Abstract
We monitored the burden of cancer in Italy and its trends over the last three decades, providing estimates of cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years (DALYs), for cancer overall and 30 cancer sites using data from the Global Burden of Disease study 2017. An overview of mortality trends between 1990 and 2017 was also provided. In 2017, there were 254,336 new cancer cases in men and 214,994 in women, corresponding to an age-standardized incidence rate (ASIR) of 438 and 330/100,000, respectively. Between 1990 and 2017, incident cancer cases, and, to a lesser extent, ASIRs significantly increased overall and for almost all cancer sites, but ASIRs significantly declined for lung and other tobacco-related neoplasms. In 2017, there were 101,659 cancer deaths in men (age-standardized death rate, ASDR, 158.5/100,000) and 78,918 in women (ASDR 93.9/100,000). Cancer deaths significantly increased between 1990 and 2017 (+ 18%), but ASDR significantly decreased (− 28%). Deaths significantly increased for many cancer sites, but decreased for stomach, esophageal, laryngeal, Hodgkin lymphoma, and testicular cancer. ASDRs significantly decreased for most neoplasms, with the main exceptions of cancer of the pancreas and uterus, and multiple myeloma. In 2017, cancer caused 3,204,000 DALYs. Between 1990 and 2017, DALYs and age-standardized DALY rates significantly declined (-3.4% and -33%, respectively). Age-standardized mortality rates in Italy showed favorable patterns over the last few decades. However, the absolute number of cancer cases and, to a lower extent, of cancer deaths increased likely due to the progressive ageing of the population, this calling for a continuous effort in cancer prevention, early diagnosis, and treatment.
Similar content being viewed by others
Introduction
Neoplasms represent the second cause of death following cardiovascular diseases, with 9.6 million deaths and 233.5 million disability-adjusted life-years (DALYs) worldwide in 20171,2. Cancer cases has been increasing over the last decades (by 33% between 2007 and 2017, reaching 24.5 million new cancer cases in 2017), largely due to population ageing and growth1,2. While age-standardized incidence rates remained relatively stable since 1990, age-standardized death rates have been decreasing globally over the last decades, due to improvements in cancer diagnosis and treatment, as well as to the control of some risk factors, such as tobacco and alcohol1,3. However, a large variability in incidence and mortality cancer rates still exists, particularly between high- and low-income countries 1,2, due to different socio-demographic level, education, prevention strategies, and access to effective treatments and specialized care4.
In Italy, according to the National Institute of Statistics and the Global Cancer Observatory, there are about 410,000 new cancer cases and 175,000 cancer deaths each year5,6. Overall cancer mortality has been declining in both sexes over the last decades, with favorable trends for most common cancer sites7,8. Data on cancer incidence and other indicators of cancer burden in Italy are scanty. A continuous monitoring of the burden of cancer is of outmost importance in order to plan proper allocation of health resources for cancer prevention, diagnosis, and management.
In this regard, using the Global Burden of Disease (GBD) 2017 study, we provide a specific focus on the burden of cancer in Italy, including information on cancer incidence, mortality, years of life lost (YLLs), years lived with disability (YLDs), and DALYs. An overview of mortality trends between 1990 and 2017, as well as a comparison of incidence, mortality, and DALYs with other countries of Western Europe is also provided.
Results
Incidence
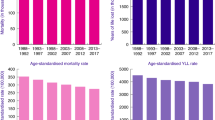
In 2017, in Italy there were 254,336 new cancer cases (95% UI 237,798–279,776) in men and 214,994 (199,575–230,541) in women, corresponding to an age-standardized incidence rate (ASIR) per 100,000 of 438 (95% UI 409–480) and 330 (95% UI 306–356), respectively (Table 1). Cancer of non-melanoma skin (43,003), prostate (40,927), trachea, bronchus, and lung (TBL; 28,651), and colorectum (28,602) were the most frequent sites in men, accounting for 56% of all male cancers. For women, the most common incident cancers were breast (49,500), non-melanoma skin (31,080), colorectal (23,626), and TBL (12,325) cancer, these representing 54% of all female cancer cases.
