Abstract
The eye lens is a unique organ as no cells can be replaced throughout life. This makes it decisive that the lens is protected against damaging UV-radiation. An ultraviolet (UV)-absorbing compound of unknown identity is present in the aqueous humor of geese (wild and domestic) and other birds flying at high altitudes. A goose aqueous humor extract, that was believed to contain the UV protective compound which was designated as “compound X”, was fractionated and examined using a variety of spectroscopic techniques including LC–MS and high field one- and two dimensional-NMR methods. A series of compounds were identified but none of them appeared to be the UV protective “compound X”. It may be that the level of the UV protective compound in goose aqueous humor is much less than the compounds identified in our investigation, or it may have been degraded by the isolation and chromatographic purification protocols used in our investigations.
Similar content being viewed by others
Introduction
The eye is often compared to a camera; it forms an image of the environment on its photoreceptive layer, the retina. The diaphragm of the camera is analogous to the iris of the eye, and the lens focuses the light onto the most central part of the retina, the macula lutea. The light pathway through the eye media consists of cornea, aqueous humor, lens, and vitreous body. One decisive premise to achieve an optimal image is that all components of the media allow visible radiation (VR: 760–400 nm wavelength) to pass through. Shorter wavelengths of ultraviolet radiation (UVR) (UV-A: 400–320 nm, UV-B: 320–290 nm, and UV-C: 290–200 nm) must be blocked to prevent phototoxic tissue damage. However, a fraction of long UV-rays (320–400 nm) is obviously penetrating to the retina, at least in some birds using those rays during mate-choice, foraging, etc.1. The most potent UVR is largely absorbed by the ozone layer, minimizing damage on biological structures in general. In fact, only some 5% of all VR/UVR energy striking our planet is in the UV-range, and of this 97% is UV-A. However, the action spectrum for DNA damage steeply increases with decreasing wavelength from some 330 nm, underlining the significant biotoxicity. It has long been known that the limited dose of actinic UVR reaching the ground is able to cause harmful effects on the eye2, and changes may occur at three different levels, i.e. the cornea (snow blindness/photokeratitis), lens (cataract), and retina (macula degeneration). Potentially UV-protecting components related to the lens should therefore be searched for in front of this organ, i.e. in the cornea and the aqueous humor.
However, the human eye has some protection, e.g. the high levels of high molar absorptivity ascorbic acid present in the aqueous humor and corneal epithelium (30–300 times the serum level, respectively)3,4,5,6. This view may explain why the ascorbic acid level in the corneal epithelium is high in diurnal and low/zero in nocturnal mammals7, that the ascorbic acid content is much higher in corneal epithelium compared to aqueous humor in diurnal mammals, and that ascorbic acid content is higher in corneal epithelium of mammals living in the mountains, such as reindeer, compared to those living close to sea level8. Furthermore, this hypothesis has also been supported experimentally as artificially increased levels of ascorbic acid in the aqueous humor of guinea pigs had a protective effect against UV-induced DNA damage to the lens epithelium9, high ascorbic acid concentration protects the basal layer of the corneal epithelium by absorbing incident UVR10, and ascorbic acid (as well as tocopherol) in the “aqueous humor” increased the viability of lens epithelial cells after UVR11. There is also some evidence that systemic ascorbic acid supplementation used prophylactically may reduce the prevalence of hazy vision after photorefractive keratotomy (commercially available excimer lasers for refractive surgery are based on energy output at 213–193 nm wavelength)12. Hence, ascorbic acid seems to act as “sunglasses” for the diurnal mammal eye. It would be natural to believe that the presence of ascorbic acid would be significant in the aqueous humor of high-flying birds, as these are exposed to vastly more UV radiation at e.g. 10,000 m altitudes. The aqueous humor is an integral part of the optic pathway of the eye media regardless of whether mammal or bird, and so an observation of low ascorbic acid content in avian aqueous humor was surprising13.
