Abstract
Microvascular disease and rarefaction are key pathological hallmarks of hypertension. The retina uniquely allows direct, non-invasive investigation of the microvasculature. Recently developed optical coherence tomography angiography now allows investigation of the fine retinal capillaries, which may provide a superior marker of overall vascular damage. This was a prospective cross-sectional study to collect retinal capillary density data on 300 normal eyes from 150 hypertensive adults, and to investigate possible associations with other organ damage markers. The average age of participants was 54 years and there was a greater proportion of males (85; 57%) than females. Multivariate, confounder adjusted linear regression showed that retinal capillary rarefaction in the parafovea was associated with increased pulse wave velocity (β = − 0.4, P = 0.04), log-albumin/creatinine ratio (β = − 0.71, P = 0.003), and with reduced estimated glomerular filtration rate (β = 0.04, P = 0.02). Comparable significant associations were also found for whole-image vascular-density, for foveal vascular-density significant associations were found with pulse wave velocity and estimated glomerular filtration rate only. Our results indicate that retinal capillary rarefaction is associated with arterial stiffness and impaired kidney function. Retinal capillary rarefaction may represent a useful and simple test to assess the integrated burden of hypertension on the microvasculature irrespective of current blood pressure levels.
Similar content being viewed by others
Introduction
Hypertension has been identified as one of the most significant risk factors for global morbidity and mortality1. It significantly increases the risks of stroke, coronary heart disease, heart failure and kidney disease and is a major cause of premature death worldwide. Microvascular disease and rarefaction are key pathological hallmarks of hypertension2. Subtle damage to organs such as the heart, kidney, eye and the peripheral vasculature can be detected early in the disease process and may help to prevent clinical events. Diagnostic assessment of hypertension mediated organ damage (HMOD) allows better prediction of cardiovascular (CV) risk3,4,5, identifying high-risk individuals for whom a more intense treatment is needed. Successful treatment can provide significant reductions in risk of subsequent CV disease6,7.
The retina uniquely allows direct, non-invasive investigation of the microvasculature, providing a valuable window into the microvascular sequelae of hypertension. Assessment of retinal damage due to hypertension (hypertensive retinopathy) is recommended in clinical guidelines for the management of patients with hypertension8. Hypertensive retinopathy progresses through various stages beginning with general arteriolar narrowing and subsequently focal areas of arteriolar narrowing, opacification of arteriolar walls, arteriovenous nipping, hemorrhages, hard exudates and cotton-wool spots. Signs of hypertensive retinopathy are predictive of incident stroke, congestive heart failure, and CV mortality—independent of traditional risk factors9,10,11,12,13,14,15,16. Even the earliest stage of hypertensive retinopathy- general arterial narrowing, is associated with stroke13,17,18, kidney disease19,20 and CV mortality21,22. Digital fundus photography can detect these retinal changes, but cannot resolve the finer retinal capillaries, which are more representative of the entire microvascular network and may provide the earliest markers of ischemia/hypoxia, before arteriolar and venular changes occur. Early detection of hypertensive damage is important to prevent further degeneration and recent evidence suggests that retinal neurodegeneration is already evident in individuals with mildly elevated blood pressure (BP) levels23.
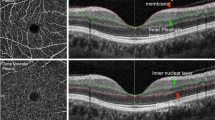
Optical coherence tomographic angiography (OCT-A) is a more recent ocular imaging technology that permits fast, non-invasive assessment of the retinal capillaries and blood flow in the retina (see Fig. 1). Importantly, OCT-A can be used to visualize all layers of the retinal vasculature in detail. Recent investigations using OCT-A technology have reported reduced retinal capillary density in diabetes24 and uncontrolled hypertension25, while another study reported restoration of normal capillary density after treatment with carotid angioplasty and stenting26.
OCT angiogram from a single eye, showing the four retinal depths (top): the superficial retinal plexus, the deep retinal plexus, the outer retina and the choriocapillaris. Bottom left shows the scan region (darker) overlaid on the broader retina. Bottom right shows the cross-section of the superior vascular plexus (SVP).
This study aimed to further investigate retinal capillary changes with respect to BP and HMOD measures related to vascular stiffness and kidney function. Kidney function was assessed using urinary albumin/creatinine ratio (ACR) and estimated glomerular filtration rate (eGFR), and arterial stiffness was assessed using carotid-femoral pulse wave velocity (PWV).
