Abstract
This systematic review (SR) addressed the following common clinical question: What is more effective in reducing or eliminating endotoxin in endodontic infections—single or multiple-session treatments using calcium hydroxide medications? Literature searches of Medline/PubMed, Embase, Cochrane Library, Scielo, Science Direct, Web of Knowledge, Scopus, and Google Scholar databases. Two reviewers independently assessed the eligibility for inclusion, extracted data, and evaluated the quality of the studies using the risk of bias tools. Electronic searches resulted in 358 articles, of which 32 studies were included for full-text assessment, and nine were included in this review. Meta-analysis pooling all the nine studies revealed lower levels of endotoxin for multiple-session treatment (P < 0.001). The sub-group analysis indicated no difference between single-session and 7 days of Ca(OH)2 medication (SMD − 0.32; P = 0.22). However, 14-days (I2 = 80.5%, P < 0.001) and 30-days (I2 = 78.9%, P < 0.01) of Ca(OH)2 medication was more effective than single-session treatment (both, p < 0.001). Overall, Overall, this SR provides evidence to support that multiple-session disinfection protocols with the placement of Ca(OH)2 medications are more effective in reducing the levels of endotoxin from root canal infections compared to single-session when applied for 14 and 30 days.
Similar content being viewed by others
Introduction
Apical periodontitis is an inflammatory process of the periapical tissues caused mainly by bacteria and their byproducts in the root canal that can culminate in bone loss1,2,3,4,5. Studies have demonstrated that these infections are polymicrobial comprised both by Gram-positive and -negative bacteria1,2,3,4,5,6.
Lipopolysaccharides (LPSs), also known as endotoxins, are the major surface molecule and pathogenic factor of Gram-negative bacteria7,8. LPSs are present inside a bacterial cell attached to the outlying cell membrane. Endotoxins have been detected in 100% of teeth with root canal infections and the presence of apical periodontitis9,10,11,12. LPS is one of the most important virulent factors involved in the development of periapical inflammation13,14. Endotoxin activates immunocompetent cells present in periapical tissues leading to the release of several proinflammatory mediators14.
Higher levels of endotoxins present in root canal infections are strongly correlated to the presence of spontaneous pain9,15, clinical symptomatology such as pain on palpation and tenderness to percussion15,16, presence of exudation9,15,16, and severity of bone resorption (size/volume of periapical radiolucency)11,16.
Taken the high inflammatory potential of endotoxins to periapical tissues and their strong correlation with the presence of clinical symptoms as well as the severity of bone resorption, the removal/ elimination of endotoxins during root canal therapy is important for the remission of symptoms and healing of periapical tissues. Over the years, different studies have evaluated the ability of root canal therapies in reducing/eliminating endotoxins from infected root canals10,12,17,18,19,20,21. Single-session treatment accomplished by disinfection protocols involving chemomechanical preparation followed by prompt obturation and multiple-session therapy with the supplement of interappointment intracanal medication has been tested against endotoxins12,22.
Calcium hydroxide [Ca(OH)2] is the most commonly used intracanal medication for multiple-session therapy12,17,18,20,23,24,25. Its efficacy against endotoxins has been demonstrated by in vitro23,26 and animal studies24,27. Ca(OH)2 is able to hydrolyze lipid A molecule, an Esther-linked hydroxyl fatty acids, resulting in atoxic components19,23,24,26. A histopathological study has further corroborated this ability to convert lipid A into atoxic components. However, controversy exists on whether Ca(OH)2 intracanal medication can clinically boost the reduction or elimination of endotoxins from infected root canals achieved with root canal instrumentation10,15,18,20,21,22,25,28,29,30,31.
The assessment of the effectiveness of different root canal therapies on the reduction or elimination of endotoxins from root canal infections through this systematic review (SR) and meta-analysis is important to provide a high quality of evidence and relevant data to contribute to the establishment of evidence-based treatment protocols. Therefore, this SR aimed to compare the effectiveness of multiple-session treatment with the use of calcium hydroxide intracanal medications over single-session treatment against endotoxins present in root canal infections.
