Abstract
Cardiovascular disease (CVD) is a well-known complication of diabetes, but the association has not been studied among Inuit in Greenland. The aim was to examine the association between diabetes and incident CVD among Inuit in Greenland and determine if the common diabetogenic TBC1D4 variant confers increased risk of CVD. We followed an initial study population of 4127 adults in Greenland who had participated in at least one population-based health survey, in national registers. We used Poisson regression to calculate incidence rate ratios (IRR) of cardiovascular endpoints, comparing participants with and without diabetes and comparing homozygous TBC1D4 carriers with heterozygous carriers and non-carriers combined. Close to 10% had diabetes and age range was 18–96 years (45% male). Of the 3924 participants without prior CVD, 362 (~ 9%) had CVD events during a median follow-up of 10 years. Multivariate IRR for the effect of diabetes on CVD was 1.12 (95% CI: 0.80, 1.57) p = 0.50. Using a recessive genetic model, we compared homozygous TBC1D4 carriers with wildtype and heterozygous carriers combined, with a multivariate IRR of 1.20 (95% CI: 0.69, 2.11) p = 0.52. Neither diabetes nor the TBC1D4 variant significantly increased CVD risk among Inuit in Greenland in adjusted models.
Similar content being viewed by others
Introduction
Cardiovascular disease (CVD) and diabetes are serious and costly conditions, associated with high morbidity and mortality. CVD is the number one cause of the death worldwide, accounting for roughly one third of all deaths1. Diabetes prevalence globally has almost doubled from 1980 to 2014 with a rise from 4.7% to 8.5%2. The same trend is seen in Greenland, where diabetes was almost non-existent half a century ago with a prevalence estimate of 0.06% in 19623 in contrast to recent estimates of diabetes of 9% and prediabetes of 19%4. Diabetes increases risk of CVD two to three fold in European populations5 and the same trend is shown in Inuit populations6,7 including Inuit in Greenland8,9. However, no epidemiological studies have been conducted to this day, elucidating the effect of diabetes on CVD risk in Greenland.
Physical inactivity, obesity, ageing, poor diet, smoking, abnormal lipids, hypertension and genetic predisposition are common risk factors for diabetes10,11,12 and CVD13,14,15,16,17. More than half of the population in Greenland smoke18 and physical inactivity is much more common in modern days as a result of a social transition from a traditional active lifestyle to a modern sedentary life with access to processed high caloric foods19. Accordingly obesity is an increasing problem among Inuit in Greenland with a rise from 15 to 25% over the past two centuries20 and it is a major risk factor of diabetes21. The common myth that Inuit are exempted from CVD22,23,24 is continuously disproven with recent studies finding that CVDs in Inuit populations are prevalent and relevant public health issues25,26,27. Adding to the pool of risk factors for diabetes among Inuit is the unique genetic structure of isolated populations giving rise to common risk alleles with larger effect sizes than in populations with a more diverse genetic composition.
The TBC1D4 p.Arg684Ter is one such risk variant discovered in the Greenlandic population in 2014 with an allele frequency of 17% and 4% homozygous (HO) carriers28. The diabetogenic effects demonstrated include decreased insulin-stimulated glucose uptake in skeletal muscle leading to postprandial hyperglycemia and hyperinsulinemia, impaired glucose tolerance and diabetes28. The variant shows the strongest association using a recessive model, and HO carriers have an odds ratio of 10.3 for developing diabetes and the variant is thought to explain around 15% of all diabetes in Greenland28. Among Inuit in Nunavik, a population comparable to Inuit in Greenland, diabetes was found to be underdiagnosed among carriers of the variant29, underlining the importance of recognizing potential clinical implications associated with the TBC1D4 variant.
The pathophysiology connecting the TBC1D4 variant to diabetes involves the defective protein it produces. The long isoform of TBC1D4 expressed in skeletal and heart muscle codes for an insulin stimulated activating protein that facilitates GLUT4 translocation from intracellular vesicles to the cell membrane after insulin stimulation30. Lack of the long TBC1D4 isoform confers postprandial hyperglycemia and hyperinsulinemia. A potential link between TBC1D4 and CVD is supported by mice knock-out studies that have uncovered that TBC1D4 phosphorylation plays an important role in the electrical conduction system of the heart31 suggesting that the variant may influence cardiovascular outcomes. Furthermore, a recent TBC1D4 study found that knock-out mice exhibited impaired cardiac function, increased infarction area and decreased left ventricular wall thickness three weeks after induced cardiac ischemia, compared to wild type (WT), indicating a functional role of TBC1D4 variant regarding heart damage after myocardial infarction32. A common ground between the TBC1D4 variant, diabetes and cardiovascular outcomes, is found in the “common soil hypothesis”, a shared pathophysiological pathway of diabetes and CVD through obesity, insulin resistance, dyslipidemia and inflammation33,34.