Results
The clinical characteristics of the study population are shown in Table 1. Overall 150 patients were included in the analysis. The mean ± SD age of participants was 54 ± 16 years and there was a greater proportion of males (n = 85, 57%) than females (n = 65, 43%). Seventy nine percent of patients received treatment with some form of antihypertensive medication. Of those taking hypertensive medication, the average number of antihypertensive drugs was 1.8 ± 0.7. Thirty-two participants were on no antihypertensive medication (usually due to treatment with lifestyle interventions or intolerances to anti-hypertensive medication), 43 on only one medication, 55 on a combination of two medications and 20 on triple anti-hypertensive therapy. Further baseline characteristics of the included patients are described in Table 1. Data on body mass index (BMI) was missing in 5% of the cases, medical history (type 1 or 2 diabetes) in 8%, PWV in 15%, eGFR in 16%, ACR in 26% of cases. All other data described in Table 1 was complete.
Of the entire cohort, 46 had T2D and 1 had type 1 diabetes. Data on HbA1c was available for 76% of the cohort and was utilised to categorise a further n = 2 participants (with missing diabetes history) as having diabetes (based on HbA1c > 6.5%)27, resulting in 48 participants with diabetes in the cohort, and data on diabetes unavailable for 7% of the full cohort.
The cohort had an average BMI of 32.1 kg/m2 which placed them into the obese range. The average 24 h ambulatory BP was 135/81 mmHg.
Capillary density was calculated for 3 zones of interest, each centered on the fovea (see Fig. 2). The association between retinal capillary density at SVP and systemic factors is shown in Table 2 and Fig. 3. Univariate linear regression showed that retinal capillary rarefaction in each zone of interest was associated with increased PWV (P < 0.0001 to P = 0.006) and logACR (P = 0.0001 to P = 0.02), and with reduced eGFR (P < 0.0001 to P = 0.005). These results remained statistically significant after multiple testing adjustment.
Scatterplots of foveal density against measures of hypertension mediated organ damage, including the unadjusted linear regression model fits (blue line) with the mean (grey area) and observed (red dashed lines) 95% confidence intervals. (a) Pulse wave velocity; (b) log (albumin–creatinine ratio); (c) estimated glomerular filtration rate (eGFR > 90 excluded), (d) boxplot comparing whole image density between groups classified by estimated glomerular filtration rate < > 60).
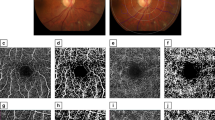
Examples of superficial macular OCT-A images are provided in Fig. 4, comparing an image with high capillary density from a participant with normal vascular stiffness and healthy kidney function to an image with low capillary density from a participant with high vascular stiffness and impaired kidney function.
Superficial macular OCT-A image samples demonstrating differences in capillary density. (a) High capillary density (foveal density 51.5) in a participant with normal vascular stiffness (PWV 8.8 m/s) and healthy kidney function (eGFR = 90 mL/min/1.73 m2). (b) Low capillary density (foveal density 21.7) in a participant with high vascular stiffness (PWV 12.2 m/s). (c) Low capillary density (foveal density 29.7) in a participant with impaired kidney function (eGFR = 29 mL/min/1.73 m2).
Group comparison for eGFR < 60 (reference eGFR ≥ 60) supported the association with retinal capillary rarefaction (P = 0.0003 to P = 0.007). Retinal capillary density was not directly associated with ambulatory systolic BP; however, it was associated with diastolic BP for the parafovea and whole image (P = 0.01). Retinal capillary rarefaction in the parafovea and whole image was associated with the diagnosis of T2D (P = 0.002 and P = 0.004 respectively). Retinal capillary rarefaction was associated with higher HbA1c in all 3 zones of interest (P = 0.01 to P = 0.005). The statistically significant results remained significant after multiple testing adjustment.
The linear associations between retinal capillary rarefaction and measures of HMOD were robust to adjustment for age, sex, SBP, DBP and T2D (see Table 3), with the exception of the foveal density and logACR association (P = 0.08). The eGFR group comparisons were also robust to similar adjustment in the parafoveal and whole image zones, but not the foveal zone (P = 0.1). The statistically significant results remained significant after multiple testing adjustment.