Between 1990 and 2017, incident cancer cases, incidence rate, and, to a lesser extent, ASIR significantly increased in both sexes combined (+ 61%, + 50%, and + 9%; Supplementary Table S1). Cancer cases and incidence rates significantly increased for almost all cancer sites, the only exceptions being stomach and laryngeal cancer, while ASIRs significantly increased for several cancers, but significantly declined for TBL, bladder, stomach, ovarian, lip and oral, laryngeal, and esophageal cancer.
Mortality
In 2017, there were 101,659 (95% UI 92,220–108,583) cancer deaths in men and 78,918 (95% UI 72,831–84,942) in women, corresponding to an age-standardized death rate (ASDR) per 100,000 of 158.5 (95% UI 148.4–169.6) in men and of 93.9 (95% UI 86.4 – 101.4) in women (Table 1). In men, the most common causes of cancer deaths were TBL, colorectum, prostate, and stomach cancer, with 24,438, 11,172, 9713, and 7011 deaths, respectively; in women, the major causes of cancer deaths were breast, colorectal, TBL, and pancreatic cancer, with 12,769, 9,810, 9,661, 6,778 deaths, respectively.
While cancer deaths and death rate significantly increased between 1990 and 2017 by 18% and 11%, respectively, ASDR significantly decreased by 28% (Supplementary Table S2). Deaths and death rates in both sexes combined significantly increased for many cancer sites, but significantly decreased for stomach, esophageal, laryngeal, Hodgkin lymphoma, and testicular cancer. ASDRs significantly decreased for most neoplasms, with the exceptions of cancer of the pancreas, multiple myeloma, and uterus.
DALYs, YLLs and YLDs
In 2017, cancer caused 3,204,000 (95% UI 3,018,000–3,396,000) DALYs in Italy, of which 94% came from YLLs (3,012,000) and 6% from YLDs (192,000; Supplementary Tables S3–S6). The proportion of DALYs due to YLLs was the highest (≥ 98%) for cancer of the TBL, pancreas, liver, gallbladder, and esophagus, while was the lowest (< 85%) for cancer of the prostate, uterus, and thyroid (Supplementary Table S3).
The leading causes of cancer DALYs and YLLs for both sexes combined in 2017 were TBL (612,000 and 602,000, respectively), colon and rectum (345,000 and 319,000), breast (276,000 and 240,000), and pancreatic (211,000 and 208,000) cancer (Supplementary Tables S4–S5). The leading causes of cancer YLDs were breast (36,200), colon and rectum (26,600), prostate (26,300), and bladder (12,300) cancer (Supplementary Table S6).
Between 1990 and 2017, a decline in DALYs (− 3.4%) and DALY rates (− 9.4%) was observed (Supplementary Table S4). DALYs and DALY rates significantly decreased for several cancer sites, including TBL, stomach, and leukemia, although they significantly increased for other cancer sites, such as pancreatic, prostate, kidney, multiple myeloma, uterine, cervical, and non-melanoma skin cancer. Age-standardized DALY rates declined overall (-33%) and for most cancer sites.
The patterns for YLLs, YLL rates, and age-standardized YLL rates were consistent with those for DALYs, DALY rates, and age-standardized DALY rates, with declines by − 6%, − 12% and − 34% for all cancers combined (Supplementary Table S5).
Overall and for all cancer sites, YLDs and YLD rates significantly increased between 1990 and 2017 (by 68% and 58%, respectively, for all cancers; Supplementary Table S6). Similarly, age-standardized YLD rates significantly increased overall (+ 20%) and for many cancer sites (including the most frequent ones), although they significantly decreased for some other cancers, such as bladder, stomach, and larynx.
