue harvesting
Fish used for dissection were selected based on bottom dwelling results (Fig. 1b). Fish were sorted as high/low stress reactive based on their bottom dwelling time in minute 2 (Fig. 1c). We selected eight fish, as a conservative estimate of the minimum number likely to yield signficant results based on results from previous studies37, from the top and bottom of the distribution for each age and sex (N = 64) (Fig. 1d). The fish were euthanised with overdose of tricaine methane sulfonate (250 mg/L, MS222, sigma). Samples of whole-brain and heart tissue were dissected out, immediately snap-frozen and stored at − 80 °C until required.
Extracting genomic DNA
Genomic DNA was isolated from whole-brain and heart samples using the HotSHOT protocol42. In summary, 100 μL of 50 mM NaOH was added to each sample, which was then incubated at 95 °C for 30 min in a heat block. Once completed, 10 μL of 1 M Tris–HCL, pH 8.0, was added to neutralise the pH.
Extracting mRNA and cDNA library generation
Total RNA was isolated from whole-brain samples using TRIzol. The protocol followed is as stated in the TRIzol Reagent User Guide (Invitrogen). Briefly, following sample homogenisation with TRIzol, RNA was isolated through precipitation, washing, and resuspension. RNA concentrations and quality were checked using a Thermo Scientific NanoDrop 2000. Corresponding cDNA libraries were generated using the ProtoScript II First Strand cDNA Synthesis Kit (NEB (UK) Ltd., Hitchin, UK).
Real-time quantitative PCR
In order to investigate differences in HPI axis and smurf2 gene expression, relative qPCR assays were then performed using SYBR Green (Applied Biosystems) and the CFX Connect Real-Time System (Bio-Rad), with all reactions performed in triplicate. Reference genes for these qPCR analyses were β-actin and rpll3α. PCR reactions were performed at 95 °C for 5 min followed by 50 cycles of 95 °C for 10 s, 60 °C for 12 s and 72 °C for 12 s. Relative mRNA expression was calculated using a modified version of the Pffafl method to account for multiple reference genes and slight variation in primer amplification efficiency43 (Supplementary Table 4). All primer sequences can be found in Supplementary Table 4. Missing data were assigned the highest measured Cq for that gene of interest plus one as performed previously44.
To analyse relative average telomere length of the samples, qPCR was again conducted using SYBR Green (Applied Biosystems) and the CFX Connect Real-Time System (Bio-Rad), with all reactions performed in triplicate. The protocol used was a modified version of the method described by Cawthon45. Telomere length qPCR reactions were performed at 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 54 °C for 2 min. Relative telomere length was expressed as 1/(Cqtelomere/CqSingleCopyGene). The single copy gene used as reference for the relative ratio was c-fos, primers without intra-exon introns (Supplementary Table 4).
Statistical analysis
All statistical analyses of behavioural data were carried out in R version 3.2.2 (R core developer team), and linear mixed effects models (LME) were fitted using the lme4 package46, generalized mixed effect models (GLMM) were fitted using the glmmTMB package47. Data distributions were initially assessed visually, and model diagnostics were subsequently checked to assure appropriate fits. We hence fitted LMEs for normally distributed data (total distance moved) and GLMMs with betabinomial error distributions where binomial models had been overdispersed48. We used the emmeans package in R (Lenth, 2019) to perform Tukey posthoc tests. Unless indicated, the details of our posthoc analysis are contained in our Supplementary Materials.
Differences in gene expression and telomere length were assessed using three-way between-subjects ANOVAs with age (aging, young), sex (male, female), and stress reactivity (low, high) as the independent variables. All follow-up tests generating simple effect comparisons were conducted with the use of SPSS syntax and, to account for multiple testing, Bonferroni corrections were applied. Pearson’s product-moment correlations were run to assess the relationship between telomere length, HPI axis gene expression and smurf2 gene expression. Normal distribution of variables was assessed by Shapiro–Wilk’s test (p > 0.05). Gene expression data were log 10 transformed to achieve normal distribution for parametric statistical analysis. Gene expression descriptive statistics for significant effects and interactions are shown as log 10 transformed. Log 10 transformed descriptive statistics are presented in Supplementary Table 2 for three-way between-subjects ANOVAs. Untransformed relative fold gene expression descriptive statistics are presented in Supplementary Table 1. All effects are reported as significant at p < 0.05. All molecular analyses were conducted using SPSS Statistics Version 26 (IBM Corporation, 2019).