Foveal density was more strongly correlated with HbA1c than T2D (in contrast to the parafovea and whole image density), hence we fitted an additional multivariate model adjusting for age, sex, SBP, DBP and HbA1c instead of diabetes. This did not change the outcomes considerably, as summarized in Table 4.
These findings indicate that retinal capillary rarefaction in SVP is associated with arterial stiffness as measured by PWV, and impaired kidney function as measured by eGFR and ACR, independent of age, sex, diabetes and best practice BP measures.
Discussion
The results from our study indicate that microvascular changes in the eye as assessed by retinal capillary density using OCT-A, are clearly associated with other well-established markers of HMOD including arterial stiffness as measured by PWV, and impaired kidney function as assessed both by eGFR and ACR. Importantly, in the context, these associations were independent of age, sex, diabetes and ambulatory BP measures. Retinal capillary rarefaction may therefore represent a useful and simple test to assess the integrated burden of hypertension on the microvasculature irrespective of current BP levels and therefore add substantially to risk stratification of hypertensive patients. Furthermore, future research will have to investigate whether longitudinal assessment and the corresponding changes in the retina may serve as an indicator of adequate/inadequate antihypertensive treatment.
A recent similar small study applying OCT-A in non-diabetic hypertensive patients of Asian ethnicity was reported by Chua et al.25. The Chua study found an association between BP and foveal density at the deep vascular plexus (DVP). It did not investigate PWV but found an association between foveal density and eGFR, similar to our result but only statistically significant for the DVP, not SVP. Findings from our study in a larger cohort of patients suggests that the association between eGFR and foveal density holds also for the SVP (and parafoveal and full image zones) and provides the first evidence that PWV is associated with retinal capillary density. There was agreement between the studies on the lack of association at the SVP layer between foveal density and 24 h ambulatory BP. However, in extending the zones of interest we found associations between retinal capillary density and DBP in the parafovea and whole image. We provide evidence therefore that hypertension may similarly affect both SVP and DVP layers, akin to other systemic disorders such as diabetes24.
Retinal capillary rarefaction has previously been reported in diabetes28, along with retinal microvascular changes in pre-diabetes29. Our results provide further evidence of retinal capillary rarefaction in diabetes (in parafovea and whole image) and indicate a linear relationship between HbA1c and capillary density in all 3 zones of interest.
A previous study found increased PWV was associated with wider retinal venular caliber in the Atherosclerosis Risk in Communities Study30. Current guidelines by the European Society of Hypertension state that a measured PWV larger than 10 m/s can be considered an independent marker of HMOD8. Interestingly, our study suggests that the fine retinal capillaries may also provide some information about the health of the large arteries, such as the aorta, as assessed by PVW. However, this is based on correlation analysis only and will require further substantiation. Nevertheless, the association of retinal capillary density with markers of both micro- and macro-vascular damage indicates that this measure may represent an attractive approach to better understand and quantify the overall/integrated burden of elevated BP over prolonged periods of time on susceptible vascular structures and provide superior assessment of HMOD with potential therapeutic implications.
The capillaries are more representative of the entire microvascular network and may provide the earliest markers of ischemia/hypoxia, before arteriolar and venular changes occur. Early detection of hypertensive damage is important to both CV risk stratification and to prevent further retinal degeneration. Indeed, recent evidence suggests that retinal neurodegeneration is already evident in individuals with only mildly elevated BP23.
Retinal capillary rarefaction was independently associated with impaired kidney function (reduced eGFR and logACR). The retinal and renal circulations exhibit shared characteristics in terms of anatomy and physiology31, hence it is possible that retinal capillary rarefaction reflects similar changes in the kidney in hypertension. The results are consistent with previously published associations between retinal arteriolar narrowing and kidney function32,33 or kidney disease19,20,34. Future research will be needed to determine if retinal capillary rarefaction, compared to arteriolar narrowing, can provide an earlier or more accurate marker of hypertensive damage including kidney dysfunction.
As OCT-A is non-invasive, accessible and relatively low cost, it could be a very useful and simple addition to the routine diagnostic workup of hypertensive patients in the future. Current guidelines recommend screening for HMOD in multiple organs8, or multiple markers for a single organ in the case of the kidney (eGFR and ACR), because CV risk increases for each extra sign of HMOD35. Future longitudinal research is required to establish whether retinal OCT-A markers improve CV risk stratification over existing markers, or provide an easier method compared to less accessible or more costly tests.
