Abstract
This study reports the high-yield production of a novel 133/135La theranostic pair at a 22 MeV proton beam energy as an attractive alternative to the recently introduced 132/135La pair, demonstrating over an order of magnitude production increase of 133/135La (231 ± 8 MBq 133La and 166 ± 5 MBq 135La at End of Bombardment (EOB)) compared to 11.9 MeV production of 132/135La (0.82 ± 0.06 MBq 132La and 19.0 ± 1.2 MBq 135La) for 500 µA·min irradiations. A new sealed solid cyclotron target is introduced, which is fast to assemble, easy to handle, storable, and contains reusable components. Radiolabeling with macrocyclic chelators DOTA and macropa achieved full incorporation, with respective apparent 133La molar activites of 33 ± 5 GBq/µmol and 30 ± 4 GBq/µmol. PET centers with access to a 22 MeV capable cyclotron could produce clinically-relevant doses of 133/135La, via natBa irradiation, as a standalone theranostic agent for PET imaging and Auger electron therapy. With lower positron energies and less energetic and abundant gamma rays than 68Ga, 44Sc and 132La, 133La appears to be an attractive radiometal candidate for PET applications requiring a higher scanning resolution, a relatively long isotopic half-life, ease of handling, and a low patient dose.
Similar content being viewed by others
Introduction
Theranostics in nuclear medicine is a technique whereby a site specific pharmaceutical is radiolabelled first with a radioisotope for diagnostic imaging. After analysis, the same pharmaceutical is labelled with a particle emitting radioisotope for therapeutic application1. The complementary isotopes used are called theranostic pairs. It is essential that the two isotopes have very similar chemical properties with the ideal case being that they are different isotopes of the same element. Auger electron-emitting isotopes have potential as a high linear energy transfer (LET) therapeutic agent to destroy cancer cells by depositing their ionizing emission energy over a very short path length, damaging DNA by inducing various types of DNA damage, including double-strand breaks. This holds advantages over lower LET therapy such as β- therapy where emissions can travel over 1 cm, and may unnecessarily irradiate healthy tissue2, 3. High LET Auger electron emissions have achieved encouraging clinical results, with 111In-DTPA-octreotide and 125I-IUdR causing tumor remissions in patients with lower normal tissue toxicity, and improvements in the survival of glioblastoma patients using 125I-mAb 425 with minimal normal tissue toxicity4. A recently developed theranostic pair is 132/135La, where positron emissions from 132La are used for PET imaging while the Auger electrons from 135La have the potential for use in Auger electron therapy (AET)5,6,7. Theranostic La pairs are not only inherently useful but also can serve as surrogates for potential future study relating to 225Ac alpha-particle therapy. 225Ac-labeled compounds have seen significant recent clinical successes in treating aggressive tumor metastases2, 8.
However, 132/135La has limitations for PET imaging due to its fundamental positron and gamma emission properties, and current cyclotron production methods. The average and maximum 132La positron energies of 1.29 MeV and 3.67 MeV are significantly higher than those of other commonly used PET isotopes such as 18F (Emean = 0.250 MeV, Emax = 0.634 MeV), 64Cu (Emean = 0.278 MeV, Emax = 0.653 MeV), 68Ga (Emean = 0.829 MeV, Emax = 1.90 MeV), or 44Sc (Emean = 0.632 MeV, Emax = 1.47 MeV)9. The higher positron energy of 132La implies reduced PET image spatial resolution for tumor imaging, especially when imaging smaller tumors and metastases. Furthermore, 132La emits high abundance gamma rays within typical 511 keV PET scanner energy windows that can contribute to spurious coincidences, as well as high energy gamma rays that may complicate handling.
Using natBa target material, current 132La cyclotron production methods via 132Ba(p,n)132La require long irradiation times and generate reduced activity due to the very low natural abundance of 132Ba (0.1%).
The present work describes high yield 133/135La production through 22 MeV proton irradiation of natBa metal encapsulated within a convenient sealed cyclotron target. Irradiating natBa at 22 MeV generates much higher yields of 133/135La compared to 132/135La production at 11.9 MeV and bypasses the majority of 132La production, avoiding contributions from its higher energy positron emissions. 133La has average and maximum positron energies of 0.461 MeV and 1.02 MeV, respectively, that are lower than those of 132La and other PET isotopes such as 68Ga and 44Sc. Gamma emissions from 133La are low intensity and energy, falling well outside the typical PET scanner energy window. These features of 133La simplify handling and reduce patient dose. This novel 133/135La isotope system and its production method have the potential to improve the image quality of smaller and metastatic tumors and allow clinically relevant production of 133/135La via shorter cyclotron beamtime irradiations without requiring isotopically enriched Ba target material. High-yield production is possible via proton irradiation of natBa on a cyclotron capable of attaining 22 MeV beam energies. The favorable 133La positron and gamma-ray emission properties suggest that 133/135La has significant potential as a theranostic pair substitute for 132/135La.