We do not know if CVD risk for Inuit with diabetes is the same as for Caucasians with diabetes or if the TBC1D4 variant confers increased risk of CVD. The aim of the study was therefore to determine if diabetes is associated with CVD risk among Inuit in Greenland. The second objective was to examine if carriers of the TBC1D4 variant have a higher risk of CVD than non-carriers.
Methods
Study population
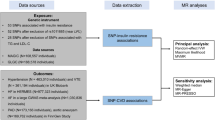
The study population included participants from two countrywide Greenlandic population-based studies: the B99 study and the Inuit Health in Transition (IHIT) study. B99 is a population-based survey of life style and disease among Greenlanders conducted from 1999–2001 and IHIT, completed from 2005–2010, is a general health study among adults in Greenland35,36. Participants over 18 years who completed an interview, a clinical examination and had blood samples drawn, were included in the study population. Participants who appeared in both surveys were included at the earliest participation date to get maximum follow up time. The population surveys are described in detail elsewhere35,36. The studies were approved by the Ethics Committee in Greenland with adherence to the Declaration of Helsinki and written informed consent was obtained from participants.
Definition of diabetes
Diabetes was defined according to World Health Organization 2006 criteria for type 2 diabetes: fasting plasma glucose > 6.9 mmol/l or plasma glucose > 11 mmol/l 2 h after an oral glucose load during an Oral Glucose Tolerance Test (OGTT) and/or self reported by questionnaire37.
TBC1D4 genotyping
Participants were genotyped for the TBC1D4 variant using the KasPAR assay (LGC Genomics, Hoddesdon, UK) and admixture proportions of European ancestral DNA were estimated for each individual, where a proportion of 1 indicates 100% Inuit ancestry and zero indicates 100% European ancestry using data generated from the Illumina MetaboChip28.
Cardiovascular outcomes
CVD outcomes were defined as fatal or non-fatal CVD diagnoses using the International Classification of Diseases 8 (ICD8) used from 1971–199338, the International Classification of Diseases 10 (ICD10) used since 199439, the International Classification of Primary Health Care 2 (ICPC2) used since 200340 and the Danish version of the NOMESCO Classification of Surgical Procedures (SKS)41. We included five CVD groups namely ischemic heart disease (including coronary surgeries), stroke, vascular disease (including ischemic amputations), heart failure and atrial fibrillation. CVD outcomes were retrieved from the Greenlandic and Danish Hospital Discharge Registers and the Greenlandic and Danish Causes of Death Registers. CVD outcomes from national registers were linked to health surveys by the Personal Identification Number unique for every Greenlandic and Danish citizen (See Appendix).
National registers
The Greenlandic Civil Registration System
The Greenlandic Civil Registration System is administered by the Danish Civil Registration System42. Amongst other things, it contains information on sex, place of birth, place of residence and emigration status.
The Greenlandic Hospital Discharge Register
Since 1987, the Greenlandic Hospital Discharge Register has been used to register discharge diagnoses from health centers and hospitals in Greenland. Medical information and information on surgical procedures are recorded. In 2014 Greenland changed to a new electronic medical record system from Æskulap to Cosmic. Cosmic was first implemented at Queen Ingrid’s Hospital in Nuuk in the beginning of 2014 and then successively among the 4 main regional hospitals and health centers throughout Greenland.
The Danish National Hospital Register
The Danish National Hospital Register was established in 1977 with the purpose of registering discharge diagnoses from Danish hospitals43. Greenlandic patients requiring specialized medical procedures or treatments unavailable in Greenland are flown to Copenhagen for treatment and their diagnoses are therefore found in this register.
The Danish Register of Causes of Death
Since 1875, deaths in Denmark have been registered by the National Board of Health and the Danish Register of Causes of Death was established in 1970 as a computerized record system44. The register includes, amongst other things, information on sex, age, causes of death and date of death for residents in Denmark dying in Denmark and Greenlandic residents dying in Denmark.
The Greenlandic Register of Causes of Death
The Greenlandic Register of Causes of Death was established in 1983 as part of the Danish Register of Causes of Deaths44. It includes causes of death of Greenlanders with a registered address in Greenland, as well as information on sex, age and date of death.