Associations between retinal capillary density and measures of HMOD were substantially stronger than those with best practice BP measures, i.e. ambulatory BP, in this treated hypertension cohort. This was true for univariate and adjusted models. Indeed, for the fovea region there was no association between retinal capillary density and BP. Hence, we hypothesize that the correlations between retinal density and measures of HMOD reflect cumulative damage due to previous BP burden.
Even ABPM, while superior in predicting CV outcomes, is only a snapshot of the BP profile at a specific point in time. It does not necessarily inform about longer term BP control and prevention of further progression or even regression of HMOD. OCT-A imaging could potentially fill this gap and provide an integrative marker for previous BP burden, similar to the use of HbA1c in assessing control of glucose metabolism over the previous ~ 3 months in patients with diabetes. An integrated measure of the BP burden on both the micro-and macro-vasculature may be most useful to improve individual management of hypertension and potentially to monitor treatment effects. This, however, will have to be addressed in appropriately designed longitudinal studies.
The OCT-A measures of retinal capillary density are based on optical changes due to blood flow; if blood flow drops below the detection threshold, the capillary will not be identified and the measured density will be reduced36. A different retinal imaging modality, laser doppler flowmetry, has also been used to demonstrate reduced retinal capillary density in hypertensive patients37, along with increased retinal vascular resistance38. Hence it is possible that atherosclerotic processes that reduce blood flow are the mechanism by which retinal capillary rarefaction occurs in hypertension. Of note, foveal vascular density is anatomically influenced by the foveal avascular zone which can lead to increased variability of density estimates. Therefore, results of the para-foveal density and whole image density may be more robust.
The recent development of OCT-A technology, permitting fast, non-invasive assessment of the retinal capillaries and blood flow, is now being utilized extensively for clinical research in many disorders including ocular disease. Our results add to the evidence of retinal capillary changes due to hypertension, highlighting that clinical researchers exploring OCT-A in ocular diseases should either exclude or adjust for systemic disorders such as hypertension and diabetes.
The strengths of this study include a well phenotyped cohort including ambulatory BP monitoring, PWV and kidney function (eGFR and ACR). The results from this cross-sectional study warrant further investigation with longitudinal studies. One limitation of the study was the inclusion of patients on different medications and different stages of hypertension, however this reflects a real-world hypertensive cohort. Additionally, the duration of hypertension was not exactly known, and the associations do not prove causality.
We have demonstrated a relationship between retinal capillary density and both macro- (PWV) and micro-vascular (eGFR and ACR) parameters in adults with treated arterial hypertension. Assessment of the microcirculation is crucial to the understanding of the effects of systemic diseases such as hypertension and diabetes39. As with other target organ damage in hypertension, recognition of the effects on the retinal capillaries could allow clinicians to better manage hypertensive patients. OCT-A is a practical, non-invasive tool for investigation of the retinal capillaries which may mirror other HMOD such as microvascular rarefaction in the kidney and stiffening of large arteries. As such, assessment of retinal capillary density may have clinically relevant advantages in that it may represent a superior marker of overall vascular damage (both micro- and macrovascular) and may serve as a stand-alone and powerful integrated measure of HMOD irrespective of the current BP level.
Materials and methods
Study design and participants
We conducted a prospective cross-sectional study including 155 participants with arterial hypertension from the Dobney Hypertension Centre at the Royal Perth Hospital, Perth, Australia. Patients aged 18 years or older underwent OCT-A imaging between February 2016 to October 2019. All images were evaluated by an eye specialist, participants with retinal disease (glaucoma, age-related macular degeneration, diabetic retinopathy) or previous retinal surgery (n = 5) were excluded, leaving n = 150 participants for the current analysis. The study was approved by the East Metropolitan Health Service Ethics and Governance Committees (EC00270, REG Number: RGS1040). The research followed the tenets of the Declaration of Helsinki for research involving human subjects. Informed consent was obtained from all subjects.
Clinical workup
Patients were referred to the Dobney Hypertension Centre for management of hypertension predominantly from primary care physicians. Clinical diagnosis for hypertension was based on current European guidelines40,41. A thorough medical history was obtained from all patients and a routine physical examination was performed. Secondary causes of hypertension were investigated where clinically indicated. Patients with identified secondary hypertension were not included in this analysis. Office BP measurements were obtained according to unattended automated office BP principles and the average of three measurements recorded.