Trends in mortality rates and ASDRs over time
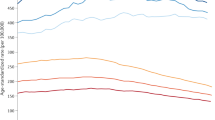
Figure 1 shows the trends in death rates and ASDRs for all cancers over the period 1990 and 2017, for men and women separately. Cancer ASDRs declined over the last decades in both men and women, while death rates levelled-off since the mid 2000s in men and since early 2010s in women (Fig. 1).
Trends in age-standardized and all ages death rates from all cancersa, Italy, 1990–2017. Legend: Solid lines show age-standardized death rates for men (light blue) and women (light red), dashed lines show all ages death rates for men (blue) and women (red). aIncludes malignant neoplasms (International Classification of Diseases 10 [ICD-10] codes C00-C96, excluding Kaposi sarcoma, ICD-10 C46), benign/in situ neoplasms (ICD-10 D00-D49), and other malignant neoplasms (ICD-10 codes C17, C30-C31, C37, C38, C40-C41, C47-C49, C4A, C51-C52, C57-C58, C60, C63, C66, C68, C69, C74-C75).
Figures 2a and b give the corresponding trends for the 30 cancer categories. Death rates increased over the last decades for several neoplasms, including female TBL, breast, pancreas, prostate, bladder, and kidney. Death rates were stable, at least over more recent calendar years, for cancer of the liver (since late 1990s), leukemia (since mid 2000s), non-Hodgkin lymphoma (since 2005), female gallbladder, cervix (since mid 1990s), and female upper aerodigestive tract cancers. Declines in death rates were observed for male TBL, male upper aerodigestive tract cancers, colorectum (since the late 1990s), stomach, female thyroid, Hodgkin lymphoma, and testicular cancer.
Trends in age-standardized and all ages death rates for 30 cancer groups (ordered by ranking in number of deaths in 2017), Italy, 1990–2017. (a) Cancer of the: trachea, bronchus, and lung; colon and rectum; breast; pancreas; stomach; liver; prostate; bladder; leukemia; non-Hodgkin lymphoma; kidney; gallbladder and biliary tract; brain and nervous system; ovary; multiple myeloma; esophagus; malignant skin melanoma; and lip and oral cavity. (b) Cancer of the: mesothelioma; cervix, uterus; larynx; non-melanoma skin; other pharynx; nasopharynx; thyroid; Hodgkin lymphoma; testis; other malignant neoplasms; and other neoplasms. Legend: Solid lines show age-standardized death rates for men (light blue) and women (light red), dashed lines show all ages death rates for men (blue) and women (red).
ASDRs declined for most cancers, with long-term reductions for TBL, male upper aerodigestive tract, male bladder, female gallbladder, breast, stomach, prostate, ovarian, mesothelioma, non-melanoma skin, thyroid and Hodgkin lymphoma, and more recent declines for colorectum (since the late 2000s), liver (since the late 1990s), leukemia (since the early 2000s), non-Hodgkin lymphoma (since the late 1990s), and cervix cancer (since the late 1990s; Figs. 2a and b). ASDRs were stable over the last decades for female TBL, other female upper aerodigestive tract cancers, female bladder cancer, kidney, male gallbladder, brain, multiple myeloma, melanoma, female mesothelioma, and uterus (since the late 1990′s). Only for pancreatic cancer, ASDRs non significantly increased up to more recent calendar years, even if a levelling-off was observed since 2015.
Comparisons with Western European countries
In 2017, Italy had lower age-standardized incidence (375.5), death (121.2), and DALY (2676.6) rates compared to Western Europe overall (407.7, 128.8, and 2877.7, respectively; Table 2). In particular, these rates were lower than those of the other most populous Western European countries (i.e., France, Germany, and the United Kingdom), with the only exception of Spain. Moreover, between 1990 and 2017 ASIR increased by a lower extent in Italy (+ 9.4%) than in Western Europe (+ 13.7%) and other Western European countries, while ASDRs and age-standardized DALY rates decreased more strongly in Italy (− 28.2% and − 32.5%) than in Western Europe (− 22.4% and − 25.6%) and the other Western European countries.