What protect the bird’s eyes when flying at high altitudes, e.g. above the Himalayas14? High flying birds are exposed to significant amounts of UV-radiation, because the UV level increases approximately 10–12% every 1000 m. In addition, the cataractogenic effect is enhanced by reflection from the terrain—when moving from snow-free to snow-covered areas the effect is increased by a factor of 1615. Several UV-absorbing components, e.g. uric acid, tryptophan and tyrosine, have been proposed as being present in bird aqueous humor. In addition to these, a single, unknown component dominates the UV absorbing profile in goose aqueous humor, as observed with liquid chromatography-UV spectroscopy (LC-UV)16. From the initial data of reference16, the compound has UV absorbance in the 250–320 nm region, and appears to have a polarity similar to the above-mentioned metabolites. A lesser level of this compound is present in lower flying/nocturnal species e.g. duck and owl, and barely visible or absent in non-flying species16. We hypothesize that this “compound X” has a protective role for the eye of birds and describe here our work towards identifying the unknown compound.
Results and discussion
Liquid chromatography-UV analysis of highly polar substances in goose aqueous humor
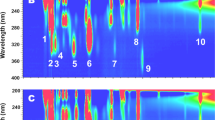
Capillary liquid chromatography-ultraviolet absorption (LC-UV) 2D plots (Fig. 1) shows chromophoric molecules (green frame), which were far more prominent in goose aqueous humor (top) compared to e.g. duck (bottom), resembling that observed previously16. A similar profile was seen in wild geese, suggesting that the prominence was not purely environmental. The green frame suggests the presence of multiple compounds. Compound 2 was however seen to form from compound 1 when samples was exposed to air, a clue that compound 1 (“compound X”) was sensitive to oxidation. The capillary LC system was coupled with electrospray-mass spectrometry (ESI–MS), a natural combination for gaining structure information of limited samples. Surprisingly, the molecules of interest had at this stage signals below the system´s detection limits, even when using high performance capillary LC-Orbitrap MS instrumentation.
Two dimensional (capillary LC X UV) plots of aqueous humor from goose (top) and duck (bottom). Conditions are described in “Methods” section (liquid chromatography and spectroscopy). 1 = “compound X”, and 2 is formed from “compound X”.
Analysis of enriched material
To obtain improved signal intensities, “compound X” was enriched with semi-preparative scale LC for subsequent elucidation, using a modified RPLC column variant (ACE AQUA) for increased resolution of the polar analytes (Fig. 2, 1D view; similar results were obtained using wild geese samples). Instant re-analysis of the isolated “compound X” revealed a pure peak, but the peak shape would be perturbed in subsequent analyses, again suggesting a reaction chemistry occurring when the analyte was chromatographed away from the aqueous humor. Moreover, when compound enrichment was attempted spending over 100 eye samples, a trace dark solid was produced. Size exclusion chromatography (SEC) could imply that the enriched material had (bio)macromolecular features (Fig. 3). Hence, the analyte was assumed to form polymeric species/aggregates when concentrated and isolated from the aqueous humor. When treating “compound X” with trypsin, no enzymatic cleavage was observed, i.e. presence of tryptic peptides using MS analysis, which could rule out the presence of a protein17. Crystallization procedures were subsequently attempted of isolated material, including developing an in-capillary crystallization procedure intended for limited samples (see Supplementary Materials for description of the procedure).
Reversed phase LC chromatography of goose aqueous humor, used for isolation of “compound X”. Conditions are described in “Methods” section (liquid chromatography and spectroscopy).
Size exclusion chromatography (SEC) of isolated “compound X” (light blue trace) and “compound X”/caffeine mixture (Vt marker, blue trace). Conditions are described in “Methods” section (liquid chromatography and spectroscopy).
However, only trace amounts of amorphous material were produced, unsuited for X-ray diffraction (XRD) experiments. 1H nuclear magnetic resonance (NMR) spectroscopy of the isolated material showed protons in the 6–8 ppm range (implying aromatic functionality), but again with insufficient sensitivity for structural analysis of the fractioned “compound X” peak.