Results
Behavioural analysis
Bottom dwelling tendency
Overall, time was a significant predictor of bottom dwelling tendency (Likelihood Ratio Test (LRT) = 44.9, p < 0.001). Pairwise comparisons of bottom-dwelling tendency at each minute, suggested that the first minute significantly differed from all other timepoints (p < 0.001 for all comparisons). However, the second minute did not significantly differ from subsequent timepoints in terms of bottom dwelling tendency (p > 0.98 for all comparisons, Fig. 1b). Since minute 2 was the earliest time point at which animals differed in behaviour, it was used to select individuals for molecular analysis (Fig. 1c,d). Zebrafish that had low bottom dwelling tendency in the second minute were classed as low stress reactive and those that maintained a high degree of bottom dwelling in minute 2 were classed as high stress reactive. When the entire 5 min period was analysed, fish that were classed as high or low stress reactive on the basis of bottom dwelling at minute 2 also grouped at the top or bottom of the population distribution for bottom dwelling over the 5 min period.
The interaction between age and minute was a significant predictor of the proportion of time per minute spent on the bottom of the tank (LRT = 23.7, p < 0.001), and post-hoc analysis revealed that aging fish exhibited less bottom dwelling over time, but young fish did not change their bottom dwelling tendency over time (Fig. 2a: see Supplementary Information for additional results). The interaction between sex and minute was not significant (LRT = 1.9, p = 0.168), however, sex was significant as a main effect (LRT = 8.2, p = 0.004), with males spending more time on the bottom (M = 0.688, SD = 0.258) than females (M = 0.583, SD = 0.315;).
Results of the novel tank diving test. (a) Overall proportion of time spent in the bottom third of the tank over the duration of the test. (b) Distance covered by males and females for each minute of the novel tank test. (c) Proportion of time aging and young, and (d) males and females spent in an immobile state over the 5 min of the test. All error bars represent ± standard error mean (SEM).
Total distance moved
The interaction between sex and minute was a significant predictor of total distance moved per minute (LRT = 7.0, p = 0.008), and post-hoc analysis revealed that males increased their distance moved at a faster rate than females (Fig. 2b). However, the interaction between age and minute was not significant (LRT = 2.2, p = 0.137), nor was age as a main effect (LRT = 2.2, p = 0.133).
Immobilization tendency
The interaction between age and minute was a significant predictor of the proportion of time per minute spent immobile (LRT = 6.5, p = 0.011). Aging fish spent more time immobile (proportion of time per minute spent immobile = 0.743, SD = 0.156) than young fish (proportion of time per minute spent immobile = 0.652, SD = 0.188; Fig. 2c), and post-hoc analysis revealed that aging fish resumed movement more slowly than younger fish. The interaction between sex and minute was also a significant predictor of the proportion of time per minute spent immobile (LRT = 17.8, p < 0.001). Females spent more time immobile (proportion of time per minute spent immobile = 0.736, SD = 0.177) than males (proportion of time per minute spent immobile = 0.659, SD = 0.173; Fig. 2d), and post-hoc analysis revealed that females resumed movement more quickly than males.
HPI axis gene expression
Fish at the extremes of the tank diving distributions were assessed for differences in the expression of HPI axis genes as well as analysis of telomere length. We performed qPCR on the expression of corticotropin-releasing factor (crf), mineralocorticoid receptor (mr) and glucocorticoid receptor α (grα) (Fig. 3a,b). Subjects identified as more stress reactive in the novel tank assay were found to have significantly higher expression of crf and mr compared with their less stress reactive siblings; they were also found to have a significantly higher Mineralocorticoid:Glucocorticoid (MR:GR) ratio, an important factor in maintaining HPA axis homeostasis and regulating the stress response49 (Fig. 3c).
Analysis of HPI axis-associated gene expression within brain extracts by qPCR validates behaviourally determined stress reactivity. qPCR analysis of the HPI axis-associated genes, corticotropin-releasing factor (crf), glucocorticoid receptor α (grα) and mineralocorticoid receptor (mr) in association with zebrafish (a) sex and (b) stress reactivity (N = 96). (c) Stress reactivity had a significant effect on the MR:GR ratio, a key indicator of HPA axis dysfunction (N = 32). The expression of SMAD Specific E3 Ubiquitin Protein Ligase (smurf2) was also analysed through qPCR and was significantly associated with both (d) sex and (e) stress reactivity (N = 32). Box plots indicate median values (middle lines), first and third quartiles (box edges) and minimum and maximum values (whiskers). *p-value < 0.05; **p-value < 0.01.
Corticotropin-releasing factor (crf)
The three-way between-subjects ANOVA revealed that the main effect of sex was significant (F(1,24) = 11.87, p = 0.002, partial η2 = 0.331). Males (M = 0.54, SD = 0.43) had significantly higher crf expression compared with females (M = − 0.03, SD = 0.58). The main effect of behavioural stress reactivity was also significant (F(1,24) = 9.20, p = 0.006, partial η2 = 0.277). Subjects with high stress reactivity (M = 0.50, SD = 0.52) had significantly higher crf expression compared with low stress reactive subjects (M = 0.00, SD = 0.54).