Discussion
This analysis of GBD cancer burden in Italy indicates that over the last decades cancer cases and incidence rates significantly increased overall and for almost all cancer sites, the main exception being stomach cancer. More favorable trends were, however, observed in ASIRs for TBL and as other tobacco-related cancers. Cancer deaths and death rates also increased, but death rates were more favorable—at least over more recent calendar years—for various neoplasms, including stomach, upper aerodigestive tract, Hodgkin lymphoma, and testicular cancer. Moreover, ASDRs steadily declined, particularly in men, for most cancer sites, the main exception being pancreatic cancer. A slight decline in DALYs and DALY rates was observed overall and for several cancer sites, and age-standardized DALY rates significantly declined for most neoplasms. Similar declines were found for YLLs, which explain about 95% of DALYs. Conversely, YLDs and YLD rates significantly increased overall and for many cancer sites, although a decrease in age-standardized YLD rates was observed for some neoplasms, including bladder, stomach, and larynx.
A few considerations may help interpret the patterns of cancer in Italy. For all neoplasms and most cancers considered, we observed an increase in the absolute number of cancer cases and cancer deaths, reflected also in an increase of crude incidence and death rates. Conversely, ASIRs, and particularly ASDRs, showed favorable patterns for most neoplasms considered. The differences between crude and age-standardized rates indicate that the increase in cancer burden in Italy is mainly due to the changes in the population structure over the last decades1,9. The decrease in overall YLLs from 1990 to 2017, while the total deaths increased, also indicates that people are dying at older ages. Moreover, the increase in the overall YLDs, along with the decrease in YLLs, are likely due to better survival rates. The decreases in the age-standardized (death) rates for many cancers observed in Italy over the last few decades, can be likely attributable to improvements in modifiable risk factors, implementation of secondary prevention programs, as well as progresses in the management and treatment for various neoplasms. With reference to primary prevention, an important role in the reduction of cancer mortality and DALYs can be due to favorable modifications in behavioral risk factors, including particularly a decrease in (male) tobacco smoking10,11,12 (the major determinant of lung and other tobacco-related neoplasms11), a reduction in alcohol consumption (strongly associated to liver and head and neck cancer, and, at a lower extent, to colorectal and breast cancer13), and a more affluent and varied diet (an important determinant of colorectal and other digestive tract cancers)14,15. The unfavorable trends in pancreatic cancer age-standardized rates may be due to the patterns in overweight/obesity16 and diabetes17—two known risk factors for this neoplasm—though the prevalence of overweight/obesity in Italy has not steady increased in Italy as in the USA and many other European countries18,19. Improved diagnosis may have also had some role, too.
The prevention and treatment of infections, such as Helicobacter pylori (which causes about 78% of stomach cancers), Hepatitis B and C virus (which accounts for most liver cancers), and Human Papilloma Virus (HPV; causally implicated in most cervical cancers) are also likely to have played a relevant role in the cancer mortality reductions20,21,22,23,24. Similarly, the improvement of working conditions and the reduction to occupational exposure to various carcinogens (such as asbestos) over the last decades may help explain the reduction in lung and bladder cancer ASDR25,26. Indoor and outdoor air pollution, a known risk factor for lung but also bladder cancer, with a synergistic effect with tobacco smoking27, has also substantially decreased in Italy over the last few decades, this further contributing to the favorable patterns in those neoplasms.
Screening has been the main responsible of the long-term decline in cervical cancer rates (through the Pap smear test and, more recently, the HPV test) in Italy as in most other Western European countries28,29,30. The implementation of organized screening programs (through fecal occult blood test, flexible sigmoidoscopy, and colonoscopy) has been shown to improve early diagnosis and reduce mortality from colorectal cancer31,32. Breast cancer screening (through breast examination and mammography) is also active in Italy since the early 2000s for women over age 50, although the coverage of such screening programs is still low in many Italian regions and its effectiveness is still debated33,34. The use of the prostate-specific antigen (PSA) test may have improved early diagnosis of prostate cancer, although the quantification of the role of the PSA test on mortality from this neoplasm is still under debate35,36.