All participants were fitted with an ambulatory BP monitor by a trained research nurse. The devices (Spacelabs 90207, Spacelabs Healthcare, Snoqualmie, Washington, United States) were programmed to take 4 measurements per hour during the day (6:00 – 22:00 h) and 2 per hour during the night (22:00–6:00 h). Arm circumferences were measured and appropriate cuffs were chosen according to Spacelabs product specifications and the first reading was performed in the presence of research staff.
Pulse wave velocity (PWV) measurements were carried out with SphygmoCor® XCEL system (AtCor Medical Pty Ltd, Australia). Standard procedures of the manufacturer were followed to obtain the parameters. Participants were asked to attend fasted on the morning of the assessment and to abstain from smoking, caffeine and alcohol consumption 12 h before the assessment and not to take their usual BP medication. Participants were instructed to take their regular BP medication after the assessment of PWV and retinal imaging, which was usually carried out in the morning (before 12:00 h). The PWV was performed after a 5-min rest period in supine position. Parallel assessment of pulse in thigh with specialized cuffs and a mechanical sensor held against the neck of the participants allowed calculation of PWV42. The distance in between the sites where the pulse was recorded was measured by trained study nurses or doctors before the measurement. The capturing time for PWV assessment was set to 10 s with a PWV distance and subtraction method. The PWV assessments was performed twice and their average used for further analysis. For analysis purposes, an additional parameter “BP control status” was defined as poorly controlled if mean ambulatory systolic blood pressure (SBP) > 140 mmHg, or diastolic blood pressure (DBP) > 90 mmHg.
Pathology
Blood samples and mid-stream urine were obtained from participants within 4–6 weeks prior to retinal imaging. If participants had already obtained the required pathology results within 3 months prior to retinal imaging these results were used for analysis.
Pathology tests included serum creatinine, spot urine albumin and calculation of eGFR (measured with the CKD-EPI algorithm43 in mL/min/1.73 m2) and ACR (Albumin/Creatinine ratio in mg/mmol creatinine) fasting glucose and HbA1c.
Retinal examination procedures
Participants completed a questionnaire regarding their ocular history, including glaucoma, macular degeneration and intraocular surgery. Retinal imaging was performed using the Optovue AngioVue® spectral-domain optical coherence tomography-angiography (OCT-A) device (Optovue, Inc., Fremont, CA, USA). Non-invasive OCT-A technology uses laser reflectance of the surface of flowing red blood cells to accurately depict vessels in the retina, including fine capillaries, without the traditional need for intravascular dyes44. As with traditional OCT, cross-sectional scans are combined to provide 3-dimensional image data, however with OCT-A, changes in signal over time are used to detect blood flow, permitting quantitative measurement of flow area and vessel density. The OCT-A algorithms produce 2-dimensional images (en face angiograms or capillary density maps) segmented into four retinal depths: the superficial retinal plexus (SVP), the deep retinal plexus (DVP), the outer retina and the choriocapillaris (Fig. 1). The retinal capillaries exist primarily in the SVP and DVP layers. The AngioVue system uses split-spectrum amplitude-decorrelation angiography algorithm, which minimizes motion noise. A high level of reproducibility for retinal capillary density measurements using OCT-A has already been published45.
The OCT-A device was used to collect macular scans with a 6 mm × 6 mm field of view. AngioVue software (version 2016.1.0.26) automatically provided segmentation of the SVP and calculated the percentage image area occupied by blood vessels with flowing erythrocytes in the selected region; the superficial retinal capillary density.
A trained grader blinded to the participant characteristics reviewed the quality of all OCT-A scans. Poor quality scans were excluded from the analysis if they exhibited segmentation failure or image artifacts due to optical or residual motion.
For this study, capillary density at SVP was measured in macula region, in a 6 mm square region centered on the fovea. The SVP includes the vasculature between the internal limiting membrane and 10 µm above the inner plexiform layer. Capillary density was calculated for 3 regions of interest, each centered on the fovea (see Fig. 2); the whole image, the fovea (a circular region 1.5 mm in diameter centered on the fovea) and parafovea (an annulus extending from the outside of the fovea region out to a diameter of 2.5 mm).
