Abstract
Loss-of-function mutations in the Sost gene lead to high bone mass phenotypes. Pharmacological inhibition of Sost/sclerostin provides a new drug strategy for treating osteoporosis. Questions remain as to how physical activity may affect bone mass under sclerostin inhibition and if that effect differs between males and females. We previously observed in female Sost knockout (KO) mice an enhanced cortical bone formation response to a moderate level of applied loading (900 με at the tibial midshaft). The purpose of the present study was to examine cortical bone adaptation to the same strain level applied to male Sost KO mice. Strain-matched in vivo compressive loading was applied to the tibiae of 10-, 26- and 52-week-old male Sost KO and littermate control (LC) mice. The effect of tibial loading on bone (re)modeling was measured by microCT, 3D time-lapse in vivo morphometry, 2D histomorphometry and gene expression analyses. As expected, Sost deficiency led to high cortical bone mass in 10- and 26-week-old male mice as a result of increased bone formation. However, the enhanced bone formation associated with Sost deficiency did not appear to diminish with skeletal maturation. An increase in bone resorption was observed with skeletal maturation in male LC and Sost KO mice. Two weeks of in vivo loading (900 με at the tibial midshaft) induced only a mild anabolic response in 10- and 26-week-old male mice, independent of Sost deficiency. A decrease in the Wnt inhibitor Dkk1 expression was observed 3 h after loading in 52-week-old Sost KO and LC mice, and an increase in Lef1 expression was observed 8 h after loading in 10-week-old Sost KO mice. The current results suggest that long-term inhibition of sclerostin in male mice does not influence the adaptive response of cortical bone to moderate levels of loading. In contrast with our previous strain-matched study in females showing enhanced bone responses with Sost ablation, these results in males indicate that the influence of Sost deficiency on the cortical bone formation response to a moderate level of loading differs between males and females. Clinical studies examining antibodies to inhibit sclerostin may need to consider that the efficacy of additional physical activity regimens may be sex dependent.
Similar content being viewed by others
Introduction
Loss-of-function mutations in the Sost gene lead to osteosclerotic phenotypes in humans (sclerosteosis, OMIM 269500 and van Buchem disease, OMIM 239100)1,2 and to high bone mass phenotypes in male and female mice3,4. Interestingly, female Sost null mice have an increased volumetric cortical bone mineral density compared to males3. In contrast, transgenic female mice overexpressing Sost exhibit low bone mass5. Sost is a negative regulator of Wnt signaling and subsequently bone formation, since canonical Wnt signaling activation increases bone mass. Pharmacological inhibition of sclerostin, the product of the Sost gene, through a sclerostin-neutralizing antibody (Scl-Ab) has shown significant bone gains in rodents6 and nonhuman primates7 and reduced fracture risk in postmenopausal women with osteoporosis8. Romosozumab (Evenity™, Amgen and UCB) was recently approved by the FDA for the treatment of postmenopausal women with osteoporosis at high risk of fracture. Other sclerostin antibodies are being tested for use in children and adults with rare diseases, such as Type I, III or IV Osteogenesis Imperfecta (Setrusumab, formerly called BPS804, Mereo BioPharma).
Questions remain as to how physical activity may enhance bone mass in young and old male and female patients undergoing sclerostin inhibition treatment. Fracture risk is of course a concern in the clinical populations where sclerostin antibodies will be used, thus moderate rather than high intensity training regimes are likely more feasible. Nevertheless, it is important to understand how the adaptive response to in vivo loading is altered by sclerostin inhibition across age and sex. Sclerostin is primarily expressed by the osteocytes and mechanical loading of cortical bone in the mouse ulnae led to substantial downregulation in endogenous Sost mRNA expression and a decreased number of stained osteocytes releasing sclerostin9. It has been suggested that loading-induced down-regulation of sclerostin increases bone formation by relieving inhibition of canonical Wnt signaling in osteoblasts as well as by suppressing resorption through the regulation of OPG. We recently showed female Sost knockout (KO) mice had a significantly greater load-induced cortical (mid-diaphysis) and trabecular (metaphysis) bone formation response compared to female littermate control (LC) mice10,11. Dkk1 expression was significantly greater in female Sost KO compared to LC mice, and was significantly downregulated with loading in both female Sost KO and LC mice. The load-induced cortical resorption response was similar in female Sost KO and LC mice. Others have reported region-specific load-induced cortical bone formation response in the absence of Sost in young female mice12,13. It was shown that sclerostin antibody treatment enhances the anabolic bone formation response to loading in young female C57BL6 mice14.
We have previously shown a net gain in trabecular bone volume fracture and trabecular thickness could be achieved with loading in Sost KO adult female mice, due to reduced resorption, which was not the case in males11. It is unknown how Wnt signaling is affected during loading under Sost inhibition in male mice, since all of the aforementioned studies were performed in females and our single study in males did not examine gene-level changes in the trabecular bone. This is important since previous studies have shown that in addition to aging, sex has an influence on Wnt/β-catenin signaling in bone, suggesting that sex hormones may be possible regulators or mediators of sclerostin function15. Modder et al. reported that men have higher serum sclerostin levels than women and that serum sclerostin levels increase markedly with age16. Callewaert et al. showed that disrupting androgen receptor signaling increased periosteal bone formation following ulnar loading, in addition to lowering Sost and sclerostin expression after loading (more than in wildtypes)17. They suggested that androgen receptor signaling may interfere with the inhibiting effect of sclerostin signaling on bone formation18. Several studies have revealed a sex-related difference in bone mass and/or the anabolic response to mechanical loading in mice that had altered Wnt/β-catenin signaling. Deletion of a single copy of β‐catenin in osteocytes abolished the anabolic response to loading in male and female mice, but interestingly the osteocyte β-catenin haploinsufficiency led to reduced bone mass in 8- and 20-week-old females, with less effect on males19,20. Sex differences were observed in the anabolic response to loading of Lrp5 null and Lrp5HBM + mice21,22. Female Lrp5-deficient mice had greater suppression of exercise-dependent bone remodeling than males21, while female Lrp5HBM + mice were more responsive to lower magnitudes of loads and had less bone loss in response to disuse compared to males23. These results demonstrate the need for further examination of the underlying basis for the observed sexual dimorphism in light of sclerostin inhibition that targets this pathway.
Since the majority of pre-clinical loading studies to date focused on females, it remains unclear if physical activity will be beneficial in older as well as young males undergoing sclerostin inhibition. Less is known in general regarding how males with or without Sost deficiency respond to mechanical loading with age. Ducher et al. showed that periosteal bone formation in male tennis players’ humeri was more pronounced in prepubertal and peripubertal boys and plateaued in postpubertal players24. Mosley et al. reported no sex-related differences in the cortical bone formation response to ulnar loading in young rats25, while other studies observed an elevated mechanoresponse in cortical bone of female wild-type mice26,27,28,29. We observed that the cortical bone formation response to loading was reduced at maturation in both female LC and Sost KO mice coincident with age-dependent expression of Wnt target genes in female Sost KO and LC mice as well as Sost gene expression in LC mice10. It remains unclear if male Sost KO and LC mice have age-related changes in mechanoresponse similar to what has been reported in female wild-type mice30,31,32,33. This knowledge is especially relevant considering that men suffer from age-related bone loss and have fragility fractures and thus would benefit from sclerostin inhibition34.
