Abstract
This study aims to evaluate the influence of myosteatosis on survival of patients after radical cystectomy (RC) for bladder cancer. We retrospectively identified 230 patients who underwent RC for bladder cancer at our three institutions between 2009 and 2018. Digitized free-hand outlines of the left and right psoas muscles were made on axial non-contrast computed tomography images at level L3. To assess myosteatosis, average total psoas density (ATPD) in Hounsfield Units (HU) was also calculated as an average of bilateral psoas muscle density. We compared cancer-specific survival (CSS) between high ATPD and low ATPD groups and performed cox regression hazard analyses to identify the predictors of CSS. Median ATPD was 44 HU (quartile: 39–47 Hounsfield Units). Two-year CSS rate in overall patients was 76.6%. Patients with low ATPD (< 44 HU) had significantly lower CSS rate (P = 0.01) than patients with high ATPD (≥ 44 HU). According to multivariate analysis, significant independent predictors of poor CSS were: Eastern Cooperative Oncology Group performance status ≥ 1 (P = 0.03), decreasing ATPD (P = 0.03), non-urothelial carcinoma (P = 0.01), pT ≥ 3 (P < 0.01), and pN positive (P < 0.01). In conclusion, myosteatosis (low ATPD) could be a novel predictor of prognosis after RC for bladder cancer.
Similar content being viewed by others
Introduction
Radical cystectomy (RC) is the standard treatment for patients with muscle-invasive bladder cancer, patients with high-risk non-muscle invasive bladder cancer, and patients with carcinoma in situ (CIS) resistant to bacillus Calmette-Guerin (BCG) treatment1,2,3. Meanwhile, five-year overall survival (OS) rates are 42–58%, despite RC4,5. Depending on the patient’s condition, bladder-preserving therapy by combined modality therapy could be a treatment option6. To select optimal management on an individual basis, it is therefore important to identify preoperative prognostic factors for patients who undergo RC.
Sarcopenia, defined as severe wasting of skeletal muscle mass, has been reported in several recent studies to be a preoperative prognostic factor in patients who undergo RC for bladder cancer, and is associated with poor rates of survival after RC7,8,9. Meanwhile, myosteatosis has recently drawn attention as a novel and objective preoperative prognostic factor in patients with various cancers, including gastric cancer, colorectal cancer, pancreatic cancer, lung cancer and ovarian cancer10,11,12,13,14. Myosteatosis is defined as increased fat infiltration in skeletal muscle15. Sarcopenia concerns muscle quantity and can be evaluated on abdominal computed tomography (CT) by the volume of skeletal muscle or psoas muscle7,8,9. Myosteatosis, by contrast, concerns muscle quality and can be evaluated on abdominal CT by the CT attenuation value of skeletal muscle or psoas muscle10,11,12,13,14.
To our knowledge, the association between myosteatosis and survival after RC for bladder cancer has not been investigated. We hypothesized that myosteatosis could be a novel preoperative prognostic factor in patients who undergo RC. In the present study, we evaluate the association between various parameters, including sarcopenia and myosteatosis, and survival after RC in patients with bladder cancer. To assess sarcopenia and myosteatosis, we measured psoas muscle index (PMI) and average total psoas density (ATPD), respectively.
Results
Patient demographics are summarized in Table 1. Median age was 73 years (quartile: 67–79 years) and 184 patients were male (80%). Median PMI and ATPD were 4.6 cm2/m2 (quartile: 3.6–5.6 cm2/m2) and 44 HU (quartile: 39–47 HU), respectively. Sixty-five patients (28%) received neoadjuvant chemotherapy. There were concerns about the possible potential impact of neoadjuvant chemotherapy on the myosteatosis measurements, but in these patients, median ATPD difference before and after neoadjuvant chemotherapy was 0 HU (quartile: − 2.5–1.0 HU) and there was no notable change in ATPD before and after neoadjuvant chemotherapy. Most patients underwent open RC (82%). Pathological diagnosis was urothelial carcinoma (UC) in 209 patients (91%) and non-UC in 21 patients (9%, squamous cell carcinoma in 10 patients, small cell carcinoma in 7 patients and other histopathological type in 4 patients). Pathological T stage of resected specimen was ≤ pT2 in 142 patients (62%) and ≥ pT3 in 88 patients (38%). Pathological lymph node metastasis was observed in 41 patients (18%).