Glucocorticoid receptor alpha (grα)
The three-way between-subjects ANOVA revealed no significant main effects nor any significant interactions (Supplementary Table 3).
Mineralocorticoid receptor (mr)
The three-way between-subjects ANOVA revealed that the main effect of behavioural stress reactivity was significant (F(1,24) = 8.18, p = 0.009, partial η2 = 0.254). High stress reactive subjects (M = 0.35, SD = 0.29) had significantly higher mr expression compared with subjects with low stress reactivity (M = 0.00, SD = 0.41).
Mineralocorticoid:glucocorticoid (MR:GR) ratio
The three-way between-subjects ANOVA revealed that the main effect of behavioural stress reactivity was significant (F(1,24) = 11.04, p = 0.003, partial η2 = 0.315). Subjects with high behaviourally determined stress reactivity (M = 0.49, SD = 0.34) had a higher MR:GR ratio compared with low stress reactive zebrafish (M = 0.00, SD = 0.48).
Telomere length
Telomere length was shorter in both brain and heart tissue from aging fish irrespective of the level of stress reactivity (Fig. 4a,b). Heart tissue of aging subjects with high stress reactivity had significantly shorter telomeres compared with individuals with low stress reactivity (Fig. 4a). No significant effect of stress reactivity on telomere length in brain tissue was seen. No sex differences were identified in the telomere analysis.
Telomere length shortens with age in both heart and brain and correlates with stress reactivity in the heart, but not brain, of zebrafish. (a) Relative telomere length ratios calculated by qPCR for heart tissue grouped by age, sex and stress reactivity (N = 31). Age and stress reactivity in aging zebrafish have a signficant effect on telomere length in heart tissue. (b) Relative telomere length ratios for brain grouped by age, sex and stress reactivity (N = 32). Only age has a significant effect. Box plots indicate median values (middle lines), first and third quartiles (box edges) and minimum and maximum values (whiskers). YFL young female low stress reactivity, YFH young female high stress reactivity, YML young male low stress reactivity, YMH young male high stress reactivity, AFL aging female low stress reactivity, AFH aging female high stress reactivity, AML aging male low stress reactivity, AMH aging male high stress reactivity. **p-value < 0.01; ***p-value < 0.001.
Heart Telomere Length
The three-way between-subjects ANOVA revealed that the main effect of age was significant (Fig. 4a; F(1,23) = 52.91, p < 0.001, partial η2 = 0.697). Shorter telomeres were found in aging subjects (M = 0.98, SD = 0.12) compared with younger subjects (M = 1.28, SD = 0.14). The interaction between age and stress reactivity was significant (F(1,23) = 10.71, p = 0.003, partial η2 = 0.318). The Bonferroni adjusted post hoc comparisons found that aging high stress reactive fish (M = 0.89, SD = 0.08) had significantly shorter telomeres in heart tissue compared with their low stress reactive siblings (M = 1.09, SD = 0.04) (Fig. 4a; p = 0.002). At both extremes of the stress reactivity spectrum, aging fish were found to have significantly shorter heart telomeres compared with their younger counterparts (young high stress reactive (M = 1.31, SD = 0.17) and young low stress reactive (M = 1.25, SD = 0.11), respectively: (p < 0.001; p = 0.011)). No other significant effects or interactions were found (Supplementary Table 3).
Brain telomere length
The three-way between-subjects ANOVA revealed that the main effect of age was significant (Fig. 4b: F(1,24) = 11.71, p = 0.002, partial η2 = 0.328). Shorter telomeres were found in aging subjects (M = 2.65, SD = 0.29) compared with younger subjects (M = 3.01, SD = 0.28). No other significant effects or interactions were found (Supplementary Table 3).
SMAD specific E3 ubiquitin-ligase 3 (smurf2) expression
smurf2 is thought to function downstream of telomere attrition to affect replicative senescence50,51. Therefore, we also tested whether the differences in telomere length were accompanied by differences in the expression of smurf2 by qPCR (Fig. 3d,e). Pearson’s product-moment correlations were conducted to assess the relationship between telomere length, in both brain and heart, and smurf2 gene expression. There was no statistically significant correlation between heart telomere length and smurf2 expression (r(29) = − 0.012, p = 0.951), nor was there a statistically significant correlation between brain telomere length and smurf2 expression (r(30) = 0.173, p = 0.345).