A relevant role in the reduction of mortality rates from several cancers (including, among others, breast, prostate, testicular, leukemia, and Hodgkin lymphoma) is also due to the progress in the management and treatment for those cancers over the last decades, with the adoption of modern diagnostic techniques, improvements in surgery, introduction of innovative treatment approaches, recent developments in personalized medicine, as well as a better case management36,37,38,39,40.
We observed a relatively lower burden of cancer in Italy, as compared to other most Western European countries, this suggesting a possible improved control of modifiable risk factors41, as well as a better management and treatment for cancer in our country. The access to the best treatment options for cancer care in Italy is guaranteed by the presence of an efficient National Health System (NHS) with universal coverage, which assures since 1978 free of charge access to health care for all citizens42. Indeed, the Italian NHS ranked second globally by the World Health Organization in 2010, first by the Bloomberg Global Health Index in 2013, and ninth by the GBD Healthcare Access and Quality Index 20169,43,44.
Limitations
As in prior GBD studies, estimates presented in this study depend on the quality and quantity of the data sources available to inform the estimates45. Because of the lag time for data reporting, estimates for 2017 were mainly based on data and trends from recent years. Cancer mortality estimates are predominantly based on vital registration data, cancer registry data, and to a much lesser extent other data sources. In Italy, vital registration data are available since long time, has a 99% coverage, and cancer certification is reasonably reliable and valid, particularly for most common neoplasms7. Italian cancer registries cover approximatively 70% of the Italian population46. Incidence estimates used in this paper were predicted values from models that used observed mortality data as inputs and the use of MIRs. Although MIRs can change (for example as a consequence of COVID-19), incidence from mortality is estimated after careful estimation of MIRs using selected data sources1. Moreover, MIRs allow for a uniform method to estimate incidence and have been used in other cancer estimation frameworks which have detailed its benefits, including greater representativeness, especially in the absence of quality or complete population-based cancer registry systems47. Although the proportion of miscoded deaths in Italy is low, the redistribution of unspecified codes (the so-called “garbage” codes) may have somehow affected mortality, as well as incidence, estimates. Misclassification of metastatic sites as primary cancer sites (e.g., liver, TBL, and brain) is another source of potential bias, but again it should be limited in Italy, where sufficient diagnostic resources exist. Further, changes in coding practices or coding systems may also have an effect, even though mapping to the GBD causes list includes adjustments to account for different coding systems. GBD incidence and mortality estimates were somewhat higher than those provided by the Global Cancer Observatory, but differences were below 10% for many cancers, except for selected cancer sites (such as corpus uteri, brain, and stomach for incidence, and nasopharynx, cervix, and prostate for mortality)6. Such differences may be due to different estimation methods used and, in particular, to the redistribution of “garbage codes” made by GBD.
Conclusions
Age-standardized cancer rates in Italy showed favorable patterns over the last few decades, particularly for mortality, thanks to improvements in lifestyles, early diagnosis and treatment. However, the absolute number of cancer cases and, to a lower extent, of cancer deaths increased, likely due to the progressive ageing of the population1. Such increase may threaten the already progressively reducing country health resources48. Therefore, health care professionals, researchers, and policy makers should made a continuous effort in health promotion and prevention, to maximize the control of behavioral and environmental risk factors (namely tobacco, alcohol, low physical activity, high body mass index, and air pollution) for cancer, but also of other non-communicable diseases, as well as to promote early diagnosis, and further improve cancer treatment and management.