Materials and methods
Chemicals
Natural barium (99.99% trace metals basis) dendritic pieces, ACS reagent grade concentrated hydrochloric acid (37%) and nitric acid (70%), and ICP-OES elemental standards were purchased from Sigma-Aldrich (St. Louis, MO, USA). Silver rod (99.9%) was purchased from Metal Supermarkets (Mississauga, ON, Canada). Branched DGA resin (50–100 µm) was purchased from Eichrom (Lisle, IL, USA). NIST traceable γ-ray sources used for high-purity germanium detector (HPGe) energy and efficiency calibration were acquired from Eckert & Ziegler Isotopes (Valencia, California, USA). Thin-layer chromatography silica gel sheets were purchased from Merck (Darmstadt, HE, Germany).
High purity water (18 MΩ·cm) was obtained from a MilliporeSigma Direct-Q 3 UV system (Burlington, MA, USA). The macrocyclic chelator DOTA was purchased from Macrocyclics (Plano, TX, USA), and the macrocyclic chelator macropa was purchased from MedChemExpress (Monmouth Junction, NJ, USA).
Instrumentation
Sample activity was measured using an Atomlab 500 Dose Calibrator (Biodex, Shirley, NY, USA). Radionuclidic purity was assessed using a GEM35P4-70-SMP high-purity germanium detector (ORTEC, Oak Ridge, TN, USA) with ORTEC GammaVision software. Elemental purity was assessed using a 720 Series ICP-OES (Agilent Technologies, Santa Clara, CA, USA). A NEPTIS Mosaic-LC synthesis unit (Optimized Radiochemical Applications, Belgium) was used to separate and purify the 133/135La from the dissolved Ba target solution. An AR-2000 Radio-TLC Imaging Scanner (Eckert & Ziegler, Hopkinton, MA, USA) was employed to quantify the fraction of chelator-bound 133/135La after the reaction. The solid targets were manufactured using a Model 6318 hydraulic press (Carver, Wabash, IN, USA), and the natBa metal was pressed inside a 10 mm (I.D.) EQ-Die-10D-B hardened steel die (MTI Corporation, Richmond, CA, USA). A S90013A optical light microscope (Fisher Scientific, Waltham, MA, USA) was employed to inspect the seal integrity of each sealed solid target after manufacturing.
Cyclotron targetry and irradiation
A completed sealed cyclotron target is depicted in Fig. 1. Cyclotron targets were prepared from 200 mg of natBa metal, an Ag disc (24 mm diameter, 1.5 mm thick) cut from an Ag rod, In wire (1 mm diameter), and Al foil (25 µm thick). A 10 mm diameter depression was machined into the center of each disc to a 100 µm depth, and a 1 mm wide annulus with an inner diameter of 15 mm was machined to a depth of 100 µm. Using a method similar to the target production described in10, natBa metal was quickly loaded into a hardened stainless steel die to minimize exposure to the atmosphere, and a force of 15 kN was applied using a hydraulic press, producing a 10 mm diameter pellet with a thickness of 0.8 mm. Pellets were produced in large quantities (> 10/batch) and removed quickly from the die and sealed in a vial with an argon atmosphere to prevent oxidation during storage.
A 23 mm diameter Al foil cover was cut out with a flap extension to facilitate post-irradiation removal by peeling. Individual pellets were then placed in the central Ag disc depression and pressed at a force of 20 kN on the hydraulic press to secure the pellets in the depression. 5.5 cm of In wire was then laid into the annulus depression with 1 mm of overlap at the ends, the target assembly was quickly covered by the Al cover, and a force of 25 kN was applied using the hydraulic press to compress the In wire to form an air-tight bond between the Ag disc and Al cover. Following pressing, the target was observed under an optical light microscope to confirm target seal integrity, verifying there were no pinholes present in the Al cover. The target was stored under regular atmospheric conditions ready for on-demand irradiation.
Targets were irradiated at 22 MeV using a 24 MeV TR-24 cyclotron (Advanced Cyclotron Systems Inc., Richmond B.C., Canada) for 25–200 min with a maximum proton beam current of 20 µA at current densities of 25.5 µA/cm2. A pneumatically actuated TA-1186 solid target assembly (Advanced Cyclotron Systems Inc., Richmond B.C., Canada) was used with the target disc perpendicular to the proton beam. O-rings within the assembly provided a helium gas seal on the front and water seal on the back for both cooling streams. The Ag target was designed to be at least 0.6 mm thick behind the 0.8 mm thick natBa pellet so that the exit beam energy leaving the Ag disc was degraded below 6 MeV, as simulated by SRIM 201311. This design consideration was to avoid the production of 13N (t1/2 = 9.97 min) in the cyclotron cooling water circuit via the 16O(p,α)13N reaction. A 250 µm thick Ag degrader was added to the cyclotron beamline after the Al vacuum foil so that extracting the cyclotron beam at 17 MeV resulted in the target incident energy being degraded to 11.9 MeV. These irradiations at 11.9 MeV served to provide a comparison to the 132/135La isotope production introduced by Aluicio-Sarduy et al.5.
After allowing 1–2 h post-irradiation for decay of short-lived La isotopes, the target assembly was opened pneumatically, and the sealed target slid down a plastic guide tube into a lead shield. The lead shield was brought to a dose calibrator where its activity was measured, followed by placement into a lead castle containing a NEPTIS automated separation unit.