Motivated by our earlier observations in females10, we hypothesized that (1) Sost deficiency in male mice would lead to an enhanced anabolic response to loading and (2) the bone formation response in males would be reduced at skeletal maturation. To test these hypotheses, we first performed in vivo strain gauging on male young growing (10-week-old), adult skeletally mature (26-week-old) and middle-aged (52-week-old) mice, and developed finite element (FE) models for the young and adult mice. We then applied two weeks of in vivo dynamic axial compressive loading to the tibiae of male young and adult Sost KO and LC mice, and quantified changes in cortical bone morphology by microCT, bone formation and resorption by 3D time-lapse in vivo morphometry using registered microCT data and conventional 2D histomorphometry. We also measured the gene expression of several Wnt target genes and Wnt inhibitors at 3, 8, and 24 h after a single loading session in male young and middle-aged mice of both genotypes. It should be noted that we did not examine gene expression in adult mice, but focused only on young and middle-aged mice to see a potential greater difference. Also, we did not examine the cortical bone formation/resorption changes in response to loading over two weeks in middle-aged male mice, since the load levels required to engender known osteogenic strain levels (e.g. 900 µɛ) were discovered to be above those that led to limping and joint damage in previous pilot studies in female Sost KO mice10.
Materials and methods
Animals
Sperm from four male Sost -/- mice was provided by Novartis. Intracytoplasmic sperm injection with the oocytes from female C57BL/6 J mice was performed and a breeding colony was maintained. The first heterozygous generation was mated among themselves. In following generations, homozygous Sost KO and LC mice were identified using a Multiplex PCR with mice tail cuts, according to a protocol provided by Novartis. Sost KO and LC male mice at age of 10, 26 and 52 weeks were used in this study. All animal experimental procedures were approved by the local animal welfare ethics committee (LAGeSo Berlin, G0021/11). All methods were carried out in accordance with relevant guidelines and regulations of the local animal welfare ethics committee.
In vivo load-strain calibration
To conduct a strain-matched in vivo tibial loading experiment, age- and genotype-specific load-strain relationships were determined by a separate strain gauging experiment (n = 7/age/genotype; 42 mice in total). At 10, 26, and 52 weeks of age, single element strain gauges (EA-06-015LA-120/E2, Micromeasurements, USA) were surgically attached to the medial surface of the tibial midshaft in LC and Sost KO male mice while mice were under anesthesia. A range of axial dynamic compressive loads (peak loads ranging from − 2 to − 14 N in LC and − 2 to − 20 N in Sost KO mice) was applied to the tibia using a custom-made fixture built in a loading device (Testbench Electro Force LM1, TA Instruments, USA), while strain measurements were recorded simultaneously using WinTest software associated with the loading device. Strain gauge measures were performed on both left and right tibiae of each mouse. In vivo stiffness (load/strain) was calculated as the change in load over the change in strain as described previously35,36 and used to calculate the peak compressive load required to induce + 900 με at the gauge site of the tibial midshaft (Supplementary Table 1).
Finite element analysis
Since the strain gauging experiment only provided strain measures at a very local area (gauge site) on bone surface and the gauge-measured strain does not always represent the peak or average strain at the tibial midshaft, microCT-based FE analyses were conducted to determine the load-induced strain environment across the entire tibia. The FE modeling approach used here had been developed and validated in our previous studies examining female C57BL/6 and female Sost KO mice37,38,39. Briefly, FE models of typical 10- and 26-week-old Sost KO and LC tibiae (n = 1/age/genotype) were built based on ex vivo microCT scanning of the strain-gauged whole tibiae (Amira, Thermo Fisher Scientific), at an isotropic voxel resolution of 9.91 μm (Skyscan 1172, Brukers, Belgium; 100 kVp, 100 µA, 360°, 0.3° rotation step, 3 frame averaging). Heterogeneous material properties (Young´s modulus) based on spatial distribution of tissue mineral density in the microCT scans were assigned to the FE models consisting of tetrahedral elements38,39,40. Poisson´s ratio was set to 0.35 for all models39. Loading and boundary conditions were applied to mimic the experimental in vivo axial loading of the tibia. Linear elastic FE analyses were performed (Abaqus 6.13, Dassault Systemés Simulia, MA). The predicted strain value at the gauge site for each tibia was calculated by averaging the strain in the longitudinal direction of the strain gauge at its mounting position, which was visible on the scans. The predicted strains in cortical bone were calculated in a volume of interest (VOI) centered at the tibia midshaft, containing 5% of the total tibia length, analogue to the region used for the microCT analysis. The maximum absolute value between the maximal (tensile, εMax) and minimal (compressive, εMin) principal strains was calculated for each element within the heterogeneous FE models. Thereafter, the mean values of the tensile (\(\bar{\varepsilon }_{\text{Max}}\)) and compressive (\(\bar{\varepsilon }_{\text{Min}}\)) strains in the midshaft VOI were determined. Elements for which abs (εMax) > abs (εMin ) were used to calculate \(\bar{\varepsilon }_{\text{Max}}\) and SD (εMax), while elements for which abs (εMax) ≤ abs (εMin ) were used to calculate \(\bar{\varepsilon }_{\text{Min}}\) and SD (εMin). The SD (standard deviation) reflects the range of εMax for tension and εMin for compression within the VOI.
In vivo tibial mechanical loading
The left tibiae of 10- and 26-week-old Sost KO and LC mice (n = 8/age/genotype) underwent daily in vivo cyclic compressive loading (900 με at strain gauge site, 216 cycles/day at 4 Hz) for 2 weeks (5 days/week, Monday-Friday) (Fig. 1). The strain level of 900 με was the same as that used in our previous study examining the effect of Sost deficiency on cortical and trabecular bone adaptation in female mice10,11. Although this strain is lower than the more commonly used peak strain of 1200 με, it is higher than strain levels engendered in wild-type C57BL6 mice during normal walking (200–600 µɛ)41,42. The loading frequency (4 Hz) represents the stride frequency of the mouse43. The loading regime included triangle waveforms with 0.15 s symmetric active loading/unloading, a 0.1 s rest phase (at − 1 N) between every load cycle and a 5 s rest inserted between every four cycles35. At day 15, the mice were euthanized through an overdose of potassium chloride while under anesthesia (ketamine 60 mg/kg and medetomidine 0.3 mg/kg). The weight was measured daily throughout the experiment and no abnormal weight loss was observed; there was no significant difference in start or end weight within a group (Supplementary Table 2). No mice exhibited any complications or signs of limping throughout the loading experiment. After dissection, tibial bone length was measured (Supplementary Table 2).
Schematic illustration of the experimental in vivo loading setup, including timeline for in vivo microCT imaging (day 0, 5, 10, 15), fluorochrome labeling for histomorphometry and procedure for 3D time-lapse in vivo morphometry. The region of interest for microCT analysis, 3D time-lapse in vivo morphometry and conventional histomorphometry was centered at the tibial midshaft.