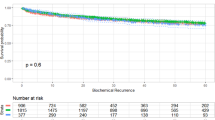
During the observation period (median 25.5 months, quartile: 10.8–49.3 months), 62 patients died of bladder cancer (27%) and 18 patients died of another cause (8%). The two-year OS rate and two-year cancer specific survival (CSS) rate were 73.4% and 76.6%, respectively (Fig. 1). We classified the patients into high ATPD (≥ 44 HU) and low ATPD (< 44 HU) groups using the median ATPD as cutoff value, and we compared OS and CSS between the two groups. The patients with low ATPD had significantly lower rate of OS (P = 0.04) and lower rate of CSS (P = 0.01) than patients with high ATPD (Fig. 2). Patient demographics are compared in Table 2. The patients in the low ATPD group were significantly older and had lower ratio of males, higher body mass index (BMI), higher percentage of poor Eastern Cooperative Oncology Group performance status (ECOG PS) and lower psoas muscle index than those in the high ATPD group.
Table 3 shows the results of univariate and multivariate cox proportional analyses of associations between various parameters and OS. In univariate analysis, the following were significantly associated with poor OS: older age (P = 0.01), ECOG PS ≥ 1 (P < 0.01), Charlson Comorbidity Index (CCI) ≥ 1 (P = 0.04), low PMI (P = 0.02), low ATPD (P = 0.01), non UC (P < 0.01), pT ≥ 3 (P < 0.01) and pN positivity(P < 0.01). Multivariate analysis showed that ECOG PS ≥ 1 (P = 0.03), pT ≥ 3 (P < 0.01) and pN positive (P < 0.01) were significant independent predictors of poor OS. Moreover, increasing age was a marginally significant predictive factor of OS (P = 0.06). On the other hand, PMI (P = 0.11) and ATPD (P = 0.18) were not independently significant.
Table 4 shows the results of univariate and multivariate cox proportional analyses of associations between various parameters and CSS. According to univariate analysis, the following were significantly associated with poor CSS: ECOG PS ≥ 1 (P = 0.01), low ATPD (P < 0.01), non-UC (P < 0.01), pT ≥ 3 (P < 0.01), and pN positivity (P < 0.01). Meanwhile, significant independent predictors of poor CSS according to multivariate analysis were: ECOG PS ≥ 1 (P = 0.03), low ATPD (P = 0.03), non-UC (P = 0.01), pT ≥ 3 (P < 0.01), and pN positivity (P < 0.01).
To develop a risk classification to predict CSS after radical cystectomy in patients with bladder cancer, five risk factors, (ECOG PS ≥ 1, ATPD < 44HU, non-UC, pT ≥ 3, and pN positivity) were used, and the cohort was classified into five groups according to the presence of these five risk factors. This model effectively stratified patients in terms of CSS according to the number of risk factors (P < 0.01), as shown in Fig. 3.
Table 5 shows the results of comparison of OS and CSS between high ATPD and low ATPD groups by using various cutoff values of ATPD. When using 35HU and 44HU (median value in the present study) as cutoff values, OS and CSS rates in low ATPD group were significantly lower than those in high ATPD group. On the other hand, when using other values, there was no statistically significant difference between two groups in OS and CSS rates.
Discussion
We examined low ATPD, namely myosteatosis, as a possible preoperative predictor of prognosis after RC in patients with bladder cancer. To the best of our knowledge, this is the first report about the clinical significance of myosteatosis for predicting prognosis after RC. Patients with low ATPD had lower OS and CSS rates after RC than those with high ATPD. Low ATPD was a significant independent predictor of poor CSS in patients who underwent RC for their bladder cancer.
Muscle depletion has recently drawn attention as a prognostic factor in patients with various forms of cancer. It is classified into reduced muscle volume (sarcopenia) and declined muscle quality (myosteatosis)15,16 and can occur in any weight category, from underweight to obese10,17,18. Sarcopenia has been reported to be associated with prognosis for patients with various forms of cancer, including bladder cancer9. In addition, myosteatosis has been shown to be a novel predictive factor in patients with other types of cancer, such as gastric cancer, colorectal cancer, pancreatic cancer, ovarian cancer and breast cancer10,11,12,14,19. Little is known, however, about the association between myosteatosis and the prognosis of patients with bladder cancer.