Statistical analyses
We examined the association of diabetes with CVD risk and the effect of the TBC1D4 p.Arg684Ter variant on CVD risk. Date of entry into the study was defined as date of the clinical examination during the health survey and participants were followed from entry until first CVD event, death, emigration or end of follow-up, whichever came first. CVD incidence rates were estimated using Poisson regression with log-person-time as offset variable and a significance level at 5%. Since CVD rates are not constant over time, the LEXIS macro was used to cut follow-up time into 1-year age bands and current age and calendar year were used as time scales45. Poisson regression was run on complete cases and participants with CVD events prior to entry were excluded from analyses. The effect of diabetes on CVD risk was estimated in a crude analysis and then adjusting for age, calendar year and sex in model 1. In model 2 we additionally adjusted for body mass index (BMI), systolic and diastolic blood pressure, low density lipoprotein (LDL) cholesterol, triacylglycerol (TAG) levels and smoking. In model 3 we further adjusted for the effect of TBC1D4 variant and admixture. The effect of TBC1D4 was estimated using a recessive model (the model with the best fit28) comparing HO with wildtype (WT) and heterozygous (HT) combined, in a crude model and adjusting for the same confounders as in model 3. As CVD outcomes related to diabetes are not consistently defined in the literature, we did a sensitivity analysis excluding atrial fibrillation and heart failure and ran analyses again (CVD outcomes then only included ischemic heart disease, stroke and vascular disease).
To avoid exclusion of individuals with missing data on OGTT and TBC1D4 genotyping covariates, which may infer biased results46, we ran Multivariate Imputation by Chained Equation47 with missing-at-random assumptions. For both OGTT and TBC1D4 missing data, we independently assessed five copies of the data, each with imputed missing values and estimates of parameters were averaged across the copies according to Rubin rules48. For imputation, we used R statistical program version 3.6.0 and the R-package mice49. Data management and Poisson regression was done using SAS Statistical Software (version 9.4)50.
Results
The baseline study population included 4127 participants who had completed lifestyle questionnaires and clinical examinations in the two health surveys B99 (n = 1317) and IHIT (n = 2810). Age range was 18 to 96 years and 1837 (44%) were male. We found 367 (~ 10%) participants with diabetes out of 3653 who had complete OGTT information (Table 1) and as such 474 participants had missing data on fasting and/or 120 min blood glucose values of the OGTT. Participants with diabetes had higher median age, BMI, systolic and diastolic blood pressure and fewer were smokers than in the normoglycemia group. HO carriers of the TBC1D4 variant were in higher number in the diabetes group compared to the normoglycemia group, n = 47 (13.7%) and n = 71 (2.3%) and conversely TBC1D4 WT genotype was lower in the diabetes group than the normoglycemia group n = 205 (59.8%) and n = 2205 (70.3%) respectively.
Cardiovascular outcomes
We followed the initial study population of 4127 individuals in national registers for a median of 10.2 years interquartile range (IQR) [8.3, 13.8] until CVD event, death, emigration or end of follow-up (December 31st 2016) whichever came first. A total of 622 participants died during follow-up and 111 were CVD registered deaths. We excluded 203 individuals who had a CVD event registered prior to inclusion and of the 3924 individuals that remained 362 (9.2%) had incident CVD events (Table 2). We divided participants with complete OGTT data n = 3460 (464 with missing OGTT data) into diabetes (n = 319) and normoglycemia (n = 3141) groups that accounted for 60 (18.8%) and 283 (9.0%) CVD events respectively. In total, CVD outcomes were most commonly stroke (35.1%) and ischemic heart disease (34.8%) and to a lesser extent heart failure (13.8%), atrial fibrillation (13.3%) and vascular disease (3.0%). In the diabetes group compared to the normoglycemia group, we found a higher frequency of diagnoses of heart failure (16.7% vs 13.1%), atrial fibrillation (16.7% vs 13.1%) and CVD deaths (33.3% vs 29.7%) and fewer diagnoses of stroke (30.0% vs 36.4) and vascular disease (1.7% vs 3.5%).
TBC1D4 genotype distribution among CVD events
Of the 3924 participants we included without previous CVD events 3705 were genotyped for the TBC1D4 variant and 330 of the genotyped participants developed CVD events (Table 3). TBC1D4 genotypes of those who had CVD events were 217 (65.8%) WT, 98 (29.7%) HT and 15 (4.5%) HO. This corresponds to 8.5% of WT, 9.7% of HT and 11.4% of HO genotypes developing CVD events. Types of CVD events seemed equally distributed among genotypes, except that HO genotype had fewer strokes (20% vs 35.5%) and more ischemic heart disease (46.7% vs 32.7%) compared to WT genotype. CVD deaths were also more frequent among HO than WT (33.3% vs 27.2).