Methods
Data were extracted from the GBD 2017 results tool (http://ghdx.healthdata.org/gbd-results-tool). Methods and data sources are described in detail in previous GBD publications1,2,49,50 and are compliant with the Guidelines for Accurate and Transparent Health Estimates Reporting. Briefly, GBD 2017 comprehensively and systematically analyzed 282 causes of death, 359 diseases and injuries, and 84 behavioral and environmental risks for 195 countries and territories. Here, we focused specifically on the Italian cancer burden and present estimates of incidence, mortality, YLLs, YLDs, and DALYs in Italy for 30 cancer categories (corresponding to GBD Level 3, including malignant neoplasms, International Classification of Diseases 10 [ICD-10] codes C00-C96, excluding Kaposi sarcoma, ICD-10 C46, benign/in situ neoplasms, ICD-10 D00-D49, and other malignant neoplasms, ICD-10 codes C17, C30-C31, C37, C38, C40-C41, C47-C49, C4A, C51-C52, C57-C58, C60, C63, C66, C68, C69, C74-C75)1 in the years 1990 and 2017. For a comparative purpose, incidence, morality, and DALYs are also presented for all Western European countries combined, as defined by GBD (i.e., Andorra, Austria, Belgium, Cyprus, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Luxembourg, Malta, Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, United Kingdom), and for the most populated countries of Western Europe (i.e., France, Germany, Spain, and the United Kingdom). This analysis allowed us to compare the Italian cancer burden, with that of other European areas with similar socio-economic conditions.
Estimation framework
Details of the GBD estimation framework are provided in the eAppendix of the GBD 2017 Cancer Collaboration paper1. Briefly, the GBD cancer estimation process starts with mortality. Mortality estimates are based on vital registration data using an ensemble model approach2,51. Single-cause mortality estimates are scaled into the separately estimated all-cause estimate2. Cancer incidence estimates are derived from mortality estimates, dividing them by a mortality to incidence ratio (MIR). MIR is separately estimated using a spatio-temporal Gaussian process regression for each cancer type, sex, 5-year age group, location, and year52. The correlation between survival data and the MIR is used to estimate 10-year cancer prevalence; total prevalence is then partitioned into four sequelae (i.e., diagnosis/treatment, remission, metastatic/disseminated, and terminal phase), and each sequela prevalence is multiplied by a disability weight to estimate YLDs. Lifetime prevalence of procedure-related disability is estimated for larynx, breast, colorectal, bladder, and prostate cancer. YLLs are calculated as the difference between the corresponding standard life expectancy for a person’s age, sex, and year of actual age at death2. DALYs are the sum of YLDs and YLLs and represent the loss in years due to premature death or morbidity (one DALY can be regarded as one lost year of “fully healthy life”).
The GBD world population standard is used for the calculation of age-standardized rates2. All rates are reported per 100,000 person-years. We also provide 95% uncertainty intervals (UI) for all estimates.
Data availability
Data are available in the GBD 2017 results tool (http://ghdx.healthdata.org/gbd-results-tool).
References
GBD Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life Years for 29 Cancer Groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol 5, 1749–1768 (2019).
GBD Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1736–1788 (2018).
Lowy, D. R. & Collins, F. S. Aiming high-changing the trajectory for cancer. N. Engl. J. Med. 374, 1901–1904 (2016).
Vineis, P. & Wild, C. P. Global cancer patterns: causes and prevention. Lancet 383, 549–557 (2014).
Istituto Statistico Nazionale (ISTAT). I.Stat. Available at: http://dati.istat.it/ (last accessed June 2019).
Ferlay, J. et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 144, 1941–1953 (2019).
Bosetti, C. et al. Cancer mortality in Europe, 2005–2009, and an overview of trends since 1980. Ann. Oncol. 24, 2657–2671 (2013).
Rosso, T., Bertuccio, P., La Vecchia, C., Negri, E. & Malvezzi, M. Cancer mortality trend analysis in Italy, 1980–2010, and predictions for 2015. Tumori 101, 664–675 (2015).
OECD. Italy: Country Health Profile 2017 – State of Health in the EU. European Observatory on Health Systems and Policies, 2017. (OECD Publishing, 2017).
La Vecchia, C. et al. Trends in alcohol consumption in Europe and their impact on major alcohol-related cancers. Eur J Cancer Prev 23, 319–322 (2014).