Nonetheless, the three-way between-subjects ANOVA revealed that the main effect of sex was significant (F(1,24) = 6.16, p = 0.020, partial η2 = 0.204). Males (M = 0.18, SD = 0.34) had significantly higher smurf2 expression compared with females (M = − 0.02, SD = 0.43). The main effect of behavioural stress reactivity was also significant (F(1,24) = 4.38, p = 0.047, partial η2 = 0.154). Zebrafish with high stress reactivity (M = 0.21, SD = 0.29) had significantly higher smurf2 expression compared with fish with low stress reactivity (M = 0.00, SD = 0.35). The two-way interaction between age and behavioural stress reactivity was significant (F(1,24) = 4.67, p = 0.041, partial η2 = 0.163). The Bonferroni adjusted post hoc comparisons found that aging high stress reactive fish (M = 0.29, SD = 0.23) had significantly higher smurf2 expression compared with aging zebrafish with low stress reactivity (M = − 0.13, SD = 0.41, p = 0.006). The three-way interaction between age, sex and behavioural stress reactivity was also significant (F(1,24) = 4.46, p = 0.045, partial η2 = 0.157). The Bonferroni adjusted post hoc pairwise comparisons revealed that aging female subjects with high stress reactivity (M = 0.29, SD = 0.21) had significantly higher smurf2 expression compared with aging female fish with low stress reactivity (M = − 0.34, SD = 0.35, p = 0.004). Post hoc comparisons also revealed that aging high stress reactive female zebrafish had significantly higher smurf2 expression compared with younger high stress reactive female fish (M = − 0.12, SD = 0.12, p = 0.048) whilst aging low stress reactive female zebrafish were found to have significantly lower smurf2 expression compared with younger females with low stress reactivity (M = 0.09, SD = 0.17, p = 0.039). Additionally, aging low stress reactive male zebrafish (M = 0.08, SD = 0.40) demonstrated significantly higher smurf2 expression compared with aging low stress reactive female fish (p = 0.046). Finally, younger high stress reactive male zebrafish (M = 0.37, SD = 0.29) exhibited significantly higher smurf2 expression compared with younger high stress reactive female fish (p = 0.020).
Pearson’s product-moment correlations were performed to assess the relationship between HPI axis genes, including the MR:GR ratio, and smurf2 expression. The correlation between crf and smurf2 was significant (r(30) = 0.487, p = 0.005), with crf expression accounting for 24% of the variance in smurf2 expression. No other correlations were statistically significant (p > 0.05).
Discussion
The present study used zebrafish to test the hypothesis that stress reactivity exacerbates age-dependent telomere shortening in tissues relevant to age-related disease. Importantly, our findings suggest that high stress reactivity may contribute to an increased rate of telomere attrition in a tissue-specific manner, with stress reactivity correlating with telomere length in the heart, but not brain, of zebrafish. Furthermore, our study demonstrates the value of zebrafish as a translational model to explore associations between stress reactivity, cellular aging, and age-related disease.
Zebrafish have been shown to demonstrate persistent individual differences in behaviour across both time and context and, as seen in humans52,53 and rodents54,55,56, show increased anxiety-like behaviour with age57. Thus, zebrafish provide a promising animal model for studying associations between behaviours associated with specific personality traits and aging41. Our finding that aging zebrafish exhibited increased anxiety-like behaviours is in line with previous findings. In agreement with others58, we also observed sexually dimorphic behavioural responses in both young and aging zebrafish; males showed increased bottom-dwelling, covered longer distances, and were more mobile than females when exposed to a novel environment. Importantly, males also had significantly higher crf expression, a key regulator of locomotor activity59, thus reflecting consistency between behavioural and molecular data. In contrast to other research60, no sex differences in telomere length were observed. Given that both behavioural stress reactivity and HPI activity were found to be sexually dimorphic, the lack of sex differences in telomere length may indicate that females are more sensitive to the effect of HPI activity on telomere length. Alternatively, it may be that the subtle differences in HPI activity identified between the sexes were insufficient to elicit a significant effect for sex on telomere length with the sample size used here. However, as we did not directly test the impact of HPI activity on telomere length in male and female fish, further experiments are necessary to test these hypotheses.
Cardiovascular diseases have been widely associated with aging61, anxious disposition62, and telomere shortening63. The data reported here support such an association and points to a possible mechanism: zebrafish with high stress reactivity, and therefore a likely increased lifetime of perceived stress, have an increased HPI axis response which, over their lifetime, may lead to an increased rate of telomere shortening in heart tissue. Importantly, increased levels of stress hormone, such as cortisol and glucocorticoids, have been previously reported to have a negative effect on telomere length in peripheral tissues63,64,65. Furthermore, the expression of telomerase, the enzyme with the ability to counteract telomere erosion, is susceptible to modulation by different hormones39,65,66,67, including glucocorticoids68, thus providing further support for such a molecular mechanism. Interestingly, the balance of cardiomyocyte mr and gr has been shown to be paramount for cardiovascular health in mice69, which is consistent with the higher MR:GR ratio found here in aging zebrafish with shorter telomeres.