Material and methods
This SR followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines32 and registered in the PROSPERO database (Registration number: CRD42017077160).
Research question
What is more effective in reducing or eliminating endotoxin in endodontic infections—single or multiple-session treatments using calcium hydroxide medications? This review question was based on the PICO format as follows: Population—patients with endodontic infection; Intervention—endodontic treatment; Comparison—multiple-session using calcium hydroxide medication versus single-session; Outcome—level of endotoxin.
Search strategy
The literature search was conducted to identify clinical studies that compared the effectiveness of single- and multiple-session root canal therapy in reducing endotoxin from root canal infections. Electronic searches were conducted in Medline/PubMed, Embase, Cochrane Library, Scielo, Science Direct, Web of Knowledge, Scopus databases, and Google Scholar. The literature search was performed retrieving articles published until Aug 2020. In addition to the electronic search, the reviewers undertook a hand search in the references list of each included study. Search was conducted with the following MeSH and free terms: (“periapical periodontitis”[all], OR “periapical diseases”[all], OR “dental pulp diseases”[all], OR “apical periodontitis”[all], OR “endodontic infection”[all]) AND (“endotoxin”[all], OR “LPS”[all], OR “lipopolysaccharide”[all]) AND (“intracanal medication”[all], OR “calcium hydroxide”[all]).
Eligibility criteria
Clinical studies in humans that compared the effect of single-session versus multiple-session using Ca(OH)2 intracanal medications were included in this SR. Studies were eliminated if they presented any of the following exclusion criteria: in vitro or animal study, case report, review or opinion article; absence of the endotoxin levels; absence of the description of the type of endodontic infection; lack of a clear definition of the method used for endotoxin detection; absence of the amount of endotoxin in the same study before and after intracanal medication.
Study selection procedure
All references were tabulated by using the software EndNote X7 (Thomson Reuters, New York, NY, US). Duplicate references were excluded. Titles, abstracts, and keywords were screened by two reviewers (DDR and BJC) based on the eligibility criteria. The Kappa coefficient was performed to determine the agreement between the reviewers (kappa = 0.90). The screened lists were compared, and in case of any disagreement, a consensus was reached through discussion or consultation of a third author (GGN). After initial screening of titles and abstracts, the full articles were evaluated by the same two reviewers (DDR and BJC).
Data collection process
Data extraction was performed independently by two reviewers (DDR and BJC). In case of disagreements, these were solved by discussion or seeking the opinion of a third author (GGN).
Data extraction
The following information was gathered from the included articles: Publication details: authors and year of publication; Characteristics of the study: sample size and study design; Characteristics of the patients: diagnosis, age range of the patients, and teeth treated; Characteristics of the endodontic treatment: type of instrumentation, irrigation solution, type of vehicle used for the Ca(OH)2 medication, dosage [ratio of Ca(OH)2, number of renewal sessions, period of time of application Ca(OH)2 intracanal medication, and method used to deliver the medication inside the canal; and Characteristics of the endotoxin detection: method and test used for the detection of endotoxin.
Risk of bias in individual studies
Quality assessment was performed independently by two reviewers (DDR and BJC). The risk of bias in randomized clinical trials (RCTs) was performed using the Cochrane risk of bias tool (RoB 2.0)33. The RoB 2.0 tool assesses the risks of bias according to 6 domains as follows: randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcomes, selection of reported result and, overall. The included studies were judged as low risk of bias, high risk of bias, or some concerns. The study was considered a low risk of general bias if all domains were classified as low risk. If the study raised any concerns in at least one domain, it was considered to have some general concerns. If the study were at ‘high risk’ in any domain or three or more of the same in some concerns, it would be considered a ‘high risk bias.’ The risk of bias classification in non-randomized clinical studies was performed using the Cochrane risk of bias tool (ROBINS-I)34, according to 7 domains as follow: confounding; selection of participants; classification of interventions; deviations from the intended interventions; missing outcome data; measurement of outcomes; and selection of the reported results. For each domain, the studies were classified as low, moderate, serious, critical, or no information available for risk of bias. The general bias assessment was determined by combining the level bias in each domain. When the study was judge as ‘unclear’ in their key domains, an attempt was made to contact the authors to obtain more information and enable definitive judgment of ‘low’ or ‘high’ risk.