Analysis of un-separated aqueous humor
The analytes were stable in capped aqueous humor, investigated with samples stored in a freezer, refrigerator, and subjected to freeze–thaw cycles, so analyses of un-chromatographed samples were undertaken. UV analysis confirmed that goose samples had elevated absorbance (Fig. 4, left). Next, high field NMR (800 MHz) of non-chromatographed samples was performed, as this approach can be used for structural elucidation of un-separated molecules. The one dimensional 1H NMR spectra of un-chromatographed aqueous humor from various bird species goose, chicken, turkey resembled each other (Fig. 4). Two-dimensional NMR spectroscopy enabled the identification several metabolites present, e.g. alanine, valine, leucine, isoleucine, tyrosine, phenylalanine, tryptophan and hypoxanthine. However, from inspection of the 1H spectrum and 2D nmr spectra we could not observe any aromatic substance with increased amounts in goose compared to other birds, in contrast to that observed with UV-based approaches (Fig. 4, right). Unfortunately, the identity of any possible compound responsible for increased UV-absorption in goose samples was not established in our NMR experiments.
Left: UV absorbance of aqueous humor of goose, chicken and turkey. Right: 800 MHz NMR of the same samples. Conditions are described in “Methods” section (liquid chromatography and spectroscopy and NMR Spectroscopy).
Analysis of moderately enriched “compound X” fractions
The size exclusion chromatography experiments implied a polymerization occurring during intensive enrichments, so moderately enriched fractions of “compound X” were instead examined (5–10 fractions collected). Fluorescence spectroscopy experiments were performed, showing that the fraction had emitting properties (Fig. 5).
Fluorescence spectra overlay in 2D of “compound X” and tryptophan. Conditions are described in “Methods” section (liquid chromatography and spectroscopy).
Revisiting mass spectrometry, now using conventional LC (2.1 mm ID column) coupled with an ESI source and a Q-Exactive mass spectrometer, the amino acid methionine was readily identified (also by the aid of an internal standard), see Fig. 6. No other compounds in the fraction were detected/identifiable. However, we believe it is unlikely that methionine (MW 149.21 g/mol) is “compound X”, as this amino acid has few UV-absorbing properties. During our investigation we did speculate that the “compound X” fraction could have contained 5,6-dihydroxyindole (DHI), a monomer (MW 149.15 g/mol) of the biomacromolecule melanin18,19,20, a hypothesis which could be supported by the indole-like properties and polymer-like features when performing isolation. Retention time comparisons with external standards were not possible, as commercially available DHI (Sigma) arrived in our lab as a partly polymerized/decomposed solid dark material (2 different batches). In addition, no indication of the presence of DHI was observed when NMR peaks were compared with those exhibited by another specimen of DHI which was not completely decomposed/polymerized. Furthermore, no NMR-signals in our isolated samples or in the pure eye liquid resembled nmr values reported in the literature21. No HRMS peaks were clearly observed either on side of those arising from the M+ ion of methionine, effectively discounting the possibility that DHI was compound X.
Methionine presence in fractionated “compound X” as observed with LC–MS and aided by an internal standard for retention time confirmation. Conditions are described in “Methods” section (mass spectrometry).
Discussion
While a UV-absorbing compound is present in the eyes of birds the identity of the compound remains unknown. Although hundreds of samples were extracted, and analyzed by numerous techniques, the certain identification of this endogenous metabolite was not possible at this stage. This study serves as an example of the challenges that can be encountered when performing metabolomics of limited amounts analytes, and raises the possibility that degradation of the believed UV protective "compound X" may have occurred during the isolation and disappeared in our investigation.