Although zebrafish retain telomerase activity during their adult life38,40, our data shows a significant decline in telomere length with age, which may be exacerbated by HPI axis dysfunction. In most vertebrates, including zebrafish, the major source of stem cells in the heart are the resident cardiomyocytes, which are capable of temporarily increasing their telomerase activity in response to injury40,70,71,72. However, when this peak is weakened by stress-related hormones26,39,63, it may diminish their ability to counteract cardiac damage, thus leading to shorter telomeres and the associated increased risk of cardiomyopathies. It should also be noted that our research cannot rule out a telomerase-independent process resulting from a chronically activated HPI axis, such as the postulated direct effect of reactive oxygen species on telomere length73. Nonetheless, the present investigation demonstrates that zebrafish are a promising animal model in which to study the association between stress reactivity and cardiomyopathies in aging individuals.
Upregulation of the ubiquitin ligase enzyme smurf2 is suggested to be a consequence of telomere attrition in human fibroblasts, and to be sufficient to drive senescence in the absence of DNA damage or detectable cellular stress50,51. However, telomere shortening did not predict smurf2 expression in our study. Instead, we found that smurf2 expression was increased in the anxious phenotype regardless of age or telomere length. HPA activity has been previously linked with the upregulation of ubiquitin ligases. For example, the expression of smurf2, among other ligases, is associated with social stress in the ventromedial prefrontal cortex of monkeys74, and glucocorticoid receptor imbalance is associated with the ubiquitin ligase Pellino-175. Here, we find an association between crf and the ubiquitin ligase smurf2. Thus, our findings suggest that the increase of smurf2 is not a consequence of telomere shortening, but rather of increased HPI activity. In accordance with others50, we also observed a sexual dimorphic pattern with increased expression of smurf2 in males.
A growing body of literature links shorter telomeres from peripheral tissues, such as leukocytes, with the onset and progression of neurodegenerative and psychiatric disorders28,29,30,76. Importantly, perceived stress, or stress-reactivity, is hypothesised to be a mediating factor in this relationship25,66,76,77. However, the present study finds no effect for stress reactivity on the telomere length of brain tissue. This disparity of effect on heart and brain telomere length suggests that stress reactivity influences telomere length in a tissue-specific manner. Thus, the assessment of leukocyte telomere length, or other proxy tissues, may not faithfully reflect telomere dynamics in the brain. It is therefore important to not overinterpret telomere length results obtained from peripheral tissues in clinical studies. Our use of a zebrafish model here has overcome this hurdle, enabling human-like telomere length39,40 to be assessed directly in disease-relevant tissues.
Although we demonstrate a clear association between stress reactivity and telomere length in heart tissue and show that stress reactivity affects telomere length in a tissue specific manner, there are limitations to our study. Differential rates of telomere shortening within different brain regions in humans have been previously described, with telomere length in the hippocampus significantly different between individuals with depression and controls67,78. Similarly, differences in the rate of telomere erosion have been observed for different cell types79,80. Therefore, it is possible that tissue heterogeneity could be masking anxiety-driven differences within zebrafish whole brain extracts in our study, a consideration that requires further investigation. It is also worth noting that whilst we report telomere shortening at 18 months of age in both brain and heart tissue, previous research using zebrafish has not observed age-dependent differences in telomere length until fish are at least 2 years of age39,81, with other research finding no telomere length effect of age in the zebrafish brain36. This discrepancy may highlight possible experimental differences between studies, namely the technique of telomere length analysis or strain differences38,39, or may reflect a lower rate of cell renewal and neurogenesis in the adult brain compared to heart36,73,80, making detection of shortening challenging. Therefore, if stress reactivity does lead to exacerbated telomere shortening in the brain, it may only be possible to observe this at much older stages in the zebrafish lifespan.
Taken together, we have demonstrated, using a population-based zebrafish strategy, that elevated stress reactivity affects telomere length in a tissue-specific manner, exacerbating age-dependent telomere shortening in heart but not brain tissue. As such, we demonstrate the importance of assessing telomere length in disease-relevant tissues to obtain meaningful results, and highlight the possible importance of both stress reactivity, and the resulting exacerbated telomere shortening, in the onset and progression of cardiovascular diseases. Finally, we demonstrate the utility and importance of zebrafish as a translational model that can be used in future studies to assess associations between stress reactivity, cellular aging and age-related disease.
References
Lyons, A. & Chamberlain, K. The effects of minor events, optimism and self-esteem on health. Br. J. Clin. Psychol. 33, 559–570 (1994).
Rasmussen, H. N., Scheier, M. F. & Greenhouse, J. B. Optimism and physical health: A meta-analytic review. Ann. Behav. Med. 37, 239–256 (2009).
Schoormans, D., Verhoeven, J. E., Denollet, J., van de Poll-Franse, L. & Penninx, B. Leukocyte telomere length and personality: Associations with the Big Five and Type D personality traits. Psychol. Med. 48, 1008–1019 (2018).