Analytical approach
Articles that fulfilled all the inclusion criteria were considered for quantitative synthesis by means of meta-analysis. First, a meta-analysis was performed to combine a broad range of studies that used calcium hydroxide intracanal medications for 7, 14, and 30 days compared to single-session protocols to obtain. Subsequently, a subgroup meta-analysis was performed to stratify the pooled results by the period of time application of Ca(OH)2 intracanal medication: 7, 14, or 30 days compared to single-session therapy. Pooled estimates were calculated using both the fixed- and the random-effects model, and in the presence of heterogeneity (I2 > 50%; Chi-square and P value < 0.1), the latter was preferred35. The analyses were conducted using the software Stata SE 16.0 (StataCorp., College Station, TX, USA).
Results
Summary of included studies
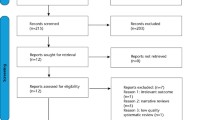
The PRISMA flow chart summarizes the study selection process (Fig. 1). The initial electronic search revealed 358 articles. After excluding 133 duplicates, 225 articles remained for the title and abstract screening. Thirty-two studies were included for full-text assessment and, of those, 22 were excluded. Accordingly, nine studies fulfilled the inclusion criteria for this review. Table S1 displays the excluded studies and the main reason for exclusion.
General characteristics
The nine studies included in this SR and meta-analysis10,12,18,20,21,22,25,36,37 consisted of a total of 426 patients, with 223 patients treated in single-session and 203 patients treated in multiple-session with Ca(OH)2 intracanal medications. All 9 clinical studies analyzed patients with primary endodontic infections with the presence of apical periodontitis. Out of the 9 studies, 5 were randomized clinical trials10,12,22,25,37 and four NRSis18,20,21,36. All studies used the Limulus Amebocyte Lysate (LAL) method for the quantification of endotoxin. The values of endotoxin were determined in Units of Endotoxin/mL (EU/mL).
Root canal instrumentation was performed using GT rotary files + k-files (Malleifer-Dentsply, Switzerland)25, Endo-Eze (Ultradent Products, USA) + k-files18,21,22, Mtwo (VDW, Germany)10,36,37, ProTaper (Dentsply, Switzerland)20, and Reciproc (VDW, Germany)12.
Root canal irrigation was performed using chlorhexidine (2% CHX-gel)10,18,22,25,36,37 or sodium hypochlorite (1% or 2.5% NaOCl)10,12,20,21,22,37. Final irrigation with 17%EDTA was performed in all studies except for Vianna 200725 and Adl 201520.
Calcium hydroxide intracanal medication was used for 7 days20,25, 14 days12,18,21,22 and 30 days10,36,37. The type of vehicle for the medication was either 2% CHX-gel18,21,25,36, saline solution10,12,20,25,37 or propylene glycol22. The medication dosage, the ratio of Ca(OH)2 to the vehicle, was 1:110,18,21,37 or not described12,20,22,25,36. Additionally, the lentulo spiral was used to deliver the Ca(OH)2 medication in all 9 studies. The included studies are summarized in Table 1.