The effect of diabetes on CVD risk and all-cause mortality
The effect of diabetes on CVD risk estimated by Poisson regression is shown in Table 4. We found significantly increased risk of CVD for participants with diabetes with a crude incidence rate ratio (IRR) of 2.45 (95% CI: 1.85, 3.23) p < 0.0001. However, in model 1 (adjusted for age, sex and calendar year) IRR decreased to 1.32 (95% CI: 0.99, 1.75) p = 0.061 and in model 2 (further adjusting for BMI, systolic and diastolic blood pressure, LDL cholesterol, TAG and smoking) IRR decreased to 1.16 (95% CI: 0.85, 1.59) p = 0.34. In model 3 (further adjusting for TBC1D4 and admixture) IRR was 1.12 (95% CI: 0.80, 1.57) p = 0.50. We did multiple imputation on missing OGTT data (n = 464 with 19 CVD events) and new Poisson regression on imputed datasets did not change IRR significantly (data not shown) suggesting that participants with missing OGTT data are not much different from complete cases. In model 3 with all-cause mortality as outcome, the effect of diabetes increased risk of death by an IRR of 1.32 (11.04,1.67) p = 0.0.
The effect of the TBC1D4 variant on CVD risk and all-cause mortality
Assuming a recessive effect of the TBC1D4 variant on CVD risk we found a crude IRR of 1.37 (95% CI: 0.81, 2.29) p = 0.24 for HO vs WT + HT. In model 3 (Table 4), we adjusted for admixture in addition to all previous covariates and IRR was 1.20 (95% CI: 0.69, 2.11) p = 0.52. We found an Inuit protective but statistically insignificant effect of admixture on CVD risk, with IRR of 0.66 (95% CI: 0.37, 1.17) p = 0.15 per percentage increase in Inuit admixture. In order to address missing data on genotypes (n = 219 with 32 CVD events) we did multiple imputation. In both crude models and model 3 run again with imputed data, IRR remained almost unchanged (data not shown), suggesting that participants with missing genotype data are not markedly different from complete cases. In model 3 with all-cause mortality as outcome, the effect of HO carrier status vs non-HO was an IRR of 0.84 (0.52, 1.35) p = 0.47.
Sensitivity analysis of CVD outcomes
In a sensitivity analysis of CVD outcomes, we omitted atrial fibrillation and heart failure and kept stroke, ischemic heart disease and vascular disease. With fewer CVD outcomes effect sizes became lower with IRR of 2.18 (95% CI: 1.61, 2.96) p < 0.0001 in a crude analysis and in model 1 IRR was 1.21 (95% CI: 0.89, 1.65) p = 0.23 (age, sex and calendar year significantly associated with increasing CVD risk, data not shown). In model 2 IRR was 1.12 (95% CI: 0.80, 1.56) p = 0.51 (age, sex, calendar year and LDL significantly associated with increased risk, data not shown). In model 3 using the recessive model IRR for HO vs WT + HT was 1.07 (95% CI: 0.74, 1.54) p = 0.72 (age, sex, calendar year, LDL cholesterol, smoking and European admixture increased CVD risk significantly, data not shown).
Discussion
In this study we tested if diabetes and the TBC1D4 variant were associated with CVD risk in Inuit in Greenland. We found that diabetes was associated with elevated CVD risk in a crude analysis, however, in adjusted models the effect was not maintained. Assuming a recessive effect the TBC1D4 variant showed a maintained trend of increased CVD risk, however this trend was not significant and a protective effect on all-cause mortality was not significant either. Age, male sex and LDL cholesterol significantly increased CVD risk in adjusted models as has been described in Inuit populations before51. In total 9% of normoglycemic participants and 19% of participants with diabetes developed incident CVD events. Altogether ~ 10% of participants had diabetes, as has been described before in the Greenlandic population52. Diabetes conferred a significant increase of all-cause mortality in a multi-adjusted model. It was unexpected that diabetes did not confer statistically significant increased CVD risk, as is the case in many other non-Inuit populations5,53,54,55 and in Inuit in Alaska51. We propose several reasons to explaining this.