Sadahiro, R. et al. Relationship between leukocyte telomere length and personality traits in healthy subjects. Eur. Psychiatry. 30, 291–295 (2015).
Ikeda, A. et al. Pessimistic orientation in relation to telomere length in older men: The VA normative aging study. Psychoneuroendocrinology. 42, 68–76 (2014).
Zalli, A. et al. Shorter telomeres with high telomerase activity are associated with raised allostatic load and impoverished psychosocial resources. Proc. Natl. Acad. Sci. USA. 111, 4519–4524 (2014).
Blackburn, E. H. Switching and signaling at the telomere. Cell 106, 661–673 (2001).
Saretzki, G., Sitte, N., Merkel, U., Wurm, R. E. & von Zglinicki, T. Telomere shortening triggers a p53-dependent cell cycle arrest via accumulation of G-rich single stranded DNA fragments. Oncogene 18, 5148–5158 (1999).
Zhang, X., Mar, V., Zhou, W., Harrington, L. & Robinson, M. O. Telomere shortening and apoptosis in telomerase-inhibited human tumor cells. Genes Dev. 13, 2388–2399 (1999).
Herbig, U., Jobling, W. A., Chen, B. P., Chen, D. J. & Sedivy, J. M. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol. Cell. 14, 501–513 (2004).
Salpea, K. D. & Humphries, S. E. Telomere length in atherosclerosis and diabetes. Atherosclerosis. 209, 35–38 (2010).
Wong, L. S. et al. Anaemia is associated with shorter leucocyte telomere length in patients with chronic heart failure. Eur. J. Heart Fail. 12, 348–353 (2010).
Giltay, E. J., Geleijnse, J. M., Zitman, F. G., Buijsse, B. & Kromhout, D. Lifestyle and dietary correlates of dispositional optimism in men: The Zutphen Elderly Study. J. Psychosom. Res. 63, 483–490 (2007).
Kelloniemi, H., Ek, E. & Laitinen, J. Optimism, dietary habits, body mass index and smoking among young Finnish adults. Appetite. 45, 169–176 (2005).
Chida, Y. & Steptoe, A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biol. Psychol. 80, 265–278 (2009).
Endrighi, R., Hamer, M. & Steptoe, A. Associations of trait optimism with diurnal neuroendocrine activity, cortisol responses to mental stress, and subjective stress measures in healthy men and women. Psychosom. Med. 73, 672–678 (2011).
Tomiyama, A. J. et al. Does cellular aging relate to patterns of allostasis? An examination of basal and stress reactive HPA axis activity and telomere length. Physiol. Behav. 106, 40–45 (2012).
Lin, J., Epel, E. & Blackburn, E. Telomeres and lifestyle factors: roles in cellular aging. Mutat. Res. 730, 85–89 (2012).
Wolkowitz, O. M. et al. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress–preliminary findings. PLoS ONE 6, e17837 (2011).
Johansson, L. et al. Midlife personality and risk of Alzheimer disease and distress: A 38-year follow-up. Neurology. 83, 1538–1544 (2014).
Bower, J. H. et al. Anxious personality predicts an increased risk of Parkinson’s disease. Mov. Disord. 25, 2105–2113 (2010).
Denollet, J. et al. Personality as independent predictor of long-term mortality in patients with coronary heart disease. Lancet 347, 417–421 (1996).
Rosmond, R. & Bjorntorp, P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J. Intern. Med. 247, 188–197 (2000).
de Pablos, R. M. et al. Chronic stress enhances microglia activation and exacerbates death of nigral dopaminergic neurons under conditions of inflammation. J. Neuroinflammation. 11, 34 (2014).
Epel, E. S. et al. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 101, 17312–17315 (2004).
Monaghan, P. & Haussmann, M. F. Do telomere dynamics link lifestyle and lifespan?. Trends Ecol. Evol. 21, 47–53 (2006).
Simon, N. M. et al. Telomere shortening and mood disorders: Preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 60, 432–435 (2006).
Vakonaki, E. et al. Common mental disorders and association with telomere length. Biomed. Rep. 8, 111–116 (2018).
Martin-Ruiz, C. et al. Senescence and inflammatory markers for predicting clinical progression in parkinson’s disease: The ICICLE-PD study. J. Parkinsons. Dis. 10, 193–206 (2020).
Forero, D. A. et al. Meta-analysis of telomere length in Alzheimer’s disease. J. Gerontol. A. 71, 1–5 (2016).
D’Mello, M. J. et al. Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circ. Cardiovasc. Genet. 8, 82–90 (2015).
Xiong, F. & Zhang, L. Role of the hypothalamic-pituitary-adrenal axis in developmental programming of health and disease. Front. Neuroendocrinol. 34, 27–46 (2013).