Nuclear reaction cross-sections of interest
Nuclear reaction cross-sections simulated by TENDL 2019 for the 13xBa(p,xn)13xLa reactions of interest for 132/133/135La are shown in Fig. 2. These same cross-sections, weighted for natBa isotopic abundance, are displayed in Fig. 3. The cyclotron beam was extracted at an energy of 22.2 MeV and degraded to a target incident energy on natBa of 22 MeV. The target incident energy of 22 MeV was selected using TENDL 2019 cross-section simulation data12.
Nuclear reaction cross-section simulation data of the proton-induced nuclear reaction on 132/134/135/136/137Ba for 132/133/135La12.
Nuclear reaction cross-section simulation data of the proton-induced nuclear reaction on 132/134/135/136/137Ba for 132/133/135La weighted for natBa isotopic abundance12.
At a 22 MeV target incident beam energy, the simulation suggests significant 135La and 133La cross sections for the 137Ba(p,3n)135La, 136Ba(p,2n)135La, 135Ba(p,3n)133La, and 134Ba(p,2n)133La reactions. The 132Ba(p,n)132La cross-section is over two orders of magnitude lower at 22 MeV compared to at 11.9 MeV, and the 134Ba(p,3n)132La reaction cross-section does not begin until just above 22 MeV. Irradiating natBa at 22 MeV should therefore maximize the production of 133La and 135La, bypass the majority of 132La production from the 132Ba(p,n)132La reaction, and just avoid the onset of the significant 134Ba(p,3n)132La reaction. Due to the higher natural abundances of 134Ba (2.42%) and 135Ba (7.59%) compared to 132Ba (0.10%), 133La production potential is much greater compared to 132La, illustrated in the difference between the absolute and isotopically weighted cross-sections shown in Figs. 2 and 3, respectively.
To compare 133/135La to 132/135La in this study, irradiations were performed with a target incident beam energy of 11.9 MeV. Figure 2 suggests irradiations at 11.9 MeV would result in the production of 135La and 132La via the 135Ba(p,n)135La, 136Ba(p,2n)135La, and 132Ba(p,n)132La reactions, while just avoiding the start of 133La production via the 134La(p,2n)133La reaction, as also described by Aluicio-Sarduy et al.5.
Other prominent cross sections at either 22 MeV or 11.9 MeV that are not depicted in Figs. 2 and 3 suggest unavoidable production of short-lived 130La (t1/2 = 8.7 min) via the 130Ba(p,n)130La reaction, 131La (t1/2 = 59.2 min) via the 132Ba(p,2n)131La reaction, 134La (t1/2 = 6.45 min) via the 134Ba(p,n)134La and 135Ba(p,2n)134La reactions, and 136La (t1/2 = 9.87 min) via the 136Ba(p,n)136La and 137Ba(p,2n)136La reactions. Significant cross sections are also present for the long-lived 137La (t1/2 = 6.2·104 y) via the 137Ba(p,n)137La and 138Ba(p,2n)137La reactions, and 138La (t1/2 = 1.03·1011 y) via the 138Ba(p,n)138La reaction.
Automated separation of 133/135La
133/135La was separated using modified aspects of a method described by Aluicio-Sarudy et al.5. The reactor vessel within its shield was transferred into the lead castle, the sealed target was opened by peeling back the Al cover, and a suction line was attached. The reactor vessel was filled with 10 mL of 18 MΩ·cm water, dissolving the natBa target material in 5 min. The Ag target disc was removed, and 10 mL of 6 N HNO3 was added to the reactor to bring the overall concentration to 3 N HNO3. 3 N HNO3 was selected to reduce possible degradative effects of concentrated 6 N HNO3 on the branched DGA resin. The target solution was withdrawn from the reactor and passed through two Acrodisc 32 mm diameter filters with 5 µm Supor membranes in parallel to capture any solid material such as natBa salts and oxides resulting from the dissolution stage. Following filtration, the target solution was passed through a SPE cartridge containing 0.25 g of branched DGA resin, and washed with 50 mL of 3 N HNO3 to remove residual Ba and other metal impurities, followed by 5 mL of 0.5 N HNO3. [133/135La]LaCl3 was eluted using 1 mL of 0.1 N HCl. Following a decay period of 5 days (to permit the decay of the short-lived 107Cd and longer-lived 106mAg) the Ag disc was removed and cleaned in reagent grade 10 N HCl for reuse. For the comparative aspects, 132/135La was separated using the same process.
Activity measurement and radionuclidic purity analysis
After separating the [133/135La]LaCl3 product, its radionuclidic purity was determined by gamma-ray spectroscopy using a high purity germanium (HPGe) detector. Calibrations for efficiency and energy were performed using NIST traceable Eckert & Ziegler Isotope Products Inc. γ-ray sources. Activities of La isotopes of interest were quantified using the efficiency-corrected HPGe measurements.
Elemental purity analysis
Inductively-coupled plasma optical emission spectrometry (ICP-OES) analysis was performed to quantify elemental impurities in the [133/135La]LaCl3 samples after allowing 10 days for residual 135La to decay. The amounts of Zn, Fe, Al, Ba, Ag, In, Sn, and Cu were determined for each sample using calibrations obtained by measuring dilutions of elemental standards of known concentrations.