Sarcopenia has been evaluated by measuring the area or volume of skeletal muscle or psoas muscle on CT images7,8,9. Myosteatosis, meanwhile, has been defined as decreased muscle attenuation values and evaluated by measuring the CT attenuation values of skeletal muscles or psoas muscles10,20. In this study we measured ATPD on the CT image at level L3, and examined the association between ATPD and the prognosis after RC in patients with bladder cancer. Patients with low ATPD had poorer OS and CSS than those with high ATPD. Moreover, low ATPD was an independent significant predictor of CSS after RCC in patients with bladder cancer. These results suggest that myosteatosis could be a novel predictive factor of poor prognosis after RC in patients with bladder cancer.
We developed a risk classification model based on various parameters, including low ATPD for patients who undergo RC. To our knowledge, this is the first study to establish a risk classification or nomogram prediction of CSS based on prognostic parameters including myosteatosis in patients with bladder cancer undergoing RC. We believe that our risk classification will be helpful in predicting prognosis after RC in patients with bladder cancer.
The prognostic impact of sarcopenia is thought to be due to a combination of vulnerability to cancer and its treatments, due to low physical reserves, or to sub-optimal treatment options in patients with limited physical reserves19,21. Meanwhile, the reason for myosteatosis leading to poor prognosis in patients with malignant diseases, remains unclear. To examine the association between sarcopenia and myosteatosis, we investigated the relationship between PMI and ATPD, but there was no significant correlation (Fig. 4, Spearman’s rank correlation coefficient 0.11, P = 0.09). This suggested that myosteatosis worsened the prognosis after RC in patients with bladder cancer by a mechanism different to sarcopenia. Several possible mechanisms have been previously suggested. Skeletal muscle is known to be secretory and muscle cells secrete cytokines and other peptides, which may influence the growth and metastasis of tumor cells10,22. Reduced muscle quality by myosteatosis can therefore lead to an altered myokine response and deficient regulation of tumor cells. Moreover, myosteatosis is associated with hyperinsulinemia and insulin resistance23,24. Hyperinsulinemia can promote tumor cell proliferation through insulin receptor25. The decline in synthesis of insulin-like growth factor-1 (IGF-1) binding protein and activation of IGF-1 by hyperinsulinemia can also lead to tumor cell proliferation25. Furthermore, myosteatosis promotes an elevated systemic inflammatory response. Inflammation stimulates tumor cell proliferation and can lead to poorer chance of cancer survival26,27,28. Further studies will seek to clarify how myosteatosis influences the prognosis of patients with bladder cancer.
The current study has several limitations. It was a retrospective study and the results require verification by a large-scale prospective study. The timing of preoperative CT scans was also inconsistent, although only patients with preoperative CT examination within 30 days of RC were included in the present study. To perform large-scale prospective studies, it is therefore necessary to recruit a large number of patients who will undergo radical cystectomy for their bladder cancer and to standardize the timing of preoperative CT scans and post-operative follow-up protocol. Moreover, although we used median ATPD (44 HU) as cutoff value, there is no consensus about optimal cutoff value of ATPD or skeletal muscle density. Interestingly, the used cutoff values of muscle attenuation value for evaluating myosteatosis status differ among previous studies. Alexio et al. used 37.8 HU as cutoff value in their studies of patients with breast cancer19, while Aust et al. used 39 HU for ovarian cancer14. Martin et al. adopted 33–41 HU according to BMI in a cohort with various cancers13. Cutoff value was decided in some studies by gender (male: 35.5–38.8 HU, female: 28.6–32.5 HU)10,11. As shown in Table 5, the results of comparison of OS and CSS between high ATPD and low ATPD differ depending on the cutoff value used. In Cox proportional analyses, however, ATPD was analyzed as a continuous variable and, as a result, poor prognostic impact of decreasing ATPD was shown. We therefore believe that myosteatosis is associated with poor prognosis in patients that have undergone radical cystectomy. Further consideration will be required to decide the optimal cutoff value of ATPD to define myosteatosis.