Polar metabolites are often extra challenging for studying/handling with chromatographic approaches22. We can expect that further developments in analytical separation technology will allow for simpler analysis. Future studies can include the investigation of alternative ionization sources for mass spectrometric analysis, as certain metabolites can have very poor signal when using conventional electrospray ionization. A significant obstacle has been obtaining adequate amounts of aqueous humor, both for identification purposes, but also for collecting data of potential clinical relevance; comparisons of levels of compounds as function of age, health, metabolic profile, etc. would have been be highly relevant, considering that e.g. cataracts are dependent on such factors.
Methods
Sample collection/preparation
Goose eyes (Anserine, White Italian) were made available to us each year in November, the only time when Holte Gaard (an ecological farm in Drangedal, Norway http://www.holtegard.no/) slaughter geese (for culinary purposes only). Approximate goose age was 7–8 months. The typical diet for the animals consisted of fresh grass and vegetables (potatoes, peas, corn, etc.). A veterinary would inspect the premises and routines the day of slaughter. Immediately after decapitation, aqueous humor was collected at the slaughterhouse through a limbal transcorneal canulation parallel to the iris diaphragm, 0.1–0.3 mL was obtained from each eye. Wild geese (Grey goose Anser anser, and Canadian goose Branta Canadensis) samples were donated from hunters. Chicken (Gallus gallus, Lomann), duck (Peking duck, Anatinae, English hybrid Cherry Waelly), and turkey (Meleagris gallopavo, British united turkey) were obtained under similar conditions as with the geese (farms: Prio Rakkestad and Revetal Vestfold, Norway).
Liquid chromatography and spectroscopy
Capillary LC-UV was performed using a CapLC-UV system (Waters, Milford, MA). LC analysis was performed with a Perkin Elmer (Waltham, MA) series 200 LC pump chromatograph coupled to a Perkin Elmer series 200 autosampler and a Waters 486 Tunable Absorbance Detector set to a wavelength of 254 nm. The RP column was an ACE AQ HPLC column (ACE; 250 mm × 10 mm, 5 μm diameter particles, S/N A59810, Advanced Chromatography Technologies Ltd, Aberdeen, Scotland). The SEC column was a 4.6 × 300 mm TSKgel SuperSW3000 SEC column (Tosoh Corp., Tokyo, Japan).
The mobile phase used for RPLC was an isocratic mixture (99/1, v/v) of water and acetonitrile (with 0.1% addition of formic acid (FA) to both solvents for pH ≈ 2.7), with a flow rate of 5 mL/min. SEC separations were done isocratically with a flow rate of 0.35 mL/min and a mobile phase containing 0.05 M sodium phosphate and 0.3 M NaCl pH adjusted to 7.0. Injection loops of 100 µL and 1 mL were used. Thawed samples were injected without any pre-treatment. The smaller loop was pre-installed in the instrument’s autosampler; the bigger one was manually installed using a two-position six-port valve (Model 7000, Rheodyne LLC, Rohnert Park, CA, US). The use of 1 mL loop required manual injections. Chromatograms were obtained with the TotalChrom Navigator software version 6.2.1 (Perkin Elmer, Waltham, MA, US). Evaporation of manually collected fractions was conducted using a Speed-vac concentrator (SC110, Savant Instruments Inc., Hicksville, NY, US), in 1.5 mL vials (Thumbs up microtubes, Diversified Biotech, Boston, MA, US), with medium or no heating. Evaporating 1 mL of aqueous solution to complete dryness took about 6 h. Qualitative UV–vis analysis was performed on a Thermo Scientific NanoDrop 2000 UV–vis spectrometer. 2 µL sample were applied on the instrument’s pedestal. Type 1 water with 0.1% FA was analyzed as a blank. The collected data were processed using the Chromeleon Chromatography Studio software (version 7.1.0.898, Dionex Corporation, Sunnyvale, CA, US). For fluorescence analysis an FP-8500 spectrofluorometer from Jasco with a Julabo F25-ED Refrigerated/Heating Circulator was employed. It was equipped with an ETC-815 Peltier thermostated single cell holder (water-cooled) for temperature control. The excitation wavelength was from 200 to 740 nm and emission wavelength from 305 to 750 nm without polarizing filters. Scan speed was 10,000, temperature 25 °C. Data collection occurred with Spectra Manager (version 2.13.00). For measurements 350 μL of sample was pipetted into a quartz sample holder.