Risk of bias within studies
The assessment of the risk of bias of the selected randomized clinical trials (RCTs) was performed using the Cochrane risk of bias tool (RoB 2.0) assessing 6 different domains33 (Fig. 2). Of the 5 RCTs included, 3 of them were ‘overall’ classified as “some concerns” of bias and 2 “low risk” of bias. All 5 RCTs10,12,22,25,37 included in this SR were classified as ‘low risk’ of bias according to RoB 2.0. The assessment of the risk of bias of the non-randomized studies of interventions (NRSis)18,20,21,36 was performed according to ROBINS-I (Fig. 3). All NRSis included in this SR were classified as “low risk’ of bias.
Quantitative synthesis
Among the 9 studies included in this SR, four studies provided data in median values1,12,22,36. Such authors were contacted to obtain raw data for the calculation of the mean values. Seven out of 9 studies reported significantly higher levels of endotoxin reduction achieved with the placement of Ca(OH)2 intracanal medications when compared to single-session irrespective period of time of application10,12,18,20,22,36,37. Whereas, Vianna 200725 and Carvalho 201621 showed no significant difference in the reduction of endotoxin after the placement of Ca(OH)2 intracanal medication for 7 days and 14 days, respectively. The authors indicated an increase in the reduction of endotoxin levels in 14%25 and 25.6%21, respectively. Figure 4 shows the forest plot for the endotoxin levels for single- and multiple-session treatments irrespective the period of Ca(OH)2 intracanal medication application. Despite the heterogeneity across the 9 studies (I2 = 77.5%; P < 0.001), the overall meta-analysis indicated lower levels of endotoxin achieved with multiple-session than in single-session treatment (SMD − 0.98; P < 0.001) (Fig. 4). The sub-group analysis indicated no difference between single-session and 7 days of Ca(OH)2 medications (SMD − 0.32; P = 0.22). However, 14-days (I2 = 80.5%, P < 0.001) and 30-days (I2 = 78.9%, P < 0.01) of Ca(OH)2 medications was more effective than single-session treatments (both, P < 0.001). Figure 5 shows the forest plot for the endotoxin levels found in single and multiple-session treatment (7, 14, and 30 days) as well as the overall estimated effect for all the period of times combined.
Discussion
This SR addressed the following common clinical question—What is more effective in reducing or eliminating endotoxin in endodontic infections—single or multiple-session treatments using calcium hydroxide medications? For that, we combined clinical studies that used calcium hydroxide intracanal medications for 7, 14, and 30 days over single-session protocols to get a broad answer about their effectiveness against endotoxin present in root canal infections. Additionally, 7, 14, and 30 days subgroup meta-analysis were performed to estimate the effectiveness of Ca(OH)2 intracanal medication for each period of time.
Keeping participants, and healthcare providers delivering the intervention unaware of the assigned intervention—a process known as blinding or masking—can reduce the risk of bias due to deviations from the intended intervention. Depending on the type of outcome measurement selected, the blinding can influence outcomes, in particular those studies that participant reports outcomes, observer makes judgment and/or provider makes decision on outcomes. In this SR, we looked at the difference in the endotoxin levels after single and multiple-session protocols with the placement of Ca(OH)2 intracanal medications, which is considered observer-reported outcomes with no judgement involved.
Here, the overall estimated effect for all the data combined from the 9 studies indicated that when Ca(OH)2 intracanal medications was used in multiple-session treatment protocols, endotoxins levels were lower than those obtained after single-session treatment protocols irrespective the period of time of application. The pool estimated effect for all the data combined, indicated by the ‘diamond shape’ in the forest plot, fell in the left side on Fig. 4, which here means in favor of the multiple-session protocols with the placement of Ca(OH)2 intracanal medications over single-session treatment protocols as labeled. The overall meta-analysis (Fig. 4) indicated considerable heterogeneity. Such heterogeneity was expected, and for that reason, we pre-planned subgroup meta-analysis for each period of time (7, 14, and 30 days), considering that the time of application could be an important modifier effect.