Maniam, J., Antoniadis, C. & Morris, M. J. Early-life stress, HPA axis adaptation, and mechanisms contributing to later health outcomes. Front. Endocrinol. 5, 73 (2014).
Patton, E. E., Dhillon, P., Amatruda, J. F. & Ramakrishnan, L. Spotlight on zebrafish: Translational impact. Dis. Model Mech. 7, 731–733 (2014).
Alsop, D. & Vijayan, M. M. Molecular programming of the corticosteroid stress axis during zebrafish development. Comp. Biochem. Physiol. A 153, 49–54 (2009).
Arslan-Ergul, A., Erbaba, B., Karoglu, E. T., Halim, D. O. & Adams, M. M. Short-term dietary restriction in old zebrafish changes cell senescence mechanisms. Neuroscience 334, 64–75 (2016).
Carneiro, M. C. et al. Short telomeres in key tissues initiate local and systemic aging in zebrafish. PLoS Genet. 12, e1005798 (2016).
Kishi, S. et al. The zebrafish as a vertebrate model of functional aging and very gradual senescence. Exp. Gerontol. 38, 777–786 (2003).
Anchelin, M., Murcia, L., Alcaraz-Perez, F., Garcia-Navarro, E. M. & Cayuela, M. L. Behaviour of telomere and telomerase during aging and regeneration in zebrafish. PLoS ONE 6, e16955 (2011).
Carneiro, M. C., de Castro, I. P. & Ferreira, M. G. Telomeres in aging and disease: Lessons from zebrafish. Dis. Model Mech. 9, 737–748 (2016).
Toms, C. N. & Echevarria, D. J. Back to basics: searching for a comprehensive framework for exploring individual differences in zebrafish (Danio rerio) behavior. Zebrafish. 11, 325–340 (2014).
Truett, G. E. et al. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 29, 52–54 (2000).
Hellemans, J., Mortier, G., De Paepe, A., Speleman, F. & Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8, R19 (2007).
Ribas, L. et al. Heat-induced masculinization in domesticated zebrafish is family-specific and yields a set of different gonadal transcriptomes. Proc. Natl. Acad. Sci. USA 114, E941–E950 (2017).
Cawthon, R. M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 30, e47 (2002).
Bates, D., Mächler, M., Bolker, B. M. & Walker, S. C. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1 (2015).
Brooks, M. E. et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400 (2017).
Bolker, B. M. et al. Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135 (2009).
de Kloet, E. R. From receptor balance to rational glucocorticoid therapy. Endocrinology 155, 2754–2769 (2014).
Arslan-Ergul, A. & Adams, M. M. Gene expression changes in aging zebrafish (Danio rerio) brains are sexually dimorphic. BMC Neurosci. 15, 29 (2014).
Zhang, H. & Cohen, S. N. Smurf2 up-regulation activates telomere-dependent senescence. Genes Dev. 18, 3028–3040 (2004).
Beekman, A. T. et al. Anxiety disorders in later life: A report from the Longitudinal Aging Study Amsterdam. Int. J. Geriat. Psychiatry. 13, 717–726 (1998).
Wittchen, H.-U. & Hoyer, J. Generalized anxiety disorder: Nature and course. J. Clin. Psychiatry 62, 15–21 (2001).
Bessa, J., Oliveira, M., Cerqueira, J., Almeida, O. & Sousa, N. Age-related qualitative shift in emotional behaviour: Paradoxical findings after re-exposure of rats in the elevated-plus maze. Behav. Brain Res. 162, 135–142 (2005).
Boguszewski, P. & Zagrodzka, J. Emotional changes related to age in rats: A behavioral analysis. Behav. Brain Res. 133, 323–332 (2002).
File, S. Age and anxiety: Increased anxiety, decreased anxiolytic, but enhanced sedative, response to chlordiazepoxide in old rats. Hum. Psychopharmacol. Clin. Exp. 5, 169–173 (1990).
Kacprzak, V. et al. Dopaminergic control of anxiety in young and aged zebrafish. Pharmacol. Biochem. Behav. 157, 1–8 (2017).
Rambo, C. L. et al. Gender differences in aggression and cortisol levels in zebrafish subjected to unpredictable chronic stress. Physiol. Behav. 171, 50–54 (2017).
Swerdlow, N., Geyer, M. A., Vale, W. & Koob, G. Corticotropin-releasing factor potentiates acoustic startle in rats: blockade by chlordiazepoxide. Psychopharmacology. 88, 147–152 (1986).
Axson, E. L. et al. Sex differences in telomere length are not mediated by sex steroid hormones or body size in early adolescence. Gender Genome. 2, 68–75 (2018).
North, B. J. & Sinclair, D. A. The intersection between aging and cardiovascular disease. Circ. Res. 110, 1097–1108 (2012).