Radiolabeling of DOTA and macropa with 133/135La
Following processing on the NEPTIS synthesis unit, the 133/135La radionuclide was eluted in 1 mL of 0.1 N HCl. 500 µL of [133/135La]LaCl3 was withdrawn, and the activity was measured. This solution was diluted with 50 µL of NaOAc buffer (pH 9.0) to adjust to pH 4.5. 100 µL of the 133/135La solution was reacted with 0.5 µg, 5 µg, and 20 µg of DOTA and macropa dissolved in 50 µL of 18 MΩ·cm water, at 80 °C for 30 min for DOTA and room temperature (22 °C) for 10 min for macropa. Each reaction solution was analyzed using radio-TLC on silica plates to determine radiochemical purity and incorporation with 0.1 M citric acid buffer as the mobile phase, with the Rf of free 133/135La = 0.9–1.0, [133/135La]La-DOTA = 0.1–0.2, and [133/135La]La-macropa = 0–0.1.
Results
Cyclotron targetry
Prior to longer irradiations, initial tests were performed with natBa targets at beam currents ranging from 1–20 µA to investigate target properties and durability. After irradiation and automated separation, HPGe analysis was performed on the Ag targets. For 11.9 MeV runs, analysis indicated small activities of 107Cd (t1/2 = 6.5 h) and 109Cd (t1/2 = 461.4 d) were produced via the 107Ag(p,n)107Cd and 109Ag(p,n)109Cd reactions. For 22 MeV runs, following the 3-h decay period, analysis indicated small activities of 107Cd, 109Cd, and 106mAg (t1/2 = 8.28 d). For both beam energies in this study, the targets did not activate significantly, and the majority of the activity present was 107Cd and 106mAg, which decayed significantly after several days. Following a 5-day decay period the targets were deemed acceptable for handling and reuse after placing the target in 10 N HCl to clean its surface. For all irradiations, none of the sealed Ag targets showed signs of physical degradation, with multiple target discs being reused upwards of 10 times.
133/135La isotope production
Average activities (n = 3) of La isotopes of interest at 11.9 MeV and 22 MeV are given as a function of time after EOB in Table 1, and several ratios of La isotopes of interest are given as a function of time after EOB in Table 2.
At 22 MeV, 500 µA·min runs (n = 3) yielded 231 ± 8 MBq 133La, and 166 ± 5 MBq 135La. Saturated yields were 161 ± 5.5 MBq/µA for 133La, and 561 ± 17 MBq/µA for 135La. Significant amounts of 134La and 136La were present at EOB (1191 ± 96 MBq and 3914 ± 384 MBq, respectively), however owing to their short half-lives (6.45 min and 9.87 min, respectively), they decayed to negligible levels after 3-h post-EOB. Short-lived 130La (8.7 min half-life) was observed and undetectable after the 3-h decay period. 132La was produced (0.38 ± 0.03 MBq at EOB), indicating its production reactions were largely bypassed. Co-production of 131La was observed (19.0 ± 1.2 MBq at EOB), however owing to its relatively short half-life (59.2 min), it decayed significantly during the 3-h decay period. TENDL 2019 cross sections indicated production of long-lived 137La and 138La, however, this was not quantified due to their extremely long half-lives.
For the comparison 132/135La production runs at 11.9 MeV, 500 µA·min runs (n = 3) yielded 0.82 ± 0.06 MBq 132La and 17.9 ± 0.8 MBq 135La at EOB. Saturated yields were 0.70 ± 0.03 MBq/µA for 132La, and 60.6 ± 2.8 MBq/µA for 135La. Significant amounts of 134La and 136La were also observed at EOB (411 ± 37 MBq and 2462 ± 94 MBq, respectively), which decayed to undetectable levels after the 3-h decay period. Cross-sections generated by TENDL 2019 indicated the production of long-lived 137La and 138La. However, production was also not quantified owing to their long half-lives.
As shown in Table 2, the activity ratio of 135La to 133La at 22 MeV is much lower than the ratio of 135La to 132La at 11.9 MeV, resulting in a much greater PET imaging potential for a given total activity. At 22 MeV, the activity ratio of 133La to 132La remains large throughout the time intervals, suggesting that the production of the 132La impurity was minimized.
Automated separation of 133/135La
To determine dissolution time, several Ba targets were dissolved in the reactor with 10 mL of water, with the time required to completely dissolve the target ranging from 4 to 5 min. A dissolution time of 5 min was selected for production run separations to provide a sufficient time margin. The DGA resin was preconditioned with 3 N HNO3 so the NEPTIS unit was prepared to receive the activity. The final product elution in 1 mL of 0.1 N HCl was calibrated to capture the maximum 133/135La activity while avoiding excess dilution of the solution.
From the start of NEPTIS separation to the completion of product elution took ~ 35 min. Over 88% of decay-corrected 133/135La activity was consistently recovered from the automated synthesis. Residual decay activities were 3% of the total in the branched DGA resin, 3% in the dissolution reactor, 2% in the two reactor filters, with the remainder (≤ 4%) in the waste.