It is important to highlight that although only 2 studies were included in the 7-day subgroup meta-analysis, both studies are very consistent, indicating low heterogeneity between them (I2 = 20.4%; Chi2 = 0.35, P = 0.555). Both single studies indicated an increase in the reduction of endotoxin in 14%25 and 25.6%20, respectively. In contrast, considerable heterogeneity was detected among the studies for both 14 days (I2 = 82.2%; Tau2 = 0.4102; Chi2 = 16.76, P = 0.001) and 30 days (I2 = 82.3%; Tau2 = 0.5399; Chi2 = 11.24, P = 0.004) of Ca(OH)2 medication protocols. According to the I2 results, the heterogeneity for both 14 (82.2%) and 30 (82.35%) days is slightly higher than the overall combined data level at 78.1%. Therefore, in particular, to 14 and 30 days, the difference in time of application has not explained the observed heterogeneity.
Since this SR was designed to address a clinical question, two main types of heterogeneity rise (1) clinical heterogeneity, in which differences between the studies may relate to interventions, and (2) methodological heterogeneity that indicates differences in the type of study design. The clinical heterogeneity (1) among the14 and 30 days studies may be attributed mainly to the intracanal medication, including the type of vehicle, the medication dosage (ratio of preparation), the renewal of the medication, as well as the method of delivery. The type of vehicle for the medication used across the studies was either 2% CHX-gel18,21,25,36, saline solution10,12,20,25,37 or propylene glycol22. Although 2% CHX-gel has no detoxifying effect against endotoxin38, its viscous vehicle, in a gel formula, influence the dissociation of Ca+2 and Cl– ions. The medication dosage, the ratio of Ca(OH)2 to the vehicle, was 1:110,18,21,37 or not described12,20,22,25,36. Different study designs (e.g., RCT versus NRSis) might produce different results, which might result in methodological heterogeneity (2). However, such heterogeneity is limited in this SR, once all studies included were classified as ‘low’ risk of bias, and therefore with a low possibility that the effect size is overestimated39.
All 9 studies included here used the Limulus amebocyte lysate (LAL) method to quantify the levels of endotoxin in root canal infection. The LAL method uses a serine protease catalytic coagulation cascade that is activated by endotoxin. Endotoxin binds to the first component in this cascade, Factor C, which is a protease zymogen. Subsequently, the cascade induces the activation of a proclotting enzyme (coagulaten) into a clotting enzyme (coagulin). Such a clotting enzyme cleaves the synthetic peptide-pNA substrate, used in the chromogenic LAL assays (QCL or KQCL), and imparts a yellowish color to the solution. As coagulogen converts into coagulin, a gel clot begins to be formed, and therefore, increase the turbidity, which is captured by the turbidimetric kinetic assay. Then, the concentration of endotoxin is determined by the strength of the yellow color (determined at an OD = 340 nm) resulting from the chromogenic LAL and the turbidity (determined at an OD = 340 nm) resulting from the coagulogen conversion. These three tests differ in the technique to detect endotoxin. The two chromogenic tests (QCL-1000 and KQCL tests) use synthetic peptide-pNA substrate, which is cleaved by the clotting enzyme, making the solution yellowish in color. While the turbidimetric test (Pyrogent 5000 test) monitors, the increase of the turbidity resulted from the conversion of coagulogen in coagulin. The two Chromogenic LAL tests determine the concentration of endotoxin in an optical density (OD) at 405 nm and while the turbidimetric LAL test in an OD at 340 nm. Both the Kinetic methods (KQCL test and Pyrogent 5000 test) monitors the progress of the LAL reaction, which confers a wider sensitivity range (0.01–100 EU/mL−1). While for the Endpoint method (QCL-1000 test), the quantification of endotoxin is determined at a time point, specifically at 16 min after the initiation of the LAL reaction, which limits its sensitivity (0.1–1 EU/mL−1). The variations of the type of endotoxin test selection across the studies included in this SR might have influenced on the effect size in terms of endotoxin quantification. According to Martinho 201140, the KQCL test yielded a median value of endotoxin close and not significantly different from the turbidimetric test. However, the endpoint QCL test indicated ~ 5 × greater than the KQCL and turbidimetric test40.