Statistical analyses
Baseline demographic and clinical characteristics were reported as absolute numbers and relative proportions in percent for categorical data and mean and standard deviation (SD) for continuous data.
To test for associations between retinal capillary density and HMOD parameters, generalized linear modelling (GLM) was utilized, with retinal capillary density as the dependent variable. We found a moderate positive correlation between right and left eye data on retinal density (Pearson correlation coefficient, r = 0.68; P < 0.0001). Hence, we utilised the inter-eye variability by considering each eye as the unit of analysis, providing greater statistical power. The GLM analyses were modified according to the generalized estimating equation (GEE) approach46. The GEE provides increased precision of estimation results while accounting for the correlation between fellow eyes. Benjamini and Hochberg47 multiple testing adjustment (false discovery rate set to 0.1) was applied to the retinal capillary density results (foveal, parafoveal and whole image).
Where unadjusted models were significant, multivariate models were fitted including adjustment for age, sex, mean arterial pressure (MAP) and T2D; only covariates with P < 0.1 were retained in the models. An additional model was provided for foveal density, replacing the covariate T2D with HbA1c, as HbA1c displayed stronger association with foveal density than T2D. HbA1c can be used to identify pre-diabetes and diabetes27.
Data for eGFR were not numerically provided above 90 mL/min/1.73 m2, as values above 90 mL/min/1.73 m2 are less accurate. To avoid introducing bias in the linear regressions, participants with normal eGFR were excluded from this particular analysis. Additionally, parameters of renal damage were also used as categorical, independent variables for linear regression models with main retinal vascular outcome markers as dependent variables to test for group differences. Logarithmically transformed ACR was used for linear regressions.
Statistical significance was defined as p < 0.05.
All statistical analyses were conducted in the R statistical environment48.
Data availability
The data that support the findings of this study are subject to restrictions and are not being made publicly available. Data are however available from the authors upon reasonable request and with permission of the hospital and ethics committee.
References
WHO. World Health Organisation. Cardiovascular diseases (CVDs) 2016 (2016).
Struijker-Boudier, H. A. J. Retinal microcirculation and early mechanisms of hypertension. Hypertension 51, 821–822. https://doi.org/10.1161/Hypertensionaha.107.103234 (2008).
Conroy, R. M. et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur. Heart J. 24, 987–1003. https://doi.org/10.1016/s0195-668x(03)00114-3 (2003).
Wong, T. Y. & Mitchell, P. Hypertensive retinopathy. N. Engl. J. Med. 351, 2310–2317. https://doi.org/10.1056/NEJMra032865 (2004).
Mancia, G. et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J. Hypertens. 31, 1281–1357. https://doi.org/10.1097/01.hjh.0000431740.32696.cc (2013).
Collins, R. et al. Blood pressure, stroke, and coronary heart disease. Part 2, short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet 335, 827–838. https://doi.org/10.1016/0140-6736(90)90944-z (1990).
Law, M. R., Morris, J. K. & Wald, N. J. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 338, b1665. https://doi.org/10.1136/bmj.b1665 (2009).
Taylor, J. 2013 ESH/ESC guidelines for the management of arterial hypertension. Eur. Heart J. 34, 2108–2109 (2013).
Wong, T. Y. Retinal arterial signs and coronary heart disease. Am. J. Cardiol. 97, 1549. https://doi.org/10.1016/j.amjcard.2005.12.003 (2006).
Wong, T. Y. et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet 358, 1134–1140. https://doi.org/10.1016/S0140-6736(01)06253-5 (2001).
Wong, T. Y. et al. Retinal arteriolar diameter and risk for hypertension. Ann. Intern. Med. 140, 248–255 (2004).
Wong, T. Y. et al. Retinal microvascular abnormalities and cognitive impairment in middle-aged persons: the atherosclerosis risk in communities study. Stroke 33, 1487–1492. https://doi.org/10.1161/01.str.0000016789.56668.43 (2002).
McGeechan, K. et al. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. Am. J. Epidemiol. 170, 1323–1332. https://doi.org/10.1093/aje/kwp306 (2009).
McGeechan, K. et al. Meta-analysis: retinal vessel caliber and risk for coronary heart disease. Ann. Intern. Med. 151, 404–413 (2009).