One reason could be if CVD outcomes were not defined accurately. The literature is rich in studies supporting that diabetes increases CVD risk, but definition of CVD outcomes varies greatly. Agreeing upon which CVD diagnoses are diabetes relevant is difficult and especially atrial fibrillation and heart failure may cause discussions among physicians. We chose to include atrial fibrillation23,56,57 and heart failure58,59,60, supported by the literature as relevant complications to diabetes, in addition to stroke, ischemic heart disease and vascular disease. In a sensitivity analysis we omitted atrial fibrillation and heart failure. Age, male sex and LDL remained significant and in addition we found that smoking, calendar year and European admixture significantly increased risk of CVD. The effect of smoking could be explained by its pathophysiological mechanisms causing atherosclerosis, which is a dominant factor in the pathophysiology of especially ischemic heart disease, vascular disease and stroke51. Atrial fibrillation and heart failure have multiple other pathophysiological mechanisms besides atherosclerosis harmonizing well with the effect of smoking found when these were omitted. The elevated CVD risk per increase in calendar year may walk hand in hand with the traditional Inuit diet of marine animals steadily over time being replaced by imported foods of low quality, thus increasing CVD associated risk62. One study of Inuit in Alaska found that a traditional Inuit diet was associated with lower TAG, blood pressure and slightly higher LDL, concluding that it was linked to a better cardiovascular profile63. The fact that Inuit admixture had a protective effect on CVD risk in the sensitivity analysis, could also be related to the Inuit diet being more habitual among inhabitants of smaller more isolated villages in Greenland, where Inuit admixture levels are highest64.
A second reason could be that Inuit are not directly comparable to Caucasians due to genetic differences. Genes play a powerful role in isolated populations as seen among Pima Indians where close to half of the population is diagnosed with diabetes65,66. Similarly, the TBC1D4 variant convincingly increases diabetes risk in Inuit populations, but it does not seem to convincingly increase CVD risk. An explanation could be, that there could be undiscovered CVD protective variants or Inuit specific interactions with diet or metabolic traits affecting CVD risk, or the TBC1D4 variant could induce less dangerous diabetes form like GCK-MODY (Glycokinase Maturity Onset Diabetes of the Young). GCK-MODY patients have prolonged mild hyperglycemia but do not require treatment67. The TBC1D4 variant showed a protective but not statistically significant effect on all-cause mortality and diabetes significantly increased risk of all-cause mortality, supporting a theory that TBC1D4 diabetes may have fewer clinical implications than non-TBC1D4 diabetes.
A third reason could be that diabetes duration was not long enough to confer increased CVD risk, as diabetes incidence in Greenland has increased from virtually non-existent to ~ 10% over the last half a century. Perhaps in 50 years, diabetes associated CVD risk will be comparable to Caucasian populations. CVD risk increases with age and although life expectancy in Greenland has gone up from 63 to 72 years, compared to the mean of the European Union of 72 to 82 years over the past 40 years68, it remains substantially lower and a younger population will develop fewer CVD events.
A fourth reason could be changes in health behavior after receiving a diabetes diagnosis. Participants with diabetes in this study were screen detected in population-based studies and were identified early in time, possibly before any diabetes symptoms manifested themselves. Consequently, participants identified with diabetes would have had time to change their risk profile by e.g. smoking secession, weight loss, a healthier diet and so on. We found fewer smokers in the diabetes group compared to the normoglycemia group 54.8 vs 69.4%. As such CVD risk may be underestimated.
A fifth explanation could be an imprecise definition of diabetes in this population. We used self-reported and OGTT (fasting and two-hour glucose measurements) to define diabetes, as WHO guidelines suggested at the time the health surveys were conducted. Using glycated hemoglobin (Hba1c) we would have caught an overlapping but not identical group of people with diabetes. A study with Inuit in Canada with the same allele frequency and postprandial effect of TBC1D4, found that in carriers with prediabetes or type 2 diabetes, 32% would have remained undiagnosed without an OGTT29. Defining diabetes by Hba1c instead of OGTT criteria as been shown to affect ethnic groups differently69. Inuit have, for any given glucose value by OGTT, a higher Hba1c compared to Danes, suggesting different associations between Hba1c and blood glucose levels in these populations70.
A sixth reason could be if the registers with CVD outcomes were incomplete or faulty. However this seems unlikely, because two studies validated the Greenlandic Hospital Discharge Register in its entirety71 and CVD diagnoses separately72 and both found it valid for epidemiological purposes. Diagnoses in the Danish Hospital Discharge Register have also been validated with good results43.
Regarding the effect of the TBC1D4 variant, we found maintained increased but statistically insignificant risk of CVD for HO carriers. We handled missing genotypes by redoing Poisson regression after multiple imputation and estimates were not changed significantly, suggesting that the number of complete cases in the models are representative of the full sample.
Compared to non-carriers, HO carriers had more frequent ischemic heart disease (HO 46.7% vs WT 32.7%), CVD deaths (33.3% vs 27.2) and fewer stroke diagnoses (HO 20.0% vs WT 35.5%), corresponding well to the fact that TBC1D4 is expressed in heart and muscle tissue and not in the brain. Although we could not detect a significantly increased risk of CVD for the TBC1D4 variant, we cannot completely rule one out. Since the TBC1D4 variant is present with the same allele frequency among Inuit in Canada29, a way to get more power could be to pool data from Greenland and Canada. If there is an increased risk of CVD for HO carriers of the TBC1D4 variant, it would be of public health interest to uncover it.