Previous studies investigated the ability of different irrigants to reduce endotoxin levels in root canal infections41,42. Buck 200141 compared in vitro the detoxifying activity of most commonly used irrigants against lipid A present in endotoxin, and the authors found little or no detoxifying activity. In agreement, Martinho 200842, in a clinical study, revealed no difference between NaOCl and CHX-gel in the elimination of endotoxin from infected root canals. The authors attributed the removal of endotoxins from dentin walls to the mechanical action of files against the dentin walls and the irrigant's flow and backflow inside the canal rather than the irrigant's detoxifying activity. Moreover, Herrera 201743 showed that the ultrasonic activation of EDTA was effective in further reducing endotoxin levels in the root canals of teeth with pulp necrosis and apical periodontitis.
One of the limitations of this SR is the lack of information in few studies about the sample size calculation as well as the type of randomization, which could possibly result in a definitive judgment of “moderate” or “high” risk of bias. However, the authors were contacted to obtain the missing information to enable a definitive judgment of ‘low’ bias. Although few studies included here lack of information regarding sample size calculation, a minimum of ten patients per group was reported in all studies except for Vianna 200725. Ten patients per group seem to be enough samples to avoid them from being underpowered.
Conclusion
Overall, this SR provides evidence to support that multiple-session disinfection protocols with the placement of Ca(OH)2 intracanal medications are more effective in reducing the levels of endotoxin from root canal infections compared to single-session when applied for 14 and 30 days. At present, there is no substantial evidence of the advantage of the 7 days Ca(OH) intracanal medication protocol over single-session treatment.
References
Möller, A. J., Fabricius, L., Dahlén, G., Ohman, A. E. & Heyden, G. Influence on periapical tissues of indigenous oral bacteria and necrotic pulp tissue in monkeys. Scand. J. Dent. Res. 89, 475–484 (1981).
Craig Baumgartner, J. & Falkler, W. A. Bacteria in the apical 5 mm of infected root canals. J. Endod. 17, 380–383 (1991).
Sundqvist, G. Ecology of the root canal flora. J. Endod. 18, 427–430 (1992).
Assed, S., Ito, I. Y., Leonardo, M. R., Silva, L. A. B. & Lopatin, D. E. Anaerobic microorganisms in root canals of human teeth with chronic apical periodontitis detected by indirect immunofluorescence. Dent. Traumatol. 12, 66–69 (1996).
Gomes, B. P. F. A. et al. Microbiological examination of infected dental root canals. Oral Microbiol. Immunol. 19, 71–76 (2004).
Kakehashi, S., Stanley, H. R. & Fitzgerald, R. J. The effects of surgical exposures of dental pulps in germ-free and conventional laboratory rats. Oral Surg. Oral Med. Oral Pathol. 20, 340–349 (1965).
Rietschel, E. T. & Brade, H. Bacterial endotoxins. Sci. Am. 267, 54–61 (1992).
Matsushita, K. et al. Inflammatory cytokine production and specific antibody responses to lipopolysaccharide from endodontopathic black-pigmented bacteria in patients with multilesional periapical periodontitis. J. Endod. 25, 795–799 (1999).
Horiba, N. et al. Correlations between endotoxin and clinical symptoms or radiolucent areas in infected root canals. Oral Surg. Oral Med. Oral Pathol. 71, 492–495 (1991).
Marinho, A. C. S., Martinho, F. C., Zaia, A. A., Ferraz, C. C. R. & Gomes, B. P. F. A. Monitoring the effectiveness of root canal procedures on endotoxin levels found in teeth with chronic apical periodontitis. J. Appl. Oral Sci. 22, 490–495 (2014).
Cardoso, F. G. R. et al. Correlation between volume of apical periodontitis determined by cone-beam computed tomography analysis and endotoxin levels found in primary root canal infection. J. Endod. 41, 1015–1019 (2015).