Longitudinal in vivo microCT
In vivo microCT with a voxel size of 10.5 μm was performed on anesthetized mice at day 0, 5, 10, and 15 to assess the midshaft cortical bone of both the right and left tibiae (vivaCT 40, Scanco Medical, Switzerland; 55 kVp source voltage, 145 μA source current, 300 ms integration time, no frame averaging, range of 180 degrees) (Fig. 1). To prevent motion artifacts during microCT scanning, anaesthetized mice were constrained in a custom-made plastic mouse bed. Our previous study has shown the repeated radiation exposure (0, 5, 10, 15 day) from microCT at doses used in this study (approximately 0.48 Gy per scan) does not affect cortical bone parameters measured by microCT or histomorphometry in 10 and 26 week-old C57BL/6 mice35.
In vivo microCT analysis
Similar to previous studies, midshaft cortical bone (5% of tibial length) was analyzed from microCT images at day 0, 5, 10 and 15 using the IPL (Image Processing Language) based standard evaluation scripts provided by Scanco (Scanco Medical, Switzerland) (Fig. 1). Although whole-tibia analyses from previous tibial loading studies have observed relatively robust mechanoadaptive responses along ~ 10–60% of the tibia from its proximal end44,45,46, the midshaft was chosen here because it is a common location where strain gauging is performed and load-strain relations accounting for the effect of age, gender, genotype, etc. are established. It is also a common volume of interest for examining cortical bone adaptation in the tibial loading model32,33,35,41,46,47,48. A global threshold of 4626 HU (809.6 mg HA/ccm) was used to segment cortical bone from soft tissue and water in all mice. The measured cortical bone parameters included: principal moments of inertia (Imax, Imin), cortical bone area (Ct.Ar), total cross-sectional area (T.Ar), cortical area fraction (Ct.Ar/T.Ar), cortical thickness (Ct.Th), and cortical tissue mineral density (Ct.TMD).
Time-lapse in vivo morphometry
MicroCT images taken at day 0 and 15 were geometrically aligned and analyzed using a registration, segmentation and quantification algorithm using Amira software (Thermo Fisher Scientific) (Fig. 1). The method has previously been described in detail30,49,50. Briefly, the algorithm involves the following steps: (1) geometrical registration of images, (2) thresholding to extract the bone region, using the same global threshold mentioned above, (3) segmentation to exclude mineralized tissue present in the medullary cavity inside the VOI, (4) labeling regions of quiescent, newly formed and resorbed bone, and (5) quantification of volumetric dynamic (re)modeling parameters of formation and resorption normalized to values at the beginning of experiment (bone volume newly mineralized between day 0 and 15 divided by the bone volume present at day 0: MV/BVday0–15; bone volume eroded between day 0 and 15 divided by the bone volume present at day 0: EV/BVday0–15; mineralizing and eroded surface between day 0 and 15 normalized to the total bone surface at day 0: MS/BSday0–15 and ES/BSday0–15). The dynamic bone formation and resorption parameters were also quantified for the endocortical and periosteal surfaces, separately using previously described methods51.
Histomorphometry
Calcein (20 mg/kg) was administered to the LC and Sost KO mice via intraperitoneal injection at day 3 and 12 during the loading experiment (Fig. 1)52. The tibiae were dehydrated in ascending grades of ethanol to absolute, cleared in xylene, infiltrated and finally embedded in polymethyl-methacrylate. The blocks were sectioned transversal to the bone’s long axis at the cortical midshaft. The slices were ground and polished to an approximately thickness of 60 μm and viewed at a magnification of 200 × under a mercury lamp microscope (KS400 3.0, Zeiss, Germany) for evidence of fluorochrome labels (Fig. 1). Images were acquired using commercially available software (Axiovision, Zeiss, Germany). The analyzed region of interest for the cortical bone included endocortical and periosteal surface. The single- and double-labeled surface per bone surface (sLS/BS, dLS/BS), mineralizing surface (MS/BS), mineral apposition rate (MAR), and bone formation rate (BFR/BS), were analyzed as recommended and using ImageJ3. MS/BS was calculated as 0.5 × sLS/BS + dLS/BS. When a specimen had no double-labeled surface (dLS/BS = 0), it was labeled as “no data” for MAR and BFR/BS53. The amount of newly mineralized bone per day was calculated using the averaged double label distances divided by the 9-day labeling interval and expressed as the MAR in units of microns per day. For determining MAR, the entire endocortical (Ec) and periosteal (Ps) surfaces were analyzed.
qPCR analysis
The left tibiae of additional groups of 10-week-old and 52-week-old mice (n = 6/genotype/age/time point following loading) were loaded for a single loading session. The loading procedure has been described above (see section In vivo tibial mechanical loading). Mice were sacrificed at either 3, 8, or 24 h after the single loading session. Left loaded and right nonloaded tibiae were analyzed for gene expression. The ends of the bone and the bone marrow was removed and RNA was extracted using the Aurum total RNA fatty and fibrous tissue kit (Bio-Rad). RNA quality and concentration were verified by NanoDrop and reverse transcription was performed using the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). Gene expression was determined using a QuantStudio 7 Flex real-time PCR system and the following TaqMan probes (Thermo Fisher Scientific): Lef1 (Mm00550265_m1), Axin2 (Mm00443610_m1), Dkk1 (Mm00438422_m1), Sost (Mm00470479_m1), Ctsk (Mm00484039_m1), Tnfrsf11b (= Opg) (Mm00435454_m1) and Tnfsf11 (= RANKL) (Mm00441906_m1). B2m (Mm00437762_m1) and GAPDH (Mm99999915_g1) were used for normalization (averaged). Relative quantification was calculated using the ΔCt method.
Statistical analysis
The within-subject effect of loading (loaded and control limbs) and between-subject effects of age (10- and 26-week-old) and genotype (LC and Sost KO) as well as interactions between these terms were assessed using a repeated measure ANOVA (SAS 9.3, Cary, USA). A separate ANOVA was used to assess between-subject age (10- and 26-week-old) and genotype (LC and Sost KO) and interaction effects for relative values, the interlimb differences (∆interlimb = loaded limb − control limb). Paired t-tests were used to compare control and loaded limbs. Unpaired t-tests were performed to compare control limbs between age or genotype. The percent difference was presented as percentual increment [%Δ = ((loaded limb − control limb)/control limb) × 100%)]. Statistical analyses of the qPCR data were performed on the ΔCt values. Unpaired or paired t-tests were performed to determine the differences between Sost KO and LC or between loaded and control limbs, respectively. A significant difference was set for all analyses as p < 0.05.
Results
Tissue strains in cortical bone of Sost KO and age- and gauge strain-matched LC mice
The strain values at the strain gauge site predicted by the FE models were similar to those measured experimentally (~ 900 µε), supporting the validation of our FE models. Further, the FE models predicted that the mean compressive strains were 16% higher in 10-week-old than in 26-week-old Sost KO mice (Fig. 2). In contrast, the mean tensile strains were almost identical in the 10- and 26-week-old Sost KO mice. Both mean tensile and compressive strains were lower in the Sost KO mice than in their age-matched LC whereas this difference was much more pronounced in the 10-week-old (37% for tension and 70% for compression) than in the 26-week-old mice (7% for tension and 9% for compression) (Fig. 2).