NMR spectroscopy
NMR spectra were acquired in pure aqueous humor liquid from geese with 10% added D2O, 9:1 (v/v) at 25 °C using a Bruker AVIIIHD-800 spectrometer (Bruker BioSpin, Fallanden, Switzerland) equipped with a 5 mm TCI cryoprobe (1H, 13C, 15N) with automatic tuning and matching and Z-gradient accessories. 1H and 2D-HSQC spectra were recorded.
Mass spectrometry
With a Q-Exactive hybrid quadrupole-Orbitrap instrument from Thermo Scientific samples were transferred to mass analyzer using ESI in positive and negative mode with a spray voltage of 1.5 kV and a capillary temperature of 320 °C. Data were collected in a full scan mode (50 to 500 m/z and 150 to 2000 m/z). The pump flow was set to 0.02 mL/min (Fusion 100 T pump, Chemyx Inc., Stafford, TX, US). For data collection and process control the Xcalibur software (version 3.0.63, Thermo Fisher Scientific) was used.
Quantification of methionine was performed using reversed phase ion pair liquid chromatography (LC) coupled to tandem mass spectrometry (MS/MS). The sample was prepared by adding isotopically labeled internal standard (IS) and was injected onto a reverse phase LC Uptisphere BP2 C18 column (50 × 2.1 mm, 3 μm, Interchrom, Interchim, Montluçon, France) using a NexeraX2 LC system (Shimadzu, Japan). The mobile phase consisted of 1 mM tridecafluoroheptanoic acid (A) and acetonitrile (B) and the gradient program were as follows: 0–1 min, 0–20% B; 1–6 min, 20% B; 6–9 min, 20–28% B; 9–16 min, 28% B. The flow rate was 200 µL/min, the injection volume was 3 µL and the column temperature was 30 °C. The mass spectrometry analysis was performed on a Qtrap 4500 MD (AB SCIEX, Foster City, CA) using electrospray ionization in positive mode with the transitions 150.1 > 104.0 (methionine) and 153.1 > 107.0 (methionine-D3). All data collection and peak integration was performed using the Analyst software (version 1.6.2).
References
Lind, O., Mitkus, M., Olsson, P. & Kelber, A. Ultraviolet vision in birds: The importance of transparent eye media. Proc. Biol. Sci. 281, 20132209. https://doi.org/10.1098/rspb.2013.2209 (2014).
Widmark, J. Ueber die Durchdringlichkeit der Augenmedien für ultraviolette Strahlen. Skand Arch. Physiol. 3, 14–46 (1892).
Harris, L. J. Chemical test for vitamin C, and the reducing substances present in tumour and other tissues. Nature 132, 27–28 (1933).
Pirie, A. Ascorbic acid content of cornea. Biochem. J. 40, 96–100 (1946).
Ringvold, A. Aqueous humour and ultraviolet radiation. Acta Ophthalmol. 58, 69–82 (1980).
Maeda, J. et al. Ascorbic acid 2-glucoside pretreatment protects cells from ionizing radiation, UVC, and short wavelength of UVB. Genes. 11, 238. https://doi.org/10.3390/genes11030238 (2020).
Ringvold, A., Anderssen, E. & Kjønniksen, I. Ascorbate in the corneal epithelium of diurnal and nocturnal species. Investig. Ophthalmol. Vis. Sci. 39, 2774–2777 (1998).
Ringvold, A., Anderssen, E. & Kjønniksen, I. Impact of the environment on the mammalian corneal epithelium. Investig. Ophthalmol. Vis. Sci. 44, 10–15 (2003).