Rabello, D. G. D. et al. Does supplemental photodynamic therapy optimize the disinfection of bacteria and endotoxins in one-visit and two-visit root canal therapy? A randomized clinical trial. Photodiagnosis Photodyn. Ther. 19, 205–211 (2017).
Pitts, D., Williams, B. & Morton, T. Investigation of the role of endotoxin in periapical inflammation. J. Endod. 8, 10–18 (1982).
Hong, C. Y. et al. The role of lipopolysaccharide in infectious bone resorption of periapical lesion. J. Oral Pathol. Med. 33, 162–169 (2004).
Jacinto, R. C. et al. Quantification of endotoxins in necrotic root canals from symptomatic and asymptomatic teeth. J. Med. Microbiol. 54, 777–783 (2005).
Martinho, F. C., de Rabello, D. G. D., Ferreira, L. L. & Nascimento, G. G. Participation of endotoxin in root canal infections: a systematic review and meta-analysis. Eur. J. Dent. 11, 398–406 (2017).
Tanomaru, J. M. G., Leonardo, M. R., Tanomaru Filho, M., Bonetti Filho, I. & Silva, L. A. B. Effect of different irrigation solutions and calcium hydroxide on bacterial LPS. Int. Endod. J. 36, 733–739 (2003).
de Oliveira, L. D. et al. Efficacy of endodontic treatment for endotoxin reduction in primarily infected root canals and evaluation of cytotoxic effects. J. Endod. 38, 1053–1057 (2012).
Marinho, A. C. S., To, T. T., Darveau, R. P. & Gomes, B. P. F. A. Detection and function of lipopolysaccharide and its purified lipid A after treatment with auxiliary chemical substances and calcium hydroxide dressings used in root canal treatment. Int. Endod. J. 51, 1118–1129 (2018).
Adl, A., Motamedifar, M., Shams, M. S. & Mirzaie, A. Clinical investigation of the effect of calcium hydroxide intracanal dressing on bacterial lipopolysaccharide reduction from infected root canals. Aust. Endod. J. 41, 12–16 (2015).
Carvalho, A. S. et al. Limewater and polymyxin B associated with NaOCl for endotoxin detoxification in root canal with necrotic pulp. Braz. Dent. J. 27, 573–577 (2016).
Xavier, A. C. C. et al. One-visit versus two-visit root canal treatment: effectiveness in the removal of endotoxins and cultivable bacteria. J. Endod. 39, 959–964 (2013).
Safavi, K. E. & Nichols, F. C. Effect of calcium hydroxide on bacterial lipopolysaccharide. J. Endod. 19, 76–78 (1993).
Silva, L., Nelson-Filho, P., Leonardo, M. R., Rossi, M. A. & Pansani, C. A. Effect of calcium hydroxide on bacterial endotoxin in vivo. J. Endod. 28, 94–98 (2002).
Vianna, M. E. et al. Effect of root canal procedures on endotoxins and endodontic pathogens. Oral Microbiol. Immunol. 22, 411–418 (2007).
Safavi, K. E. & Nichols, F. C. Alteration of biological properties of bacterial lipopolysaccharide by calcium hydroxide treatment. J. Endod. 20, 127–129 (1994).
Nelson-Filho, P., Leonardo, M. R., Silva, L. A. B. & Assed, S. Radiographic evaluation of the effect of endotoxin (LPS) plus calcium hydroxide on apical and periapical tissues of dogs. J. Endod. 28, 694–696 (2002).
Valera, M. C. et al. Comparison of different irrigants in the removal of endotoxins and cultivable microorganisms from infected root canals. Sci. World J. 2015, 1–6 (2015).
Sousa, E. L. R., Martinho, F. C., Leite, F. R. M., Nascimento, G. G. & Gomes, B. P. F. A. Macrophage cell activation with acute apical abscess contents determined by interleukin-1 beta and tumor necrosis factor alpha production. J. Endod. 40, 1752–1757 (2014).