Sost deficiency led to an increased bone formation in male mice
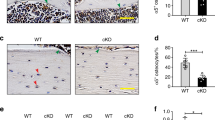
Compared to LC mice, male Sost KO mice had a greater surface area of newly mineralized tissues relative to the total surface area at the midshaft cortical bone, as indicated by time-lapse in vivo morphometry (main effect of genotype for MS/BSday0–15; Fig. 3). Further, both in vivo morphometry and histomorphometry showed that Sost deficiency-induced increase in MS/BS occurred primarily at the endocortical surfaces (main effect of genotype for Ec.MS/BS and Ec. MS/BSday0–15; Fig. 4, Supplementary Tables 3 and 4). Consistent with the enhancement of bone formation due to Sost deficiency, 10- and 26-week-old Sost KO mice had greater morphological and mechanical parameters (Ct.Ar, T.Ar, Ct.Th, Imax and Imin) relative to their littermate controls (main effects of genotype; Table 1). Additionally, those microCT-measured parameters were enhanced in 26-week-old Sost KO mice compared to 10-week-old mice (interaction of age and genotype; Table 1), likely due to an accumulation of formed bone associated with long-term Sost deficiency. Sost deficiency had little effect on bone resorption (no main effect of genotype; Fig. 3B).
(A) Visualization of newly formed (blue), resorbed (red), and quiescent (yellow) bone tissues over 15 days at the midshaft of control and loaded tibiae from young (10-week-old) and adult (26-week-old) LC and Sost KO male mice measured with 3D dynamic time-lapse in vivo morphometry. (B) MV/BVday0–15 or MS/BSday0–15 indicate the amounts of newly formed bone volume or surface area between day 0 and 15 (the loading period) relative to the total bone volume or surface at day 0. EV/BVday0–15 or ES/BSday0–15 indicate the amounts of resorbed bone volume or surface area between day 0 and 15 relative to the total bone volume or surface at day 0. ANOVA: indicates an effect of (a) genotype, (b) age, (c) loading, (d) genotype and age, (e) genotype and loading, (f) age & loading. *p < 0.05 by paired t-test. Sample size: 10-week-old LC (n = 5), 10-week-old Sost KO (n = 7), 26-week-old LC (n = 6), 26-week-old Sost KO (n = 6).
Cortical histomorphometry: (A) cross-sectional images showing fluorochrome labeling of the midshaft cortical bone at day 3 and 12; (B) mineral apposition rate (MAR) and mineralizing surface normalized to bone surface (MS/BS) are shown for both the endocortical and periosteal surfaces. MAR is the distance between the labels divided by time between labels. MS/BS indicates the extent of bone surface actively mineralizing. ANOVA: indicates an effect of (a) genotype, (b) age, (c) loading, (d) genotype & age, (e) genotype & loading, (f) age & loading. Sample size: 10-week-old LC (n = 5–6), 10-week-old Sost KO (n = 5–7), 26-week-old LC (n = 6–7), 26-week-old Sost KO (n = 4–8).
Skeletal maturation led to decreased bone formation and increased bone resorption in male Sost KO mice
Skeletal maturation led to decreased bone formation apparent in time-lapse in vivo morphometry, and histomorphometry (main effect of age by ANOVA; Figs. 3 and 4). Time-lapse in vivo morphometry showed decreased MV/BVday0–15 and MS/BSday0–15 in 26-week-old compared to 10-week-old Sost KO and LC mice (Fig. 3B). Histomorphometry measures also revealed a decrease in bone formation with skeletal maturation (Ec.dLS/BS, Ec.MS/BS, Ec.MAR, Ec.BFR/BS, Ps.sLS/BS, Ps.dLS/BS, Ps. MAR) (Fig. 4 and Supplementary Table 4). Interestingly, this reduction in bone formation with skeletal maturation was independent of Sost deficiency except in the Ec.MAR (ANOVA, interaction of genotype and age; Fig. 4B). Skeletal maturation also affected bone resorption, with 26-week-old mice having larger EV/BVday0–15 and ES/BSday0–15 than 10-week-old mice (Fig. 3B).
The moderate loading level led to only a mild anabolic response in male Sost KO mice
A significant main effect of loading was observed in MV/BVday0–15 and MS/BSday0–15) (ANOVA; Fig. 3 and Supplementary Table 3). Sub-analyses indicated that, in 26-week-old Sost KO mice, the loaded limb had a significantly greater MV/BVday0–15 (+ 76%) than its contralateral limb (p < 0.05 by paired t-test; Fig. 3B). Compared to the control limb, the loaded limb of the 10-week-old LC mice had significantly increased MV/BVday0–15 (+ 38%) and MS/BSday0–15 (+ 18%) (p < 0.05 by paired t-test; Fig. 3B), which occurred primarily at the endocortical surfaces (Ec.MV/BVday0–15: + 118%; Ec.MS/BSday0–15: + 100%; Supplementary Table 3). Additionally, loading had no effect on EV/BV or ES/BS (Fig. 3B). Although a main effect of loading on bone formation response to loading was observed, there was no interactive effect between genotype and loading (ANOVA; Fig. 3B). This was further confirmed in that there was no significant effect of genotype on the interlimb differences (loaded limb – control limb) in MV/BVday0–15 or MS/BSday0–15 for males (Fig. 5). Consistent with the observed load-induced bone formation at the endocortical surfaces by in vivo morphometry, static microCT measures at day 15 showed a significant load-induced increase in the cortical area fraction (Ct.Ar/T.Ar) (Table 1). However, bone formation indices from histomorphometry failed to show any loading effect (Fig. 4 and Supplementary Table 4).
Time-lapse in vivo morphometry measured newly formed bone between day 0 and day 15, including the total mineralizing volume (MV) and surface area (MS) normalized to the total bone volume (BV) or bone surface (BS) at day 0. Data is shown as interlimb difference (loaded-control limb) comparing male with previously published data for female mice10. ANOVA: indicates an effect of (a) genotype, (b) age, (c) genotype & age, which was performed separately for each sex. Sample size: 10-week-old LC (male = 5, female = 6), 10-week-old Sost KO (male = 7, female = 7), 26-week-old LC (male = 6, female = 7), 26-week-old Sost KO (male = 6, female = 7).
Gene expression was altered in male Sost KO mice
Ten-week-old Sost KO mice had decreased Lef1 expression in both loaded and control limbs compared to LC mice, which was significant at 8 h and 24 h and approaching significance at 3 h (Fig. 6). In contrast, Sost deficiency led to increased Lef1 expression at 24 h in 52-week-old mice (Fig. 6). We did not detect changes in Axin2 expression levels. Dkk1 was significantly increased in 52-week-old Sost KO mice at 3 h and 24 h compared to LC mice. In contrast, we detected lower expression of Dkk1 in control limbs of 10-week-old Sost KO at the 24 h time point (Fig. 6). Ctsk, Opg and RANKL mRNA levels were higher in 10-week-old Sost KO vs LC mice in many conditions at 3 h and 8 h, but not 24 h after loading (Fig. 7). In 52-week-old males, Ctsk was increased in Sost KO mice 3 h after loading in nonloaded limbs and 8 h after loading in loaded limbs; Sost depletion increased Opg expression in both loaded and control limbs at the 8 h and 24 h time points (Fig. 7). RANKL expression was increased in control and loaded limbs of 52-week-old Sost KO mice only 3 h after loading (Fig. 7).