Cheung, N. et al. Retinal fractals and acute lacunar stroke. Ann. Neurol. 68, 107–111. https://doi.org/10.1002/ana.22011 (2010).
Doubal, F. N. et al. Fractal analysis of retinal vessels suggests that a distinct vasculopathy causes lacunar stroke. Neurology 74, 1102–1107. https://doi.org/10.1212/WNL.0b013e3181d7d8b4 (2010).
Wong, T. Y. et al. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the cardiovascular health study. Arch. Intern. Med. 166, 2388–2394. https://doi.org/10.1001/archinte.166.21.2388 (2006).
Yatsuya, H. et al. Retinal microvascular abnormalities and risk of lacunar stroke: atherosclerosis risk in communities study. Stroke 41, 1349–1355. https://doi.org/10.1161/STROKEAHA.110.580837 (2010).
Yau, J. W. et al. Retinal arteriolar narrowing and subsequent development of CKD Stage 3: the Multi-Ethnic Study of Atherosclerosis (MESA). Am. J. Kidney Dis. 58, 39–46. https://doi.org/10.1053/j.ajkd.2011.02.382 (2011).
Yip, W. et al. Retinal vascular imaging markers and incident chronic kidney disease: a prospective cohort study. Sci. Rep. 7, 9374. https://doi.org/10.1038/s41598-017-09204-2 (2017).
Wong, T. Y. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology 110, 933 (2003).
Liew, G., Wong, T., Mitchell, P., Cheung, N. & Wang, J. J. Retinopathy predicts coronary heart disease mortality. Heart https://doi.org/10.1136/hrt.2008.146670 (2008).
Jung, S. et al. Evidence of neurodegeneration in individuals with only mildly elevated blood pressure. J. Hypertens. 37, 2389–2397. https://doi.org/10.1097/HJH.0000000000002164 (2019).
Ting, D. S. W. et al. Optical coherence tomographic angiography in type 2 diabetes and diabetic retinopathy. JAMA Ophthalmol. 135, 306–312. https://doi.org/10.1001/jamaophthalmol.2016.5877 (2017).
Chua, J. et al. Impact of hypertension on retinal capillary microvasculature using optical coherence tomographic angiography. J. Hypertens. 37, 572–580. https://doi.org/10.1097/HJH.0000000000001916 (2019).
Lee, C. W., Cheng, H. C., Chang, F. C. & Wang, A. G. Optical coherence tomography angiography evaluation of retinal microvasculature before and after carotid angioplasty and stenting. Sci. Rep. 9, 14755. https://doi.org/10.1038/s41598-019-51382-8 (2019).
d’Emden, M. C., Shaw, J. E., Jones, G. R. & Cheung, N. W. Guidance concerning the use of glycated haemoglobin (HbA1c) for the diagnosis of diabetes mellitus. Med. J. Aust. 203, 89–90. https://doi.org/10.5694/mja15.00041 (2015).
Jumar, A. et al. Retinal capillary rarefaction in patients with type 2 diabetes mellitus. PLoS ONE 11, e0162608. https://doi.org/10.1371/journal.pone.0162608 (2016).
Nguyen, T. T., Wang, J. J. & Wong, T. Y. Retinal vascular changes in pre-diabetes and prehypertension: new findings and their research and clinical implications. Diabetes Care 30, 2708–2715. https://doi.org/10.2337/dc07-0732 (2007).
Meyer, M. L. et al. Central arterial stiffness and retinal vessel calibers: the Atherosclerosis Risk in Communities Study-Neurocognitive Study. J. Hypertens. https://doi.org/10.1097/HJH.0000000000002252 (2019).
Schwartz, M. M., Lewis, E. J., Leonard-Martin, T., Lewis, J. B. & Batlle, D. Renal pathology patterns in type II diabetes mellitus: relationship with retinopathy. The Collaborative Study Group. Nephrol. Dial. Transpl. 13, 2547–2552. https://doi.org/10.1093/ndt/13.10.2547 (1998).
Edwards, M. S. et al. Associations between retinal microvascular abnormalities and declining renal function in the elderly population: the Cardiovascular Health Study. Am. J. Kidney Dis. 46, 214–224. https://doi.org/10.1053/j.ajkd.2005.05.005 (2005).