In conclusion, myosteatosis (low ATPD) was indicated to be independently associated with poor CSS in our patients who underwent RC for bladder cancer. The development of risk classifications or nomograms with inclusion of myosteatosis may be clinically useful for patients with bladder cancer.
Methods
Patient selection
We retrospectively reviewed the records of consecutive patients who underwent RC for bladder cancer at the Wakayama Medical University Hospital, Kinan Hospital and Rinku General Medical Center between March 2009 and March 2018. Patients were excluded from this study if preoperative abdominal CT examination was not available within 30 days of surgery, or if they did not receive post-operative follow-up at one of the institutions. Of 239 candidates, 230 patients were finally enrolled in the study. This multi-institutional retrospective study was approved by the Wakayama Medical University Institutional Review Board (approval number 3008) and conducted in accordance with the principles of the Declaration of Helsinki. All participants gave written informed consent prior to the study.
Data collection
Patient demographic data at operation, such as age, sex, BMI, ECOG PS, CCI and blood parameters were collected retrospectively from medical records. Information about neoadjuvant chemotherapy, cystectomy approach (open, laparoscopic or robotic) and urinary diversion (cutaneous ureterostomy, ileal conduit or neobladder) was also collected. We also reviewed histopathological data of resected specimens and recorded pathological diagnosis, pathological T stage, pathological lymph node metastasis and the presence of concurrent CIS.
CT image analysis
Pre-surgical abdominal CT images were used for evaluation of total psoas muscle area and density. CT scans (5 mm collimation width) were performed using a GE LightSpeed 64-slice multidetector helical CT scanner (GE Healthcare Japan Corporation, Tokyo, Japan) and scanned images were analyzed on a GE workstation by one well-trained radiologist, blinded to patient outcomes, at each institution. A digital free-hand outline of the left and right psoas muscles was made on the axial non-contrast CT image at level L3 (Fig. 5). By this procedure, the area in cm2 and density in HU of each psoas muscle at this level were automatically calculated. To assess sarcopenia, PMI in cm2/m2 was calculated by normalizing the total psoas muscle area (left and right psoas muscle area) by the square of the patient’s height7. To assess myosteatosis, ATPD in HU was also calculated as an average of left and right psoas muscle density20.
Statistical analysis
All statistical analyses were performed using JMP Pro 14. OS rate and CSS rate were determined by Kaplan–Meier method. Comparisons of OS and CSS between groups were performed using log rank tests. Comparison of patient demographics between groups were performed using chi-square tests, Fisher’s exact tests or Mann–Whitney U tests. Univariate and Multivariate Cox proportional regression analyses were performed to identify predictors of OS and CSS. In Cox proportional regression analyses, psoas muscle index and average total psoas density were analyzed as continuous variables. In all analyses, P < 0.05 was considered to be statistically significant.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Gore, J. L. et al. Use of radical cystectomy for patients with invasive bladder cancer. J. Natl. Cancer Inst. 102, 802–811 (2010).
Bochner, B. H. Optimal timing of radical cystectomy for patients with T1 bladder cancer. Urol. Oncol. 27, 329–331 (2009).
Brausi, M. Radical cystectomy for BCG failure: has the timing improved in recent years?. BJU Int. 108, 186 (2011).
Shariat, S. F. et al. Nomograms provide improved accuracy for predicting survival after radical cystectomy. Clin. Cancer Res. 12, 6663–6676 (2006).
Ploussard, G. et al. Conditional survival after radical cystectomy for bladder cancer: evidence for a patient changing risk profile over time. Eur. Urol. 66, 361–370 (2014).
Efstathiou, J. A. et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: the MGH experience. Eur. Urol. 61, 705–711 (2012).
Smith, A. B. et al. Sarcopenia as a predictor of complications and survival following radical cystectomy. J. Urol. 191, 1714–1720 (2014).
Psutka, S. P. et al. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: impact on cancer-specific and all-cause mortality. Cancer 120, 2910–2918 (2014).
Hirasawa, Y. et al. Sarcopenia as a novel preoperative prognostic predictor for survival in patients with bladder cancer undergoing radical cystectomy. Ann. Surg. Oncol. 23, 1048–1054 (2016).