Conclusions
In conclusion, this study showed that neither diabetes nor the TBC1D4 variant significantly increased CVD risk in Inuit in Greenland. However, we find it imprudent to draw hasty conclusions and suggest more studies be conducted on diabetes and CVD risk in Inuit populations.
Ethics approval and consent to participate
Oral and written consent was obtained from all participants in the two health surveys (B99 and IHIT) and the committee for Research Ethics in Greenland (Commission for Scientific Research in Greenland) granted ethical approval. The study protocols were in accordance with the Helsinki Declaration.
Data availability
Data of the health surveys is available upon request for relevant purposes.
Abbreviations
- CI:
-
Confidence interval
- CVD:
-
Cardiovascular disease
- HO:
-
Homozygous
- HT:
-
Heterozygous
- IRR:
-
Incidence rate ratio
- LDL:
-
Low density lipoprotein
- OGTT:
-
Oral glucose tolerance test
- TAG:
-
Triacylglycerol
- WT:
-
Wildtype
References
Organization, W. H. (2017).
Organization, W. H. Global report on diabetes. (World Health Organization, 2016).
Sagild, U., Littauer, J., Jespersen, C. S. & Andersen, S.J.A.M.S. Epidemiological studies in Greenland 1962–1964. 1. Diabetes mellitus in Eskimos. 179, 29–39 (1965).
Jorgensen, M. E., Borch-Johnsen, K., Witte, D. R. & Bjerregaard, P. Diabetes in Greenland and its relationship with urbanization. Diabet Med. 29, 755–760. https://doi.org/10.1111/j.1464-5491.2011.03527.x (2012).
Kannel, W. B. & McGee, D. L. Diabetes and cardiovascular disease: the Framingham study. JAMA 241, 2035–2038 (1979).
Schraer, C. D., Adler, A. I., Mayer, A. M., Halderson, K. R. & Trimble, B. A. Diabetes complications and mortality among Alaska Natives: 8 years of observation. Diabetes Care 20, 314–321 (1997).
Gohdes, D. Diabetes in North American Indians and Alaska Natives. Atlantic 43, 87 (1995).
Pedersen, M. L. Microvascular complications in Nuuk, Greenland, among Greenlanders and non-Greenlanders diagnosed with type 2 diabetes. Diabetes Res. Clin. Pract. 136, 1–6 (2018).
Pedersen, M. L., Jacobsen, J. L. & Lynge, A. R. Micro-and macrovascular complications among Greenlanders and Danes with type 2 diabetes mellitus in Nuuk, Greenland. Int. J. Circumpolar Health 69, 195–207 (2010).
Chen, L., Magliano, D. J. & Zimmet, P. Z. J. The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat. Rev. Endocrinol. 8, 228 (2012).
Chan, J. M., Rimm, E. B., Colditz, G. A., Stampfer, M. J. & Willett, W. C. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 17, 961–969 (1994).
Mokdad, A. H. et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289, 76–79 (2003).
Burns, D. M. Epidemiology of smoking-induced cardiovascular disease. Prog. Cardiovasc. Dis. 46, 11–29 (2003).
Hubert, H. B., Feinleib, M., McNamara, P. M. & Castelli, W. P. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 67, 968–977 (1983).
Kannel, W. B. Blood pressure as a cardiovascular risk factor: prevention and treatment. JAMA 275, 1571–1576 (1996).
Kannel, W. B., Castelli, W. P., Gordon, T. & McNamara, P. M. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. Ann. Intern. Med. 74, 1–12 (1971).
Wannamethee, S. G. & Shaper, A. G. Physical activity in the prevention of cardiovascular disease. Sports Med. 31, 101–114 (2001).
Bjerregaard, P. & Becker, U. Validation of survey information on smoking and alcohol consumption against import statistics, Greenland 1993–2010. Int. J. Circumpolar Health 72, 20314 (2013).
Young, T. K. Health Transitions in Arctic Populations (University of Toronto Press, Toronto, 2008).
Bjerregaard, P. & Jørgensen, M. E. & Greenland Population Study Group. Prevalence of obesity among Inuit in Greenland and temporal trend by social position. Am. J. Hum. Biol. 25, 335–340 (2013).
Jacobsen, J. L. & Lynge, A. R. Micro-and macrovascular complications among Greenlanders and Danes with type 2 diabetes mellitus in Nuuk, Greenland AU - Pedersen, Michael Lynge. Int. J. Circumpolar Health 69, 195–207. https://doi.org/10.3402/ijch.v69i2.17442 (2010).