Gene expression of Lef1, Axin2, Dkk1 and Sost was measured at 3, 8, and 24 h after a single loading session in the left loaded and right control tibiae of 10- and 52-week-old male Sost KO and LC mice. Gene expressions relative to reference genes are shown. *Indicates a significant difference between Sost KO compared to LC for each condition (t-test; p < 0.05). # Indicates a significant difference between loaded and control bones for each condition (paired t-test; p < 0.05). Sample size: n = 3–6.
Gene expression of Ctsk, Tnfrsf11b (= Opg) and Tnfsf11 (= RANKL) was measured at 3, 8, and 24 h after a single loading session in the left loaded and right control tibiae of 10- and 52-week-old male Sost KO and LC mice. Gene expressions relative to reference genes are shown. *Indicates a significant difference between Sost KO compared to LC for each condition (t-test; p < 0.05). # Indicates a significant difference between loaded and control bones for each condition (paired t-test; p < 0.05). Sample size: n = 3–6.
The Wnt effector gene Lef1 was increased in the loaded limb of 10-week-old Sost KO mice at 8 h, but the effect was abrogated at 24 h (Fig. 6). Loading did not influence Lef1 expression in 10- or 52-week-old LC mice or Axin2 expression in either Sost KO or LC mice (Fig. 6). Dkk1 expression was increased with loading at 24 h in 10-week-old Sost KO mice. At 3 h after loading we observed a decrease in the Wnt inhibitor Dkk1 expression in both 52-week-old Sost KO and LC mice (Fig. 6). We did not observe a change in Sost gene expression in LC mice following loading. There was a slight increase in Ctsk expression 3 h after loading in 10-week-old LC mice (Fig. 7). Loading caused dramatic increases in Ctsk expression in 52-week-old LC and Sost KO mice at 3 h, 8 h and 24 h. We also observed increases in Opg expression after loading in 10-week-old (LC and Sost KO, 3 h time point) and 52-week-old (Sost KO, 3 h and 8 h) mice. RANKL was affected differently by loading between 10- and 52-week-old mice. Specifically, RANKL expression was decreased at 3 h following loading in 10-week-old LC mice while it was increased at 8 h and 24 h in 52-week-old Sost KO mice (Fig. 7).
Discussion
Motivated by our earlier work on females, the goal of this study was to examine, in male mice, the effect of Sost deficiency on the cortical bone (re)modeling and adaptive response to a moderate level of applied loading. In vivo loading was applied to the tibiae of young and older male Sost KO and LC mice to engender a gauge strain of ~ 900 με at the midshaft, the same target strain as our previous female loading study10. This more moderate target strain level was chosen in the present study in males and previous study in female Sost KO mice10, since our pilot studies showed that 1200 με (requiring 17 N) determined through in vivo strain gauging of 10-week-old female Sost KO mice at the mid-diaphysis led to ankle swelling and limping in the mice during the first several days of loading.
As expected, Sost deficiency led to high cortical bone mass in young and adult male mice as a result of increased bone formation. However, the enhanced bone formation associated with Sost deficiency did not appear to diminish with skeletal maturation. An increase in bone resorption was observed with skeletal maturation in male LC and Sost KO mice. Our finding that gene expression levels for cathepsin K, Opg and RANKL are higher in Sost KO males (independent of loading in many conditions) suggests that more bone remodeling might be taking place in the absence of Sost. The applied moderate level of loading only induced a mild anabolic response in young and adult male mice, independent of Sost deficiency. Loading led to a decrease in the Wnt inhibitor Dkk1 expression at 3 h in middle-aged Sost KO and LC mice, and an increase in Lef1 expression at 8 h in young Sost KO mice. The current results suggest that in terms of bone mass, microstructure, and (re)modeling, long-term inhibition of sclerostin in male mice does not influence the adaptive response of cortical bone to moderate levels of loading.
Although Sost deficiency did not affect cortical bone response to loading in the male mice, it significantly enhanced cortical mechanoresponse in the female mice (Figs. 5 and 8)10, given the same target strain (~ 900 με) at the tibial midshaft for both males and females. Also, a reduction in bone resorption (ES/BS) in response to loading was previously observed in adult female Sost KO mice10, while it was not observed in the male Sost KO mice. These results suggest that the effect of Sost deficiency on cortical bone response to loading may be sex dependent, at least for the examined moderate level of loading which might be associated with moderate-intensity physical activities. Others have reported in 16-week-old female Sost KO mice a dose response of cortical bone to incremental loading13. Interestingly, they also found that female Sost KO mice had lower load-induced bone formation rate (rBFR/BS) relative to wild-type mice in high strain regions, but greater rBFR/BS relative to wild-type mice in low strain regions13. It remains unclear whether the effect of Sost deficiency on cortical mechanoresponse differs between males and females at a higher level of applied loading than that of the current study. However, a higher load might have less clinical relevance since patients suffering from osteoporosis might not be able to take any high-intensity activities.
MicroCT results of cortical area (ΔCt.Ar), cortical area divided by total area (ΔCt.Ar/T.Ar) and cortical thickness (ΔCt.Th) by in vivo microCT expressed as (ΔX = Xday15 − Xday0 within the same limb). Previously published data in female 10- and 26-week-old LC and Sost KO mice are shown here for comparison with male mice10. ANOVA: indicates an effect of (a) genotype, (b) age, (c) loading, (d) genotype and age, (e) genotype and loading, (f) age & loading, p < 0.05. Sample size: 10-week-old LC (male = 6, female = 6), 10-week-old Sost KO (male = 7, female = 7), 26-week-old LC (male = 7, female = 7), 26-week-old Sost KO (male = 6, female = 7).
In terms of the bone (re)modeling responses to Sost deficiency, both male and female Sost KO mice showed an elevated bone formation compared to their LCs. However, a reduction in bone resorption associated with Sost deficiency was observed in female mice10 but not in the male mice (Fig. 3B). This observation is consistent with synchronously elevated RANKL and Opg expressions at 3 and 8 h in the 10-week-old Sost KO male mice (Fig. 7). Additionally, skeletal maturation-related increase in bone resorption was diminished in female Sost KO mice relative to their LCs, whereas the effect of skeletal maturation on bone resorption was independent of Sost deficiency in male mice (Fig. 3B). These results together with those discussed above, suggest that the effect of Sost deficiency on cortical bone (re)modeling and adaptation to loading may differ between males and females.