Radionuclidic and elemental purity analysis
For irradiations at 22 MeV beam energies, small amounts of 131La and 132La were detected by HPGe gamma-ray spectroscopy performed on the 133/135La eluate product after NEPTIS separation and a 3-h decay period. For 500 µA·min runs (n = 3) at 22 MeV, the 131La and 132La activities back-calculated to EOB were 19 ± 1.2 MBq and 0.38 ± 0.03 MBq, respectively.
The decay of 133La resulted in small activities of its daughter nucleus 133Ba (t1/2 = 10.6 y). However, the resulting activity of 133Ba after the complete decay of 133La was approximately three orders of magnitude lower than the IAEA 1 MBq consignment level exemption limits13. No other radionuclidic impurities were observed in the 133/135La product.
After allowing the 133/135La eluate to decay for 10 days, an ICP-OES analysis was performed to investigate trace metal contaminants against a known mixture standard containing Zn, Fe, Al, Ba, Ag, In, Sn, and Cu. Metal impurities (n = 3 runs) are presented in Table 3.
Radiolabeling of DOTA and macropa chelators with 133/135La
Table 4 summarizes the experimental results of 133/135La radiolabeling with DOTA and macropa chelators. Radiolabeling with the tetraaza-macrocyclic chelator DOTA was performed with 133/135La at 80 ºC for 30 min and analyzed with radio-TLC. The [133/135La]La-DOTA complex remained close to the TLC baseline (Rf = 0.1–0.2) while the unreacted 133/135La migrated toward the solvent front (Rf = 0.9–1.0). The incorporation (n = 3) of 133/135La for DOTA labeling was 99.1 ± 0.6%, 98.8 ± 0.5%, and 97.9 ± 1.2% for 20, 5, and 0.5 µg, respectively. Complete labeling of DOTA with 133/135La was achieved up to 1.2 nmol of DOTA, with a corresponding apparent 135La molar activity (n = 3) of 47 ± 9 GBq/µmol and 133La molar activity (n = 3) of 33 ± 5 GBq/µmol .
Radiolabeling with the eighteen-membered macrocyclic chelator macropa was performed with 133/135La at room temperature (22 ºC) for 10 min, and analyzed with radio-TLC.
The [133/135La]La-macropa complex remained at the TLC baseline (Rf = 0–0.1) while the unreacted 133/135La migrated toward the solvent front (Rf = 0.9–1.0). The incorporation (n = 3) of 133/135La for macropa labeling was 99.3 ± 0.5%, 99.5 ± 0.7%, and 98.1 ± 1.1% for 20, 5, and 0.5 µg, respectively. Complete labeling of macropa with 133/135La was achieved up to 0.85 nmol of macropa, with a corresponding apparent 135La molar activity (n = 3) of 44 ± 8 GBq/µmol and 133La molar activity (n = 3) of 30 ± 4 GBq/µmol.
Discussion
This study presents a high-yield cyclotron production avenue for a novel 133/135La theranostic pair using a new sealed target design. Automated separation and purification produced a chemically pure product, with radiochemistry validating the feasibility of the 133/135La theranostic pair using several common radiometal chelators.
Table 5 outlines the positron decay characteristics and notable gamma rays for 133La, 132La, and several other common isotopes used for PET. 132La has a higher positron branching ratio (41.2%) compared to 133La (7.2%), producing more 511 keV emissions for a given sample activity. Initially, this higher branching ratio would seem advantageous for PET imaging. However, positrons emitted by 132La have a much higher 1.29 MeV average and 3.67 MeV maximum energy compared to 133La positron emissions, which have a low, more desirable 0.463 MeV average and 1.02 MeV maximum positron energy. Since higher positron energies are correlated with lower PET imaging spatial resolution14,15, this implies that 133La would have superior PET imaging quality compared to 132La.
The potential for improved PET scanning resolution of 133La over 132La could permit more accurate imaging to track the treatment of small tumors and metastases, complementing high LET targeted radionuclide therapy such as alpha particle or AET, which are both well suited for eradicating small metastatic tumors.
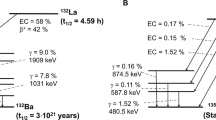
As shown in Table 5, 132La has high energy gammas with a significant abundance, whereas 133La has lower energy gammas with a much lower abundance. 132La has a maximum gamma energy of 1909.91 keV at 9% abundance, whereas 133La has a maximum gamma energy of 1099 keV with a 0.2% abundance. The lower energy and much lower abundance of the 133La gamma rays should simplify handling and reduce the dose to patients upon injection for equivalent imaging activities, even though a greater activity of 133La might be required due to the lower positron branching ratio of 133La. In addition to potentially reducing the patient dose, the gamma ray energy distribution of 133La could improve PET scanner imaging spatial resolution.
The 132La 465 keV (76%) and 567 keV (14.7%) high abundance gamma rays are within a typical 350–650 keV PET scanner energy window used to detect the 511 keV annihilation gamma rays15, which could lead to excess spurious coincidences within the scanner timing window, and interfere with image quality. 133La has no gamma rays with energies within a typical PET scanner energy window, which should result in no spurious coincidences. Additionally, as previously depicted in Table 2, the much lower activity ratio of 135La to 133La produced at 22 MeV, compared to the ratio of 135La to 132La produced at 11.9 MeV, should significantly reduce the relative amount of spurious coincidences in the PET scanner energy window from the 135La 480.5 keV gamma ray.