Wong, T. Y. et al. Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. J. Am. Soc. Nephrol. 15, 2469–2476. https://doi.org/10.1097/01.ASN.0000136133.28194.E4 (2004).
Bosch, A. et al. Retinal capillary and arteriolar changes in patients with chronic kidney disease. Microvasc. Res. 118, 121–127. https://doi.org/10.1016/j.mvr.2018.03.008 (2018).
Patel, A. et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 370, 829–840. https://doi.org/10.1016/S0140-6736(07)61303-8 (2007).
de Carlo, T. E., Romano, A., Waheed, N. K. & Duker, J. S. A review of optical coherence tomography angiography (OCTA). Int. J. Retina Vitreous 1, 5. https://doi.org/10.1186/s40942-015-0005-8 (2015).
Bosch, A. J. et al. Retinal capillary rarefaction in patients with untreated mild-moderate hypertension. BMC Cardiovasc. Disord. 17, 300. https://doi.org/10.1186/s12872-017-0732-x (2017).
Kannenkeril, D. et al. Retinal vascular resistance in arterial hypertension. Blood Press. 27, 82–87. https://doi.org/10.1080/08037051.2017.1393311 (2018).
Cheung, C. Y., Ikram, M. K., Sabanayagam, C. & Wong, T. Y. Retinal microvasculature as a model to study the manifestations of hypertension. Hypertension 60, 1094–1103. https://doi.org/10.1161/HYPERTENSIONAHA.111.189142 (2012).
Parati, G. et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J. Hypertens. 32, 1359–1366. https://doi.org/10.1097/HJH.0000000000000221 (2014).
Williams, B. et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 39, 3021–3104. https://doi.org/10.1093/eurheartj/ehy339 (2018).
Townsend, R. R. et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 66, 698–722. https://doi.org/10.1161/HYP.0000000000000033 (2015).
NICE. Chronic kidney disease in adults: assessment and management. National Institute for Health and Care Excellence Guideline CG182. (London, 2015).
Koustenis, A. Jr. et al. Optical coherence tomography angiography: an overview of the technology and an assessment of applications for clinical research. Br. J. Ophthalmol. 101, 16–20. https://doi.org/10.1136/bjophthalmol-2016-309389 (2017).
Coscas, F. et al. Normative data for vascular density in superficial and deep capillary plexuses of healthy adults assessed by optical coherence tomography angiography. Invest Ophthalmol Vis Sci 57, OCT211–OCT223. https://doi.org/10.1167/iovs.15-18793 (2016).
Ying, G. S., Maguire, M. G., Glynn, R. & Rosner, B. Tutorial on biostatistics: linear regression analysis of continuous correlated eye data. Ophthalm. Epidemiol. 24, 130–140. https://doi.org/10.1080/09286586.2016.1259636 (2017).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J. R. Stat. Soc. B Metthodol. 57, 289–300 (1995).
R: a language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, 2013).
Acknowledgements
We thank all the participants who took part in this study and the clinicians who referred participants. The generous research support by the Royal Perth Hospital Research Foundation (MRF) is also acknowledged.
Funding
This study was funded by the Department of Health, Western Australia.
Author information
Authors and Affiliations
Contributions
Figure and manuscript preparation: S.F., J.M.N.; Concept/Rationale: S.F., D.P., Y.K., M.S.; Study design: S.F., J.M.N., L.H., V.M., R.C.; Data collection: J.M.N., J.C., A.J., C.G., L.R., R.C. and J.V.; Data analysis: S.F., J.M.N., J.C., A.J., C.G., L.R. and J.V.; Interpretation: S.F., J.M.N., D.P., Y.K. and M.S.; Obtaining funding support: S.F., Y.K., L.H., V.M.; Critical review on manuscript: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frost, S., Nolde, J.M., Chan, J. et al. Retinal capillary rarefaction is associated with arterial and kidney damage in hypertension. Sci Rep 11, 1001 (2021). https://doi.org/10.1038/s41598-020-79594-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79594-3
This article is cited by
-
Simultaneously measured inter-arm blood pressure difference is not associated with pulse wave velocity in a clinical dataset of at-risk hypertensive patients
Journal of Human Hypertension (2022)
-
Opisthenar microvessel area as a sensitive predictive index of arterial stiffness in hypertensive patients
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.