Age‐related impairment of bones’ adaptive response to loading has been consistently shown in female wildtype mice27,30,32,33,35, yet the effect of sex remains contradictory across different ages. In addition, little is known regarding the effect of sex on age-related bone mechanoresponse in the absence of Sost. In one study examining the effect of ERα in osteoblast-lineage cells on bone mass and adaptation to mechanical loading, 10-week-old male littermate control mice showed less cortical and cancellous bone response compared to females29. An additional tibial loading study reported greater diaphyseal cortical and metaphyseal cancellous bone responses to loading in 17-week-old female wild-type mice compared to males of the same age28. In 19-month-old aged C57BL/6 mice, however, males and females showed similar cortical anabolic responses to loading27. Studies investigating the role of the low-density lipoprotein receptor-related protein 5 (Lrp5) in bones’ responses to loading, showed significant dose response of cortical bone in 17-week-old wild-type male mice but not in females23. Taken together, it remains unclear how sex affects bone adaptation to loading with skeletal maturation. Under the moderate loading examined, our current and previous results showed that both male and female LC mice had a reduced mechanoresponse with skeletal maturation, and this reduction appears to be greater in females than in males. Specifically, loading led to significant increases in the amount of newly mineralized bone volume and mineralizing surface area (MV/BVday0–15: + 38%, MS/BSday0–15: + 18%) in the 10-week-old LC male mice. However, no load-induced change in MV/BVday0–15 and MS/BSday0–15 was observed in 10-week-old LC male mice. We also observed a reduction of cortical bone formation response to loading with skeletal maturation in female Sost KO mice10 but not in male Sost KO mice (no interactive effect between age and loading was found by ANOVA for MV/BVday0–15 and MS/BSday0–15 in 10- and 26-week-old male Sost KO mice).
Similar to our previous study in female mice10, we observed decreased Lef1 in young male Sost KO mice compared to LC mice. A lower expression of Lef1 was measured in the loaded limbs of young female Sost KO mice at 8 h and adult Sost KO mice at 8 and 24 h compared to LC mice. Lin et al. also observed this interaction between genotype and age54, however, they reported greater Lef1 expression in young Sost KO mice compared to wild-type mice, but no difference in adult mice. We observed increased Dkk1 expression in middle-aged male Sost KO compared to LC mice, which was similar to young and adult Sost KO female mice10, which had increased Dkk1 expression compared to LC mice. Increased expression of this other Wnt inhibitor, Dkk1, may be due to a feedback mechanism intended to compensate for the loss of Sost. We did not observe a significant decrease in Sost with loading. This could be because male mice might have a greater adaptive strain threshold than females (see discussion below). Others have also reported a lack of downregulation in Sost after loading55. Recent unpublished data from our group shows that the time of day at which the mouse is loaded alters Sost expression due to circadian rhythms, which might explain our finding56. Future research is required to have a deeper understanding of the molecular mechanisms for the sex-dependent bone mechanoresponse due to sclerostin inhibition.
In general, the increases in load-induced bone formation for both Sost KO and LC mice were greater in females compared to males. One possible explanation for the dampened loading response in males could be the group housing of our male mice. A previous study demonstrated that group-housed males engaged in frequent fighting and had an attenuated response to mechanical loading compared with single-housed males26. However, since our mice are littermates, we expected limited fighting and only observed fighting on one occasion, whereby the mice were immediately separated. Another explanation could be a difference in strain threshold for stimulating new bone formation in male and females27. Since age-matched male mice are generally heavier than females (Supplementary Table 2), they may experience greater habitual loading during natural cage activities. The strain threshold already derived from habitual loading41 might be higher for males than females. The mild anabolic response to loading in males relative to females was not due to lower tissue strains. Comparing our FE analysis of male tibiae (Fig. 2), with previous FE results from females39, further demonstrates that the average and peak strains in the tibial midshaft VOI of males were greater than in female mice.
There are few limitations. Firstly, although we observed the anabolic response to loading in our time-lapse analyses, we did not observe differences between loaded and control limbs in many microCT-based morphological parameters or bone formation indices measured by histomorphometry. The reason for this discrepancy between methods might be attributed to the fact that the time-lapse analysis looks at the exact same anatomic VOI of bone, since we analyzed the registered (overlapping volume) from the initial and subsequent microCT images. In contrast, the static microCT measures might not examine the exact same VOI of bone, but rather the same relative position. This is a particular concern with longitudinal growth in the long bones, since often only a part of the tibia is scanned during in vivo microCT, to minimize radiation exposure30. Also, we have shown how histomorphometry results may depend on the position of the 2D section examined49. Time-lapse in vivo 3D morphometry overcomes these limitations via spatial registration of consecutive images and identify relatively small bone adaptive changes under a moderate level of loads. Secondly, we did not examine bone morphometric parameters in middle-aged or elderly mice, which may be more clinically relevant for osteoporosis studies, although sclerostin inhibition is also being investigated for use in children with rare bone diseases such as osteogenesis imperfecta. We did not examine gene expression in adult mice, but rather focused on young and middle-aged mice. Two-week loading studies in middle-aged and elderly Sost KO mice were not possible since loading levels required to engender 900 µε or greater would have likely damaged the ankle joint, which we observed in prior studies using 17 N to induce 1200 µε in 10-week-old female Sost KO mice10. Thirdly, the mild (re)modeling response to loading observed in our male Sost KO and LC mice is likely in part due to the use of a lower strain of 900 µɛ, which is still higher than strain levels engendered in wild-type C57BL6 mice during normal walking (200–600 µɛ)41,42. We have previously observed a much greater formation and anti-resorptive response to loading in C57BL6 mice when engendering 1200 µɛ30. However, using a higher strain level was not possible due to concerns related to overloading the mouse’s ankle joints. Regardless, comparisons between males and females were based on the same load-induced target strain at the midshaft VOI. Furthermore, the peak strains in the midshaft cortical volume of interest, determined by finite element analysis (Fig. 2), were much greater than those peak strain values that have been reported to be osteogenic for male and female animals (~ 1000 µɛ)41,57,58. More importantly, application of a target strain of 900 µɛ in male mice ensures the consistency with our previous female loading study, based on which comparisons between males and females were performed. Lastly, our previous study in females was completed shortly before we began our studies in males. However, a direct comparison at the same time would have been preferable, but we did use the same target strain and methodology including the same global threshold for microCT analysis between the studies. Repeating our results in another set of female mice was not allowed by our animal use committee.
In summary, we investigated the effect of Sost deficiency and skeletal maturation in male mice on cortical bone morphology, cortical bone (re)modeling and gene expression as well as the local mechanical strain environment induced within the bone under tibial loading. Our key findings were: (1) a moderate level of loading led to modest anabolic response in male mice, independent of Sost; (2) Lef1 expression was significantly reduced in 10-week-old Sost KO mice compared to littermates and Dkk1 expression was increased in 52-week-old male Sost KO mice compared to LC mice; (3) Comparing the findings in the current study with those from our previous study in females10 indicates that Sost deficiency influenced the bone formation response to loading not only in an age-dependent, but also in a sex-dependent manner.
References
Balemans, W. et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J. Med. Genet. 39, 91–97 (2002).
Staehling-Hampton, K. et al. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am. J. Med. Genet. 110, 144–152. https://doi.org/10.1002/ajmg.10401 (2002).
Li, X. et al. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J. Bone Miner. Res. 23, 860–869. https://doi.org/10.1359/jbmr.080216 (2008).
Balemans, W. et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum. Mol. Genet. 10, 537–543 (2001).