Comparing 133La to other PET isotopes in Table 5 shows that its respective mean and maximum positron energies of 0.461 MeV and 1.02 MeV are higher than those of 64Cu and 18F, comparable to those of 11C, and 89Zr, and lower than those of 132La, 68Ga, 44Sc, and 82Rb.
The ubiquitous 18F has a very low positron energy that provides a sharp image, and 11C has a similar positron energy to 133La. However, the shorter half-lives of 18F and 11C limit investigating longer biological processes. 64Cu has low energy positron emissions, a longer half-life, and β– emissions that enable theranostics, however cyclotron production requires expensive isotopically enriched target material due to the low 0.009% natural abundance of 64Ni. 89Zr has the longest half-life of the listed isotopes, permitting users to examine longer biological processes, however, it has several high energy gamma rays (909 keV (99%), 1713 keV (0.75%), and 1744 keV (0.12%)), which greatly increase the patient dose and shielding requirements.
68Ga has become a widely used radiometal for PET owing to its high positron branching ratio, sufficient half-life, and demonstrated chemistry. 68Ga is easily accessible via 68Ge/68Ga generators, and alternative cyclotron production routes have demonstrated potential to further enhance 68Ga supply10. However, its higher positron energies compared to 133La, 18F, and 64Cu result in lower imaging spatial resolution16, and it also has several high energy gamma rays, notably 1077 keV (3.2%), that increase shielding requirements. Despite having a longer half-life than 68Ga, the higher energy positrons of 44Sc compared to 133La, 18F, and 64Cu would also result in a lower image resolution while complicating handling and contributing significantly to patient dose with its 1157 keV (99.9%) gamma-ray emissions.
132La has a similar half-life to 133La. However, it has drawbacks including high positron emission energies and high energy and abundance gamma emissions. 82Rb also has high energy positrons, though this is acceptable given its role in imaging large cardiac structures.
From the previous comparisons, the relatively low positron energies, gamma energies, and gamma abundances of 133La imply higher imaging resolution than 132La, 68Ga, 44Sc, and a comparable imaging resolution to 11C and 89Zr. 133La appears to be an attractive radiometal candidate for PET applications requiring a high scanning resolution, with its relatively long isotopic half-life, ease of handling, and low patient dose. Quantifying 133La dosimetry in future studies is worth pursuing.
Significant advantages arise from our production method and the intrinsic properties of the 133/135La pair, compared to the currently produced 132/135La pair. Our production technique using a 24 MeV cyclotron with a new sealed target design allows high yield on-demand production.
Without an effective sealed target design, the metallic natBa ejects BaO dust into its surroundings as it rapidly oxidizes in the atmosphere, posing a potential radioactive contamination hazard during irradiation and target retrieval. Our sealed target design eliminates this issue through the secure encapsulation of the sensitive natBa target material with a durable bond between the Al target cover, In wire, and Ag disc. Furthermore, the sealed solid target design production method is robust and efficient, and the completed targets are easy to store and handle pre- and post-irradiation.
Irradiated Ag targets became activated with significant activity of 107Cd, and small activities of 109Cd, and 106mAg. Despite the 8.28-day half-life of 106mAg, after allowing for a several day decay period, residual activity in Ag targets was low enough for target reuse.
Cyclotron irradiations at 22 MeV achieved high-yield production of 133La and 135La, while only producing extremely small activities of 132La relative to 133La. Even though there was an appreciable drop in beam energy across the 0.8 mm natBa pellet (22 MeV to 18.3 MeV calculated by SRIM), this did not result in any significant increase in 132La production since the 132Ba(p,n)132La cross-section remains low across this energy range, and 132Ba has a low isotopic abundance of 0.10%. Avoiding the onset of the higher energy 134Ba(p,3n)132La reaction was important since the 2.42% isotopic abundance of 134Ba would produce a much greater activity of the 132La impurity compared to the 132Ba(p,n)132La reaction. Minimal production of 131La via the 132La(p,2n)131La reaction was observed, with any activity produced significantly decaying during the 3-h post-EOB decay period, due to its 59.2 min half-life. To further reduce radionuclidic impurities, removing the 0.1% of 132Ba natural abundance via isotopic enrichment of natBa should allow the near-complete removal of 132La production from the 132Ba(p,n)132La reaction and remove 131La from the 132La(p,2n)131La reaction, leaving only 133La and 135La after the 3-h decay period. This enriched target material would also enable cyclotrons with an energy lower than 22 MeV to produce radionuclidically pure 133/135La (although at lower production yields). Other isotopic enrichments could potentially increase production yields of 133La or 135La. However, the additional cost and availability of enriched Ba target material, as opposed to using relatively inexpensive natBa, would be an important factor to evaluate.