Ulbricht, T. & Southgate, D. Coronary heart disease: seven dietary factors. Lancet 338, 985–992 (1991).
Kannel, W. B., Hjortland, M. & Castelli, W. P. Role of diabetes in congestive heart failure: the Framingham study. Am. J. Cardiol. 34, 29–34 (1974).
Kromann, N. & Green, A. Epidemiological studies in the Upernavik district, Greenland. Incidence of some chronic diseases 1950–1974. Acta Med. Scand. 208, 401–406 (1980).
Tvermosegaard, M., Dahl-Petersen, I. K., Nielsen, N. O., Bjerregaard, P. & Jørgensen, M. E. Cardiovascular disease susceptibility and resistance in circumpolar inuit populations. Can. J. Cardiol. 31, 1116–1123 (2015).
Hutchinson, R. N. & Shin, S. Systematic review of health disparities for cardiovascular diseases and associated factors among American Indian and Alaska Native populations. PLoS ONE 9, e80973 (2014).
Bjerregaard, P., Young, T. K. & Hegele, R. A. Low incidence of cardiovascular disease among the Inuit—what is the evidence?. Atherosclerosis 166, 351–357 (2003).
Moltke, I. et al. A common Greenlandic TBC1D4 variant confers muscle insulin resistance and type 2 diabetes. Nature 512, 190–193. https://doi.org/10.1038/nature13425 (2014).
Manousaki, D. et al. Toward precision medicine: TBC1D4 disruption is common among the inuit and leads to underdiagnosis of Type 2 diabetes. Diabetes Care https://doi.org/10.2337/dc16-0769 (2016).
Sakamoto, K. & Holman, G. D. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am. J. Physiol. Endocrinol. Metab. 295, E29-37. https://doi.org/10.1152/ajpendo.90331.2008 (2008).
Quan, C., Xie, B., Wang, H. Y. & Chen, S. Pkb-mediated thr649 phosphorylation of as160/tbc1d4 regulates the r-wave amplitude in the heart. PLoS ONE 10, e0124491 (2015).
Binsch, C. et al. Absence of TBC1D4/AS160 impairs cardiac substrate metabolism and increases ischemia/reperfusion-induced myocardial damage. Diabetologie und Stoffwechsel 14, 096 (2019).
De Rosa, S. et al. Type 2 diabetes mellitus and cardiovascular disease: genetic and epigenetic links. Frontiers Endocrinol. 9, 2 (2018).
Stern, M. P. Diabetes and cardiovascular disease: the “common soil” hypothesis. Diabetes 44, 369–374 (1995).
Bjerregaard, P. et al. Inuit health in Greenland: a population survey of life style and disease in Greenland and among Inuit living in Denmark. Int. J. Circumpolar Health 62(Suppl 1), 3–79 (2003).
Bjerregaard, P. Inuit Health in Transition: Greenland Survey 2005–2010: Population Sample and Survey Methods. (Statens Institut for Folkesundhed, 2011).
Organization, W. H. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. (2006).
WHO. (World Health Organization, 1993).
Organization, W. H. International statistical classification of diseases and related health problems. Vol. 1 (World Health Organization, 2004).
Verbeke, M., Schrans, D., Deroose, S. & De Maeseneer, J. The International Classification of Primary Care (ICPC-2): an essential tool in the EPR of the GP. Am. J. Hum. Biol. 124, 809 (2006).
Munksgaard, a. f. W. H. O. (2005).
Schmidt, M., Pedersen, L. & Sorensen, H. T. The Danish Civil Registration System as a tool in epidemiology. Eur. J. Epidemiol. 29, 541–549. https://doi.org/10.1007/s10654-014-9930-3 (2014).
Andersen, T. F., Madsen, M., Jorgensen, J., Mellemkjoer, L. & Olsen, J. H. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med. Bull. 46, 263–268 (1999).
Helweg-Larsen, K. The Danish register of causes of death. Scand. J. Public Health 39, 26–29. https://doi.org/10.1177/1403494811399958 (2011).
Carstensen, B. & Center, S. D. in Annual meeting of Finnish Statistical Society. 24.
Janssen, K. J. et al. Missing covariate data in medical research: to impute is better than to ignore. J. Clin. Epidemiol. 63, 721–727 (2010).
Van Buuren, S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 16, 219–242 (2007).
Marshall, A., Altman, D. G. & Holder, R. L. Combining estimates of interest in prognostic modelling. (2009).
Groothuis-Oudshoorn, K. & Van Buuren, S. Mice: multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67 (2011).
Institute, S. Base SAS 9.4 procedures guide: Statistical procedures. (SAS Institute, 2017).