Zhuang, C. L. et al. Myosteatosis predicts prognosis after radical gastrectomy for gastric cancer: a propensity score-matched analysis from a large-scale cohort. Surgery 166, 297–304 (2019).
Kroenke, C. H. et al. Muscle radiodensity and mortality in patients with colorectal cancer. Cancer 124, 3008–3015 (2018).
Akahori, T. et al. Prognostic significance of muscle attenuation in pancreatic cancer patients treated with neoadjuvantchemoradiotherapy. World J. Surg. 39, 2975–2982 (2015).
Martin, L. et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin Oncol. 31, 1539–1547 (2013).
Aust, S. et al. Skeletal muscle depletion and markers for cancer cachexia are strong prognostic factors in epithelial ovarian cancer. PLoS ONE 10, e0140403. https://doi.org/10.1371/journal.pone.0140403 (2015).
Miljkovic, I. & Zmuda, J. M. Epidemiology of myosteatosis. Curr. Opin. ClinNutr. Metab. Care 13, 260–264 (2010).
Cruz-Jentoft, A. J. et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39, 412–423 (2010).
Prado, C. M. et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 9, 629–635 (2008).
Baracos, V. E., Reiman, T., Mourtzakis, M., Gioulbasanis, I. & Antoun, S. Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. Am. J. Clin. Nutr. 91, 1133s–1137s (2010).
Aleixo, G. F. P., Williams, G. R., Nyrop, K. A., Muss, H. B. & Shachar, S. S. Muscle composition and outcomes in patients with breast cancer: meta-analysis and systematic review. Breast Cancer Res. Treat 177, 569–579 (2019).
Kalafateli, M. et al. Muscle fat infiltration assessed by total psoas density on computed tomography predicts mortality in cirrhosis. Ann. Gastroenterol. 31, 491–498 (2018).
Williams, G. R., Rier, H. N., McDonald, A. & Shachar, S. S. Sarcopenia & aging in cancer. J. Geriatr. Oncol. 10, 374–377 (2019).
Pedersen, B. K. & Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465. https://doi.org/10.1038/nrendo.2012.49 (2012).
Miljkovic, I. et al. Abdominal myosteatosis is independently associated with hyperinsulinemia and insulin resistance among older men without diabetes. Obesity (Silver Spring) 21, 2118–2125 (2013).
Goodpaster, B. H., Thaete, F. L., Simoneau, J. A. & Kelley, D. E. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes 46, 1579–1585 (1997).
Calle, E. E. & Kaaks, R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer 4, 579–591 (2004).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
McSorley, S. T., Black, D. H., Horgan, P. G. & McMillan, D. C. The relationship between tumour stage, systemic inflammation, body composition and survival in patients with colorectal cancer. Clin. Nutr. 37, 1279–1285 (2018).
Malietzis, G. et al. Low muscularity and myosteatosis is related to the host systemic inflammatory response in patients undergoing surgery for colorectal cancer. Ann. Surg. 263, 320–325 (2016).
Acknowledgements
This document was proof-read and edited by Benjamin Phillis at the Clinical Study Support Center at Wakayama Medical University.
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to this study. S.Y., Y.I. and H.M. designed the research study. S.Y., Y.I., H.M., T.I., H.K., S.N., N.M., K.H. and K.K. collected the data. S.Y. analyzed the data and wrote the first draft of manuscript. Y.K. and I.H. revised the manuscript. All authors reviewed and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamashita, S., Iwahashi, Y., Miyai, H. et al. Myosteatosis as a novel prognostic biomarker after radical cystectomy for bladder cancer. Sci Rep 10, 22146 (2020). https://doi.org/10.1038/s41598-020-79340-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79340-9
This article is cited by
-
Computed tomography derived cervical fat-free muscle fraction as an imaging-based outcome marker in patients with acute ischemic stroke: a pilot study
BMC Neurology (2023)
-
Psoas muscle index and psoas muscle density as predictors of mortality in patients undergoing hemodialysis
Scientific Reports (2022)
-
Prognostic value of myosteatosis in patients with lung cancer: a systematic review and meta-analysis
International Journal of Clinical Oncology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.