The decay of 133La forms the daughter 133Ba (t1/2 = 10.6 y), which decays to form stable 133Cs. However, 133Ba activity resulting from the decay of its 133La is comparatively far smaller, and approximately three orders of magnitude below IAEA consignment exemption quantities13. Any additional dose from a 133La PET scan resulting from the very small amount of the 133Ba daughter would be minimal due to its extremely low activity resulting from its far longer half-life relative to 133La, low maximum gamma energy of 383 keV, and rapid excretion from the body17,18. A study by Newton et al.18 injected 72.4–79.5 kBq 133Ba into the bloodstream of healthy human volunteers and studied the full-body retention of 133Ba up to 13 y after injection. The majority of injected 133Ba was rapidly cleared from the body (74–90% within 10 d), with residual activity continuously excreted as time progressed.
Additionally, depending on the properties of the targeting vector used to deliver 133La, some of the 133La injected for a PET scan could be excreted before decaying to 133Ba, owing to its 3.92 h half-life. Therefore, pharmacokinetic studies would be useful to assess the in vivo distribution of 133La radiopharmaceuticals and its 133Ba decay daughter. As considered with cyclotron produced 99mTc, it would be useful to do a future evaluation on the significance of long-lived impurities and decay products on the patient dose19.
The automated separation of 133/135La from the natBa target material using a NEPTIS unit achieved a decay corrected activity recovery of 88% while producing a highly pure product ready for radiolabeling. In the future, 133/135La radiolabeling and radiopharmaceutical syntheses can be added to the automated synthesis process to create a final product for research or clinical use.
Radiolabeling of DOTA and macropa was successful, with high incorporations observed with each chelator. Concerning chemistry, the production of significant amounts of the “stable” isotopes 138La and 137La, could provide competition to 133/135La or 132/135La during radiolabeling, since their reaction cross sections are much larger than those of 133/135La at 22 MeV and 132/135La at 11.9 MeV. However, TENDL 2019 reaction cross-sections for the 138Ba(p,n)138La, 137Ba(p,n)137La, and 138Ba(p,2n)137La reactions indicate the amount of 137/138La relative to 133/135La produced at 22 MeV is smaller than that of 137/138La relative to 132/135La produced at 11.9 MeV12. This implies that irradiating natBa at 22 MeV could be advantageous over 11.9 MeV from a chemistry perspective, with a lower proportion of “stable” 137/138La isotopes competing during radiolabeling.
133/135La has potential as a theranostic pair for PET imaging and AET in targeted radionuclide therapy. With 11 Auger electrons per decay, 135La produces a significant amount of high LET radiation, which is especially suited for killing metastases. With an appropriate targeting vector, 133La could be used to image and 135La to kill tumor cells.
Existing low current 11.9 MeV cyclotron 132/135La production requires several-hours of long irradiations to produce small activities for limited pre-clinical applications. In contrast, much higher cross-sections for 133/135La at 22 MeV allow a significantly shorter irradiation time producing over an order of magnitude more 133/135La compared to 132/135La, and significantly, large amounts of 133La relative to 135La as previously depicted in Table 2. This large-scale 133La production compensates for the lower positron branching ratio of 133La compared to 132La. Additionally, compared to the small 132La/135La ratio shortly after EOB, the far larger 133La/135La ratio allows more flexibility with imaging and therapy.
There is a significant potential increase in PET imaging when using the 133/135La product soon after the 3-h decay period post-EOB, as well as allowing large amounts of pure Auger therapy with a longer decay period after EOB.
A typical 18F activity of 300–400 MBq is used for clinical PET imaging20, and a typical 68 Ga activity of 1.59 MBq/kg is suggested21. It would be a challenge to produce a 132La activity equivalent to a typical 18F or 68 Ga dose with current 132/135La production methods unless isotopically enriched Ba target material was used. In contrast, it should be far easier to reach a clinically relevant 133/135La activity with a 22 MeV irradiation of a natBa target. The much greater yield of 133/135La with our 22 MeV higher energy production method should enable clinically relevant amounts of activity to be produced with relatively short irradiations.
It should be noted that not all PET centers have access to a cyclotron that can reach 22 MeV, so 133/135La production will be limited to those centers with sufficiently high beam energy. However, the relatively long half-lives of 133La (3.9 h) and 135La (19.5 h) would permit regional distribution of the 133/135La theranostic pair.
Conclusion
We have developed a high yield and cost-effective method of producing a novel theranostic pair, 133/135La. Our production technique uses a new type of sealed solid target that is robust, simple to manufacture, significantly improves target handling, and contains reusable components. Production yields of 133/135La at 22 MeV are over an order of magnitude higher than existing 132/135La production techniques, enabling clinically relevant 133/135La activites to be produced at low cyclotron beam currents and relatively short irradiation times, without expensive isotopically enriched Ba target material. 133/135La shows intriguing imaging potential due to its much lower positron energy and far lower gamma-ray energies and abundances compared to 132/135La, with potential applications for treating cancer metastases as a PET/AET theranostic pair. Accordingly, 133/135La appears to be an attractive radiometal theranostic candidate for PET applications requiring high scanning resolution, a relatively long half-life, ease of handling, and lower patient dose. This study demonstrated the potential for high-yield 133/135La production via natBa irradiation at sites with a medical cyclotron that can reach 22 MeV, meeting increasing demands for pre-clinical and potential clinical applications for 133/135La radiopharmaceuticals.