GBD 2015 Tobacco Collaborators. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet 389, 1885–1906 (2017).
Lugo, A. et al. Smoking in Italy in 2015–2016: prevalence, trends, roll-your-own cigarettes, and attitudes towards incoming regulations. Tumori 103, 353–359 (2017).
Bagnardi, V. et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br. J. Cancer 112, 580–593 (2015).
Chan, A. T. & Giovannucci, E. L. Primary prevention of colorectal cancer. Gastroenterology 138, 2029–2043 (2010).
World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: a Global Perspective. Continuous Update Project Expert Report 2018. Available at: www.dietandcancerreport.org (2018).
Calle, E. E., Rodriguez, C., Walker-Thurmond, K. & Thun, M. J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N. Engl. J. Med. 348, 1625–1638 (2003).
Ben, Q. et al. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur. J. Cancer 47, 1928–1937 (2011).
Gallus, S. et al. Overweight and obesity in 16 European countries. Eur. J. Nutr. 54, 679–689 (2015).
Gallus, S. et al. Overweight and obesity prevalence and determinants in Italy: an update to 2010. Eur. J. Nutr. 52, 677–685 (2013).
Ford, A. C., Forman, D., Hunt, R. H., Yuan, Y. & Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ 348, g3174 (2014).
Roberts, S. E. et al. Review article: the prevalence of Helicobacter pylori and the incidence of gastric cancer across Europe. Aliment Pharmacol. Ther. 43, 334–345 (2016).
Plummer, M. et al. Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob. Health 4, e609-616 (2016).
Pimpin, L. et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. 69, 718–735 (2018).
Giorgi Rossi, P. et al. Cervical cancer screening in women vaccinated against human papillomavirus infection: recommendations from a consensus conference. Prev. Med. 98, 21–30 (2017).
Antoni, S. et al. Bladder cancer incidence and mortality: a global overview and recent trends. Eur. Urol. 71, 96–108 (2017).
Boffetta, P., Malvezzi, M., Pira, E., Negri, E. & La Vecchia, C. International analysis of age-specific mortality rates from mesothelioma on the basis of the international classification of diseases, 10th revision. J. Glob. Oncol. 4, 1–15 (2018).
ISPRA. Analisi dei trend dei principali inquinanti atmosferici in Italia, 2008–2017. Rapporti 302/2018. Availlable at: http://www.isprambiente.gov.it/it/pubblicazioni/rapporti/analisi-dei-trend-dei-principali-inquinanti-atmosferici-in-italia-2008-2013-2017 (2019).
Peto, J., Gilham, C., Fletcher, O. & Matthews, F. E. The cervical cancer epidemic that screening has prevented in the UK. Lancet 364, 249–256 (2004).
Cuzick, J. Long-term cervical cancer prevention strategies across the globe. Gynecol. Oncol. 117, S11-14 (2010).
Osservatorio Nazionale Screening. Rapporto 2018. Availlable at: https://www.osservatorionazionalescreening.it/content/rapporto-2018 (2018).
Manser, C. N. et al. Colonoscopy screening markedly reduces the occurrence of colon carcinomas and carcinoma-related death: a closed cohort study. Gastrointest. Endosc. 76, 110–117 (2012).
Lin, J. S. et al. Screening for colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 315, 2576–2594 (2016).
Berry, D. A. et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N. Engl. J. Med. 353, 1784–1792 (2005).
Beral, V. et al. The number of women who would need to be screened regularly by mammography to prevent one death from breast cancer. J. Med. Screen 18, 210–212 (2011).
Bray, F., Lortet-Tieulent, J., Ferlay, J., Forman, D. & Auvinen, A. Prostate cancer incidence and mortality trends in 37 European countries: an overview. Eur. J. Cancer 46, 3040–3052 (2010).
Etzioni, R. et al. The prostate cancer conundrum revisited: treatment changes and prostate cancer mortality declines. Cancer 118, 5955–5963 (2012).