Howard, B. V. et al. Cardiovascular disease prevalence and its relation to risk factors in Alaska Eskimos. Nutr. Metab. Cardiovasc. Dis. 20, 350–358 (2010).
Jørgensen, M., Borch-Johnsen, K., Witte, D. & Bjerregaard, P. Diabetes in Greenland and its relationship with urbanization. Diabet. Med. 29, 755–760 (2012).
Laakso, M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes 48, 937–942 (1999).
Grundy, S. M. et al. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 100, 1134–1146 (1999).
Franco, O. H., Steyerberg, E. W., Hu, F. B., Mackenbach, J. & Nusselder, W. Associations of diabetes mellitus with total life expectancy and life expectancy with and without cardiovascular disease. JAMA Internal Med. 167, 1145–1151. https://doi.org/10.1001/archinte.167.11.1145 (2007).
Huxley, R. R., Filion, K. B., Konety, S. & Alonso, A. Meta-analysis of cohort and case–control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am. J. Cardiol. 108, 56–62 (2011).
Movahed, M.-R., Hashemzadeh, M. & Jamal, M. M. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int. J. Cardiol. 105, 315–318 (2005).
McMurray, J. J., Gerstein, H. C., Holman, R. R. & Pfeffer, M. A. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2, 843–851 (2014).
Bell, D. S. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care 26, 2433–2441 (2003).
Nichols, G. A., Gullion, C. M., Koro, C. E., Ephross, S. A. & Brown, J. B. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 27, 1879–1884 (2004).
McGill, H. C. Jr. The cardiovascular pathology of smoking. Am. Heart J. 115, 250–257 (1988).
Bjerregaard, P. & Mulvad, G. The best of two worlds: how the Greenland Board of Nutrition has handled conflicting evidence about diet and health. Int. J. Circumpolar Health 71, 18588 (2012).
Eilat-Adar, S. et al. Dietary patterns are linked to cardiovascular risk factors but not to inflammatory markers in Alaska Eskimos. J. Nutr. 139, 2322–2328 (2009).
Moltke, I. et al. Uncovering the genetic history of the present-day Greenlandic population. Am. J. Hum. Genet. 96, 54–69 (2015).
Bennett, P., Burch, T. & Miller, M. Diabetes mellitus in American (Pima) indians. Lancet 298, 125–128 (1971).
Knowler, W. C., Pettitt, D. J., Saad, M. F. & Bennett, P. H. Diabetes mellitus in the Pima Indians: incidence, risk factors and pathogenesis. Diabetes Metab. Rev. 6, 1–27 (1990).
Steele, A. M. et al. Prevalence of vascular complications among patients with glucokinase mutations and prolonged, mild hyperglycemia. JAMA 311, 279–286 (2014).
Factbook, C. The world factbook; 2010. See also: http://www.cia gov/library/publications/the-world-factbook, accessed January 30 (2019).
Christensen, D. L. et al. Moving to an A1C-based diagnosis of diabetes has a different impact on prevalence in different ethnic groups. Diabetes Care 33, 580–582 (2010).
Jørgensen, M. E., Bjerregaard, P., Borch-Johnsen, K. & Witte, D. New diagnostic criteria for diabetes: is the change from glucose to HbA1c possible in all populations?. J. Clin. Endocrinol. Metab. 95, E333–E336 (2010).
Koch, A., Nielsen, N. & Melbye, M. Validering af Det Grønlandske Landspatientregister (Afdeling for Epidemiologisk Forskning, Statens Serum Institut, 2011).
Tvermosegaard, M. et al. Validation of cardiovascular diagnoses in the Greenlandic Hospital Discharge Register for epidemiological use. Int. J. Circumpolar Health 77, 1422668. https://doi.org/10.1080/22423982.2017.1422668 (2018).
Acknowledgements
We want to thank the participants of the health surveys.
Funding
MO was funded by the Danish Heart Association, the University of Southern Denmark and the Independent Research Fund Denmark (FSS).
Author information
Authors and Affiliations
Contributions
Conception: M.E.J., M.L.P. Data analysis: M.O., L.J.D, N.S. Data collection: M.E.J., P.B., C.V.L.L. Manuscript draft writing: M.O. Data verification: M.O., L.J.D., N.S. Manuscript editing and supervision: M.E.J., M.L.P., N.G. and T.H. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Overvad, M., Diaz, L.J., Bjerregaard, P. et al. The effect of diabetes and the common diabetogenic TBC1D4 p.Arg684Ter variant on cardiovascular risk in Inuit in Greenland. Sci Rep 10, 22081 (2020). https://doi.org/10.1038/s41598-020-79132-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79132-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.