References
Velikyan, I. Molecular imaging and radiotherapy: theranostics for personalized patient management. Theranostics 2, 424–426 (2012).
Poty, S., Francesconi, L. C., McDevitt, M. R., Morris, M. J. & Lewis, J. S. α-Emitters for radiotherapy: from basic radiochemistry to clinical studies-part 1. J. Nucl. Med. 59, 878–884 (2018).
Kassis, A. I. Molecular and cellular radiobiological effects of Auger emitting radionuclides. Radiat. Prot. Dosimetry 143, 241–247 (2011).
Ku, A., Facca, V. J., Cai, Z. & Reilly, R. M. Auger electrons for cancer therapy—a review. EJNMMI Radiopharm. Chem. 4, 27 (2019).
Aluicio-Sarduy, E. et al. Production and in vivo PET/CT imaging of the theranostic pair 132/135La. Sci. Rep. 9, 10658 (2019).
Aluicio-Sarduy, E. et al. Establishing radiolanthanum chemistry for targeted nuclear medicine applications. Chemistry 26, 1238–1242 (2020).
Fonslet, J. et al. 135La as an Auger-electron emitter for targeted internal radiotherapy. Phys. Med. Biol. 63, 015026 (2017).
Kratochwil, C. et al. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 57, 1941–1944 (2016).
Sonzogni, A. & Shu, B. Nudat 2.8 (Nuclear Structure And Decay Data). National Nuclear Data Center https://www.nndc.bnl.gov/nudat2/reCenter.jsp?z=56&n=77 (2020).
Nelson, B. J. B. et al. Taking cyclotron 68Ga production to the next level: expeditious solid target production of 68Ga for preparation of radiotracers. Nucl. Med. Biol. 80–81, 24–31 (2020).
Ziegler, J.F., Ziegler, M.D., Biersack, J.P. The Stopping and Range of Ions in Matter. SRIM 2013 http://www.srim.org (2013).
Koning, A. J. et al. TENDL: complete nuclear data library for innovative nuclear science and technology. Nucl. Data Sheets 155, 1–55 (2019).
Regulations for the Safe Transport of Radioactive Material, IAEA Safety Standards Series No. SSR-6 (Rev.1), International Atomic Energy Agency https://www.iaea.org/publications/12288/regulations-for-the-safe-transport-of-radioactive-material (2018).
Levin, C. S. & Hoffman, E. J. Calculation of positron range and its effect on the fundamental limit of positron emission tomography system spatial resolution. Phys. Med. Biol. 44, 781 (1999).
Ferguson, S., Jans, H. S., Wuest, M., Riauka, T. & Wuest, F. Comparison of scandium-44 g with other PET radionuclides in pre-clinical PET phantom imaging. EJNMMI Phys. 6, 23 (2019).
Pourmand, A. & Dauphas, N. Distribution coefficients of 60 elements on TODGA resin: application to Ca, Lu, Hf, U and Th isotope geochemistry. Talanta 81, 741–753 (2010).
Moffett, D. et al. Toxicological profile for barium and barium compounds. Agency for Toxic Substances and Disease Registry https://www.atsdr.cdc.gov/toxprofiles/tp24.pdf (2007).
Newton, D., Ancill, A. K., Naylor, K. E. & Eastell, R. Long-term retention of injected barium-133 in man. Radiat. Prot. Dosimetry 97, 231–240 (2001).
Andersson, J. D. et al. Robust high-yield ~ 1 TBq production of cyclotron based sodium [99mTc]pertechnetate. Nucl. Med .Biol. 60, 63–70 (2018).
Fludeoxyglucose F 18 Injection—FDA NDA 21-870. US Food and Drug Administration https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021870lbl.pdf (2005).
Highlights of Prescribing Information—Ga 68 DOTATOC Injection. US Food and Drug Administration https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210828s000lbl.pdf (2019).
Acknowledgements
The authors would like to thank Jonathan Doupe and Hans-Sönke Jans for providing guidance throughout this project, and John Duke and Simon Ferguson for reading and commenting on the manuscript. We also thank the Dianne and Irving Kipnes Foundation for supporting this work.
Author information
Authors and Affiliations
Contributions
B.J.B.N. performed experimental design, cyclotron target preparation and irradiations, radiochemistry, and prepared the manuscript. J.W. performed experimental design, cyclotron irradiations, and prepared the manuscript. J.D.A. and F.W. provided project supervision, contributed to experimental design, and prepared the manuscript. All authors discussed data interpretation and manuscript preparation together throughout the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nelson, B.J.B., Wilson, J., Andersson, J.D. et al. High yield cyclotron production of a novel 133/135La theranostic pair for nuclear medicine. Sci Rep 10, 22203 (2020). https://doi.org/10.1038/s41598-020-79198-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79198-x
This article is cited by
-
Cutting edge rare earth radiometals: prospects for cancer theranostics
EJNMMI Radiopharmacy and Chemistry (2022)
-
Highlight selection of radiochemistry and radiopharmacy developments by editorial board
EJNMMI Radiopharmacy and Chemistry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.