Loots, G. G. et al. Genomic deletion of a long-range bone enhancer misregulates sclerostin in Van Buchem disease. Genome Res. 15, 928–935. https://doi.org/10.1101/gr.3437105 (2005).
Li, X. et al. Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass, and bone strength in aged male rats. J. Bone Miner. Res. 25, 2647–2656. https://doi.org/10.1002/jbmr.182 (2010).
Ominsky, M. S. et al. Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J. Bone Miner. Res. 25, 948–959. https://doi.org/10.1002/jbmr.14 (2010).
Cosman, F. et al. Romosozumab treatment in postmenopausal women with osteoporosis. N. Engl. J. Med. 375, 1532–1543. https://doi.org/10.1056/NEJMoa1607948 (2016).
Robling, A. G. et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J. Biol. Chem. 283, 5866–5875. https://doi.org/10.1074/jbc.M705092200 (2008).
Pflanz, D. et al. Sost deficiency led to a greater cortical bone formation response to mechanical loading and altered gene expression. Sci. Rep. 7, 9435. https://doi.org/10.1038/s41598-017-09653-9 (2017).
Albiol, L. et al. Effects of long-term sclerostin deficiency on trabecular bone mass and adaption to limb loading differ in male and female mice. Calcif. Tissue Int. 106, 415–430. https://doi.org/10.1007/s00223-019-00648-4 (2020).
Morse, A. et al. Mechanical load increases in bone formation via a sclerostin-independent pathway. J. Bone Miner. Res. 29, 2456–2467. https://doi.org/10.1002/jbmr.2278 (2014).
Robling, A. G. et al. Sost, independent of the non-coding enhancer ECR5, is required for bone mechanoadaptation. Bone 92, 180–188. https://doi.org/10.1016/j.bone.2016.09.001 (2016).
Morse, A. et al. Sclerostin antibody augments the anabolic bone formation response in a mouse model of mechanical tibial loading. J. Bone Miner. Res. 33, 486–498. https://doi.org/10.1002/jbmr.3330 (2018).
Di Nisio, A. et al. Regulation of sclerostin production in human male osteocytes by androgens: experimental and clinical evidence. Endocrinology 156, 4534–4544. https://doi.org/10.1210/en.2015-1244 (2015).
Modder, U. I. et al. Regulation of circulating sclerostin levels by sex steroids in women and in men. J. Bone Miner. Res. 26, 27–34. https://doi.org/10.1002/jbmr.128 (2011).
Callewaert, F. et al. Androgen receptor disruption increases the osteogenic response to mechanical loading in male mice. J. Bone Miner. Res. 25, 124–131. https://doi.org/10.1359/jbmr.091001 (2010).
Callewaert, F., Sinnesael, M., Gielen, E., Boonen, S. & Vanderschueren, D. Skeletal sexual dimorphism: relative contribution of sex steroids, GH-IGF1, and mechanical loading. J. Endocrinol. 207, 127–134. https://doi.org/10.1677/JOE-10-0209 (2010).
Kramer, I. et al. Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Mol. Cell Biol. 30, 3071–3085. https://doi.org/10.1128/MCB.01428-09MCB.01428-09[pii] (2010).
Javaheri, B. et al. Deletion of a single beta-catenin allele in osteocytes abolishes the bone anabolic response to loading. J. Bone Miner. Res. 29, 705–715. https://doi.org/10.1002/jbmr.2064 (2014).
Sawakami, K. et al. The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J. Biol. Chem. 281, 23698–23711. https://doi.org/10.1074/jbc.M601000200 (2006).
Javaheri, B. et al. Lrp5 is not required for the proliferative response of osteoblasts to strain but regulates proliferation and apoptosis in a cell autonomous manner. PLoS ONE 7, e35726. https://doi.org/10.1371/journal.pone.0035726PONE-D-11-23945[pii] (2012).
Saxon, L. K., Jackson, B. F., Sugiyama, T., Lanyon, L. E. & Price, J. S. Analysis of multiple bone responses to graded strains above functional levels, and to disuse, in mice in vivo show that the human Lrp5 G171V High Bone Mass mutation increases the osteogenic response to loading but that lack of Lrp5 activity reduces it. Bone 49, 184–193. https://doi.org/10.1016/j.bone.2011.03.683S8756-3282(11)00791-5[pii] (2011).
Ducher, G., Daly, R. M. & Bass, S. L. Effects of repetitive loading on bone mass and geometry in young male tennis players: a quantitative study using MRI. J. Bone Miner. Res. 24, 1686–1692. https://doi.org/10.1359/jbmr.090415 (2009).
Mosley, J. R. & Lanyon, L. E. Strain rate as a controlling influence on adaptive modeling in response to dynamic loading of the ulna in growing male rats. Bone 23, 313–318 (1998).
Meakin, L. B. et al. Male mice housed in groups engage in frequent fighting and show a lower response to additional bone loading than females or individually housed males that do not fight. Bone 54, 113–117. https://doi.org/10.1016/j.bone.2013.01.029 (2013).
Meakin, L. B., Galea, G., Sugiyama, T., Lanyon, L. & Price, J. S. Age-related impairment of bones’ adaptive response to loading in mice is associated with sex-related deficiencies in osteoblasts but no change in osteocytes. J. Bone Miner. Res. 29, 1859–1871. https://doi.org/10.1002/jbmr.2222 (2014).
Saxon, L. K., Galea, G., Meakin, L., Price, J. & Lanyon, L. E. Estrogen receptors alpha and beta have different gender-dependent effects on the adaptive responses to load bearing in cancellous and cortical bone. Endocrinology 153, 2254–2266. https://doi.org/10.1210/en.2011-1977 (2012).
Melville, K. M. et al. Effects of deletion of eralpha in osteoblast-lineage cells on bone mass and adaptation to mechanical loading differ in female and male mice. J. Bone Miner. Res. 30, 1468–1480. https://doi.org/10.1002/jbmr.2488 (2015).
Birkhold, A. I. et al. Mineralizing surface is the main target of mechanical stimulation independent of age: 3D dynamic in vivo morphometry. Bone 66, 15–25. https://doi.org/10.1016/j.bone.2014.05.013 (2014).
Razi, H. et al. Aging leads to a dysregulation in mechanically driven bone formation and resorption. J. Bone Miner. Res. 30, 1864–1873. https://doi.org/10.1002/jbmr.2528 (2015).
Holguin, N., Brodt, M., Sanchez, M. & Silva, M. Aging diminishes lamellar and woven bone formation induced by tibial compression in adult C57BL/6. Bone 65, 83–91. https://doi.org/10.1016/j.bone.2014.05.006 (2014).
Lynch, M. E. et al. Tibial compression is anabolic in the adult mouse skeleton despite reduced responsiveness with aging. Bone 49, 439–446. https://doi.org/10.1016/j.bone.2011.05.017 (2011).
Khosla, S., Amin, S. & Orwoll, E. Osteoporosis in men. Endocr. Rev. 29, 441–464. https://doi.org/10.1210/er.2008-0002 (2008).