Reddy, V. N., Giblin, F. J., Lin, L. R. & Chakrapani, B. The effect of aqueous humor ascorbate on ultraviolet-B-induced DNA damage in lens epithelium. Investig. Ophthalmol. Vis. Sci. 39, 344–350 (1998).
Brubaker, R. F., Bourne, W. M., Bachman, L. A. & McLaren, J. W. Ascorbic acid content of human corneal epithelium. Investig. Ophthalmol. Vis Sci. 41, 1681–1683 (2000).
Reddy, G. B. & Bhat, K. S. Protection against UVB inactivation (in vitro) of rat lens enzymes by natural antioxidant. Mol. Cell. Biochem. 194, 41–45 (1999).
Stojanovic, A., Ringvold, A. & Nitter, T. Ascorbate prophylaxis for corneal haze after photorefractive keratectomy. J. Refract. Surg. 19, 338–343 (2003).
Reiss, G. R., Werness, P. G. & Brubaker, R. F. Aqueous ascorbic acid levels in diurnal birds (ARVO Abstract). Investig. Ophthalmol. Vis. Sci. 26, 101 (1985).
Scott, G. R., Egginton, S., Richards, J. G. & Milsom, W. K. Evolution of muscle phenotype for extreme high altitude flight in the bar-headed goose. Proc. R Soc. B Biol. Sci. 276, 3645–3653 (2009).
Ambach, W., Blumenthaler, M. & Schöpf, T. Increase of biologically effective ultraviolet radiation with altitude. J. Wilderness Med. 4, 189–197 (1993).
Ringvold, A. et al. UV-absorbing compounds in the aqueous humor from aquatic mammals and various non-mammalian vertebrates. Ophthalmic Res. 35, 208–216 (2003).
Raper, H. S. The tyrosinase-tyrosine reaction. Biochem. J. 21, 89–96 (1927).
Riley, P. A. Melanin. Int. J. Biochem. Cell Biol. 29, 1235–1239 (1997).
Thathachari, Y. T. & Blois, M. S. Physical studies on melanins II. X-ray diffraction. Biophys. J. 9, 77–89 (1969).
Pezzella, A. et al. Short-lived quinonoid species from 5,6-dihydroxyindole dimers en route to eumelanin polymers: Integrated chemical, pulse radiolytic, and quantum mechanical investigation. J. Am. Chem. Soc. 128, 15490–15498 (2006).
Murphy, B. P. & Schultz, T. M. Synthesis and physical properties of 5,6-dihydroxyindole. J. Org. Chem. 50, 2790–2791 (1985).
Zahn, D., Neuwald, I. J. & Knepper, T. P. Analysis of mobile chemicals in the aquatic environment—Current capabilities, limitations and future perspectives. Anal. Bioanal. Chem. 412, 4763–4784 (2020).
Acknowledgements
SRW is a member of the National Network of Advanced Proteomics Infrastructure (NAPI), which is funded by the Research Council of Norway INFRASTRUKTUR-program (project number: 295910). SRW is supported by the Research Council of Norway through its Centre of Excellence scheme, project number 262613. This work was partly supported by the Research Council of Norway through the Norwegian NMR Platform, NNP (226244/F50).
Author information
Authors and Affiliations
Contributions
Spectroscopy: D.A., F.R., A.R., J.P.M., A.L.W.; Mass spectrometry: M.Z., B.R., A.Ø., K.B.P.E.; Sample collection and preparation: A.R., S.R.W.; Chromatography: S.R.W., M.Z., B.R., E.L.; Crystallography: J.P.M., C.H.G., M.Z. All authors contributed to data analysis/feedback to the writing of the manuscript, which was primarily done by S.R.W. and A.R.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zawadzka, M., Ràcz, B., Ambrosini, D. et al. Searching for a UV-filter in the eyes of high-flying birds. Sci Rep 11, 273 (2021). https://doi.org/10.1038/s41598-020-79533-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79533-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.