Giordano, S. B. & Gradishar, W. Breast cancer: updates and advances in 2016. Curr. Opin. Obstet. Gynecol. 29, 12–17 (2017).
Hellerstedt, B. A. & Pienta, K. J. Testicular cancer. Curr. Opin. Oncol. 14, 260–264 (2002).
Sharma, S. & Rai, K. R. Chronic lymphocytic leukemia (CLL) treatment: So many choices, such great options. Cancer 125, 1432–1440 (2019).
Raj, R. V., Abedin, S. M. & Atallah, E. Incorporating newer agents in the treatment of acute myeloid leukemia. Leuk. Res. 74, 113–120 (2018).
Gallus, S., Bosetti, C., Chatenoud, L. & La Vecchia, C. Long live the Italians!. Prev. Med. 70, 76–77 (2015).
GBD 2017 Italy Collaborators. Italy’s health performance, 1990–2017: Findings 1 from the Global Burden of Disease Study 2017. Lancet Public Health (2019).
World Health Organization. World Health Report 2010: Health systems financing - the path to universal coverage. (WHO, 2010).
Lu, W. & Del Giudice, V. Italy's struggling economy has world's healthiest people. Bloomberg 2017. Available at: https://www.bloomberg.com/news/articles/2017-03-20/italy-s-struggling-economy-has-world-s-healthiest-people (Last accessed July 18, 2019).
GBD Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 3, 524–548 (2017).
AIRTUM. Copertura dei Registri Tumori in Italia. Available at: https://www.registri-tumori.it/cms/notizie/copertura-dei-registri-tumori-italia. Last accessed: July 15, 2019.
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359-386 (2015).
OECD. Health expenditure and financing: Health expenditure indicators. OECD Health Statistics (database). Availlable at: http://www.oecd.org/els/health-systems/health-data.htm (Last accessed July 18, 2019).
GBD Disease Injury Incidence Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1789–1858 (2018).
DALYs, G. & Collaborators, H. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1859–1922 (2018).
Foreman, K. J., Lozano, R., Lopez, A. D. & Murray, C. J. Modeling causes of death: an integrated approach using CODEm. Popul. Health Metr. 10, 1 (2012).
GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392, 1923–1994 (2018).
Funding
This study was funded by the Bill & Melinda Gates Foundation. The funder of the study had no role in study design, data collection, analysis and interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility to submit for publication.
Author information
Authors and Affiliations
Contributions
C.B. and L.M. conceptualized the study. C.B. drafted the manuscript. E.T. provided the analysis of the estimates and prepared tables and figures. C.A.A., J.D.H., J.M.K., M.N., A.P., and R.X. contributed in the production of GBD 2017 Cancer mortality and morbidity estimates. T.A. and C.F. contributed in the oversight of GBD 2017 Cancer estimation process and in the production of GBD 2017 Cancer mortality and morbidity estimates. K.C. provided corrections to 2017 Italy Cancer manuscript text and tables to update incidence numbers. L.M.F. revised and edited manuscript text, tables, and figures. E.T., G.C., S.G., G.G., C.L.V., A.L., C.P., L.R., and L.M. contributed to reviewing and finalizing the paper. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bosetti, C., Traini, E., Alam, T. et al. National burden of cancer in Italy, 1990–2017: a systematic analysis for the global burden of disease study 2017. Sci Rep 10, 22099 (2020). https://doi.org/10.1038/s41598-020-79176-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79176-3
This article is cited by
-
The non-fatal burden of cancer in Belgium, 2004–2019: a nationwide registry-based study
BMC Cancer (2022)
-
National Burden and Trend of Cancer in Ethiopia, 2010–2019: a systemic analysis for Global burden of disease study
Scientific Reports (2022)
-
Alarmists at the Gates: Esophageal Adenocarcinoma after Sleeve Gastrectomy is Not Different than with Other Bariatric/Metabolic Surgeries
Obesity Surgery (2022)
-
Returning to work after a sickness absence due to cancer: a cohort study of salaried workers in Catalonia (Spain)
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.