Willie, B. M. et al. Diminished response to in vivo mechanical loading in trabecular and not cortical bone in adulthood of female C57Bl/6 mice coincides with a reduction in deformation to load. Bone 55, 335–346. https://doi.org/10.1016/j.bone.2013.04.023 (2013).
Main, R. P., Lynch, M. E. & van der Meulen, M. C. In vivo tibial stiffness is maintained by whole bone morphology and cross-sectional geometry in growing female mice. J. Biomech. 43, 2689–2694. https://doi.org/10.1016/j.jbiomech.2010.06.019 (2010).
Yang, H. et al. Characterization of cancellous and cortical bone strain in the in vivo mouse tibial loading model using microCT-based finite element analysis. Bone 66, 131–139. https://doi.org/10.1016/j.bone.2014.05.019 (2014).
Razi, H. et al. Skeletal maturity leads to a reduction in the strain magnitudes induced within the bone: a murine tibia study. Acta Biomater. 13, 301–310. https://doi.org/10.1016/j.actbio.2014.11.021 (2015).
Albiol, L. et al. Sost deficiency leads to reduced mechanical strains at the tibia midshaft in strain-matched in vivo loading experiments in mice. J. R. Soc. Interface https://doi.org/10.1098/rsif.2018.0012 (2018).
Yang, H. et al. Examining tissue composition, whole-bone morphology and mechanical behavior of Gorab(Prx1) mice tibiae: a mouse model of premature aging. J. Biomech. 65, 145–153. https://doi.org/10.1016/j.jbiomech.2017.10.018 (2017).
Sugiyama, T. et al. Bones’ adaptive response to mechanical loading is essentially linear between the low strains associated with disuse and the high strains associated with the lamellar/woven bone transition. J. Bone Miner. Res. 27, 1784–1793. https://doi.org/10.1002/jbmr.1599 (2012).
De Souza, R. L. et al. Non-invasive axial loading of mouse tibiae increases cortical bone formation and modifies trabecular organization: a new model to study cortical and cancellous compartments in a single loaded element. Bone 37, 810–818. https://doi.org/10.1016/j.bone.2005.07.022 (2005).
Clarke, K. A. & Still, J. Gait analysis in the mouse. Physiol. Behav. 66, 723–729. https://doi.org/10.1016/s0031-9384(98)00343-6 (1999).
Carriero, A. et al. Spatial relationship between bone formation and mechanical stimulus within cortical bone: combining 3D fluorochrome mapping and poroelastic finite element modelling. Bone Rep. 8, 72–80. https://doi.org/10.1016/j.bonr.2018.02.003 (2018).
Javaheri, B. et al. Transient peak-strain matching partially recovers the age-impaired mechanoadaptive cortical bone response. Sci. Rep. 8, 6636. https://doi.org/10.1038/s41598-018-25084-6 (2018).
Yang, H., Embry, R. E. & Main, R. P. Effects of loading duration and short rest insertion on cancellous and cortical bone adaptation in the mouse tibia. PLoS ONE 12, e0169519. https://doi.org/10.1371/journal.pone.0169519 (2017).
Main, R. P., Lynch, M. E. & van der Meulen, M. C. Load-induced changes in bone stiffness and cancellous and cortical bone mass following tibial compression diminish with age in female mice. J. Exp. Biol. 217, 1775–1783. https://doi.org/10.1242/jeb.085522 (2014).
Weatherholt, A. M., Fuchs, R. K. & Warden, S. J. Cortical and trabecular bone adaptation to incremental load magnitudes using the mouse tibial axial compression loading model. Bone 52, 372–379. https://doi.org/10.1016/j.bone.2012.10.026 (2013).
Birkhold, A. et al. The influence of age on adaptive bone formation and bone resorption. Biomaterials 35, 9290–9301. https://doi.org/10.1016/j.biomaterials.2014.07.051 (2014).
Birkhold, A. I. et al. Monitoring in vivo (re)modeling: a computational approach using 4D microCT data to quantify bone surface movements. Bone 75, 210–221. https://doi.org/10.1016/j.bone.2015.02.027 (2015).
Birkhold, A. I. et al. The periosteal bone surface is less mechano-responsive than the endocortical. Sci. Rep. 6, 23480. https://doi.org/10.1038/srep23480 (2016).
van Gaalen, S. M. et al. Use of fluorochrome labels in in vivo bone tissue engineering research. Tissue Eng. Part B Rev. 16, 209–217. https://doi.org/10.1089/ten.TEB.2009.0503 (2010).
Foldes, J., Shih, M. S. & Parfitt, A. M. Frequency distributions of tetracycline-based measurements: implications for the interpretation of bone formation indices in the absence of double-labeled surfaces. J. Bone Miner. Res. 5, 1063–1067. https://doi.org/10.1002/jbmr.5650051010 (1990).
Lin, C. et al. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J. Bone Miner. Res. 24, 1651–1661. https://doi.org/10.1359/jbmr.090411 (2009).
Chermside-Scabbo, C. J. et al. Old mice have less transcriptional activation but similar periosteal cell proliferation compared to young-adult mice in response to in vivo mechanical loading. J. Bone Miner. Res. 35, 1751–1764. https://doi.org/10.1002/jbmr.4031 (2020).
Bouchard, A. et al. in American Society for Bone and Mineral Research. 34: 213.
Turner, C. H., Forwood, M. R., Rho, J. Y. & Yoshikawa, T. Mechanical loading thresholds for lamellar and woven bone formation. J. Bone Miner. Res. 9, 87–97. https://doi.org/10.1002/jbmr.5650090113 (1994).
Rubin, C. T. & Lanyon, L. E. Regulation of bone mass by mechanical strain magnitude. Calcif. Tissue Int. 37, 411–417. https://doi.org/10.1007/bf02553711 (1985).
Acknowledgements
The authors would like to thank Anne Seliger for helping with the animal experiments, Mario Thiele for assistance with the microCT imaging, Marie-Hélène Gaumond for help with qPCR experiments, and Mark Lepik for help with the figures. This study was partially supported by the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung: TP5/DIMEOs) and National Natural Science Foundation of China (11702008), Shriners Hospitals for Children, Canadian Institutes of Health Research (Grant PJT-165939), and the Fonds de recherche du Québec en Santé de Programme de bourses de chercheur.
Author information
Authors and Affiliations
Contributions
B.W. conceived and designed the study. I.K. and M.K. provided the initial breeding pairs of the Sost KO mice. A.B., T.T. and C.F. managed the mouse colony and performed genotyping. A.B., H.Y., T.T., B.W., L.A., S.C., C.J. performed data collection and analysis. H.Y., A.B., and B.W. performed the statistical analysis. H.Y., A.B., L.A., C.J., T.T., C.F., I.K., M.K., G.D., S.C. and B.W. interpreted the data. H.Y., A.B., L.A., and B.W. drafted the manuscript and all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, H., Büttner, A., Albiol, L. et al. Cortical bone adaptation to a moderate level of mechanical loading in male Sost deficient mice. Sci Rep 10, 22299 (2020). https://doi.org/10.1038/s41598-020-79098-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-79098-0
This article is cited by
-
Using Finite Element Modeling in Bone Mechanoadaptation
Current Osteoporosis Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.