Marinho, A. C. S., Martinho, F. C., Leite, F. R. M., Nascimento, G. G. & Gomes, B. P. F. A. Proinflammatory activity of primarily infected endodontic content against macrophages after different phases of the root canal therapy. J. Endod. 41, 817–823 (2015).
Martinho, F. C. et al. Clinical influence of different intracanal medications on Th1-type and Th2-type cytokine responses in apical periodontitis. J. Endod. 41, 169–175 (2015).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & PRISMA Group, T. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097 (2009).
Sterne, J. A. C. et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898 (2019).
Sterne, J. A. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355, i4919 (2016).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 7, 177–188 (1986).
Sousa, E. L. R., Martinho, F. C., Nascimento, G. G., Leite, F. R. M. & Gomes, B. P. F. A. Quantification of endotoxins in infected root canals and acute apical abscess exudates: monitoring the effectiveness of root canal procedures in the reduction of endotoxins. J. Endod. 40, 177–181 (2014).
Marinho, A. C. S., Martinho, F. C., Gonçalves, L. M., Rabang, H. R. C. & Gomes, B. P. F. A. Does the Reciproc file remove root canal bacteria and endotoxins as effectively as multifile rotary systems?. Int. Endod. J. 48, 542–548 (2015).
Martinho, F. C., Gomes, C. C., Nascimento, G. G., Gomes, A. P. M. & Leite, F. R. M. Clinical comparison of the effectiveness of 7- and 14-day intracanal medications in root canal disinfection and inflammatory cytokines. Clin. Oral Investig. 22, 523–530 (2018).
Schulz, K. F. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 273, 408–412 (1995).
Martinho, F. C. et al. Comparison of endotoxin levels in previous studies on primary endodontic infections. J. Endod. 37, 163–167 (2011).
Buck, R. A., Cai, J., Eleazer, P. D., Staat, R. H. & Hurst, H. E. Detoxification of endotoxin by endodontic irrigants and calcium hydroxide. J. Endod. 27, 325–327 (2001).
Martinho, F. C. & Gomes, B. P. F. A. Quantification of endotoxins and cultivable bacteria in root canal infection before and after chemomechanical preparation with 2.5% sodium hypochlorite. J. Endod. 34, 268–272 (2008).
Herrera, D. R. et al. Clinical efficacy of EDTA ultrasonic activation in the reduction of endotoxins and cultivable bacteria. Int. Endod. J. 50, 933–940 (2017).
Author information
Authors and Affiliations
Contributions
F.C.M. and G.G.N.—statistical analysis and wrote the main manuscript; D.D.R. and B.J.C.—searched for the articles, reviewed the articles, and prepared the figures; A.P.M.G. and E.G.S.—wrote the main manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nascimento, G.G., Rabello, D.G.D., Corazza, B.J.M. et al. Comparison of the effectiveness of single- and multiple-sessions disinfection protocols against endotoxins in root canal infections: systematic review and meta-analysis. Sci Rep 11, 1226 (2021). https://doi.org/10.1038/s41598-020-79300-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79300-3
This article is cited by
-
Clinical efficacy of endodontic protocols on reducing cultivable bacteria and endotoxin in infected root canal in patients submitted to head and neck radiotherapy: a randomised clinical trial
Clinical Oral Investigations (2023)
-
An overview of systematic reviews on endotoxins in endodontic infections and the effectiveness of root canal therapy in its removal
Evidence-Based Dentistry (2022)
-
Comparison of GentleWave system and passive ultrasonic irrigation with minimally invasive and conventional instrumentation against LPS in infected root canals
Scientific Reports (2022)
-
Impact of N-acetylcysteine (NAC) and calcium hydroxide intracanal medications in primary endodontic infection: a randomized clinical trial
Clinical Oral Investigations (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.