Suls, J. & Bunde, J. Anger, anxiety, and depression as risk factors for cardiovascular disease: The problems and implications of overlapping affective dispositions. Psychol. Bull. 131, 260 (2005).
Epel, E. S. et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 31, 277–287 (2006).
Mayer, S. E. et al. Cumulative lifetime stress exposure and leukocyte telomere length attrition: The unique role of stressor duration and exposure timing. Psychoneuroendocrinology. 104, 210–218 (2019).
Choi, J., Fauce, S. R. & Effros, R. B. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav. Immun. 22, 600–605 (2008).
Gotlib, I. et al. Telomere length and cortisol reactivity in children of depressed mothers. Mol. Psychiatry. 20, 615–620 (2015).
Wei, Y. B., Backlund, L., Wegener, G., Mathé, A. A. & Lavebratt, C. Telomerase dysregulation in the hippocampus of a rat model of depression: Normalization by lithium. Int. J. Neuropsychopharmacol. 18, 002 (2015).
Athanasoulia-Kaspar, A. P., Auer, M. K., Stalla, G. K. & Jakovcevski, M. Shorter telomeres associated with high doses of glucocorticoids: the link to increased mortality?. Endocr. Connect. 7, 1217–1226 (2018).
Oakley, R. H. et al. Cardiomyocyte glucocorticoid and mineralocorticoid receptors directly and antagonistically regulate heart disease in mice. Sci. Signal. 12, 9685 (2019).
Bär, C. et al. Telomerase expression confers cardioprotection in the adult mouse heart after acute myocardial infarction. Nat. Commun. 5, 1–14 (2014).
Gemberling, M., Bailey, T. J., Hyde, D. R. & Poss, K. D. The zebrafish as a model for complex tissue regeneration. Trends Genet. 29, 611–620 (2013).
Bednarek, D. et al. Telomerase is essential for zebrafish heart regeneration. Cell Rep. 12, 1691–1703 (2015).
Fouquerel, E. et al. Targeted and persistent 8-oxoguanine base damage at telomeres promotes telomere loss and crisis. Mol. Cell. 75, 117–130 (2019).
Karssen, A. et al. Stress-induced changes in primate prefrontal profiles of gene expression. Mol. Psychiatry. 12, 1089–1102 (2007).
Petrillo, M. G., Oakley, R. H. & Cidlowski, J. A. β-Arrestin-1 inhibits glucocorticoid receptor turnover and alters glucocorticoid signaling. J. Biol. Chem. 294, 11225–11239 (2019).
Monroy-Jaramillo, N., Dyukova, E. & Walss-Bass, C. Telomere length in psychiatric disorders: Is it more than an ageing marker?. World J. Biol. Psychiatry. 19, S2–S20 (2018).
Wolkowitz, O. M., Epel, E. S., Reus, V. I. & Mellon, S. H. Depression gets old fast: Do stress and depression accelerate cell aging?. Depression Anxiety. 27, 327–338 (2010).
Mamdani, F. et al. Variable telomere length across post-mortem human brain regions and specific reduction in the hippocampus of major depressive disorder. Transl. Psychiatry. 5, e636–e636 (2015).
Lin, J. et al. Systematic and cell type-specific telomere length changes in subsets of lymphocytes. J. Immunol. Res. 2016, 1–9 (2016).
Nakamura, K.-I. et al. Telomeric DNA length in cerebral gray and white matter is associated with longevity in individuals aged 70 years or older. Exp. Gerontol. 42, 944–950 (2007).
Lund, T. C., Glass, T. J., Tolar, J. & Blazar, B. R. Expression of telomerase and telomere length are unaffected by either age or limb regeneration in Danio rerio. PLoS ONE 4, e7688 (2009).
Acknowledgements
Funders: Human Frontiers Scientific Program (RGP 0008/2017, CHB, JVTP), Leverhulme Trust. (RPG-2016-143, RR, CHB), BBSRC (BB/M007863, CHB). MEMP is funded by the European Union’s Horizon 2020 research and innovation programme (Marie Sklodowska-Curie Action 750200) and by STARS@UNIPD-2019 (MetaZeb). CHB is a member of the Royal Society Industry Fellows’ College.
Author information
Authors and Affiliations
Contributions
J.R.E.: behavioural and molecular experiments, data analysis and manuscript writing. J.V.T.P.: molecular experiments, data analysis and manuscript writing. M.E.M.P.: behavioural experiments, data analysis and manuscript writing. R.R.: data analysis and manuscript writing. C.H.B.: experimental design and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Evans, J.R., Torres-Pérez, J.V., Miletto Petrazzini, M.E. et al. Stress reactivity elicits a tissue-specific reduction in telomere length in aging zebrafish (Danio rerio). Sci Rep 11, 339 (2021). https://doi.org/10.1038/s41598-020-79615-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79615-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.