Abstract
Since patients often experience pain and unpleasantness during a colonoscopy, the present study aimed to evaluate the efficacy and safety of sublingually administered fentanyl tablets for pain treatment. Furthermore, since the use of intravenous drugs significantly increases colonoscopy costs, sublingual tablets could be a cost-effective alternative to intravenous sedation. We conducted a prospective placebo-controlled randomized study of 158 patients to evaluate the analgesic effect of a 100 µg dose of sublingual fentanyl administered before a colonoscopy. Pain, sedation, nausea, and satisfaction were assessed during the colonoscopy by the patients as well as the endoscopists and nurses. Respiratory rate and peripheral arteriolar oxygen saturation were monitored throughout the procedure. There were no differences between the fentanyl and placebo groups in any of the measured variables. The median pain intensity values, as measured using a numerical rating scale, were 4.5 in the fentanyl group and 5 in the placebo group. The sedation and oxygen saturation levels and the respiratory rate did not differ between the groups. The majority of the colonoscopies were completed.Our results indicate that a 100 µg dose of sublingual fentanyl is not beneficial compared to the placebo in the treatment of procedural pain during a colonoscopy.
Similar content being viewed by others
Introduction
A colonoscopy is an invasive procedure that can cause pain and discomfort in patients. Various drugs are used to prevent and treat discomfort during a colonoscopy, but the optimal drugs and dosing regimens are still unknown. Patients want to have a painless colonoscopy1, which can be achieved with analgesics and/or sedatives. Even though a colonoscopy can be successfully performed with drugs that do not alter the patient’s level of consciousness or cause nociception2,3,4, the use of intravenous sedation or analgesia has become the standard practice in many countries5,6.
Most patients find procedural sedation effective, but it may increase the risks and costs of the procedure7,8. Repeated dosing of sedatives can cause prolonged sedation, hypoxia, and respiratory depression by decreasing the patient’s respiratory response to carbon dioxide9. Since oxygenation monitoring is required in such cases, patient follow-up is essential, and intravenously sedated patients cannot be discharged immediately after the colonoscopy.
Typically, intravenous midazolam is used to induce sedation for a colonoscopy. However, it has been shown that low-dose midazolam neither relieves discomfort nor produces amnesia in patients10. A recent meta-analysis suggested that propofol is superior to other sedative agents; recovery and discharge times were shorter and patient satisfaction scores were greater than with benzodiazepines11. However, sedation with propofol is associated with longer recovery times than sedation with midazolam and fentanyl, especially in the elderly12. Meanwhile, previous studies have interestingly demonstrated that patient comfort appears to be unrelated to the use of sedatives or analgesics10,13. Obviously, further studies are needed on procedural sedation during colonoscopy.
Although many patients report pain during a colonoscopy, only a few studies have focused on the use of opioid analgesics during this procedure14,15,16. Fentanyl is rather short acting compared to other strong opioids (e.g., sufentanil) that are commonly used during the intraoperative period. Transmucosal sublingual fentanyl has been developed to improve the management of breakthrough pain in cancer patients. Achieving analgesia using a transmucosal tablet during colonoscopy is a novel approach, and a recent study reported promising results that indicated that oral transmucosal fentanyl is a safe and effective premedication in patients undergoing surgery under general anesthesia17. Similarly, low doses of intravenous fentanyl seem to be effective in achieving a satisfactory level of comfort during a colonoscopy14. Sublingual administration is an easy and non-invasive method of fentanyl administration prior to a colonoscopy. Furthermore, a single dose of sublingual fentanyl does not require follow-up after the procedure. The only limitation is that the patient is not allowed to drive a vehicle after the administration of strong opioids.
The purpose of the present study was to compare the efficacy and safety of sublingually administered fentanyl and a placebo in patients undergoing a colonoscopy. We hypothesized that sublingual fentanyl would provide sufficient analgesia and increase patient satisfaction during the colonoscopy.
Methods
Study design
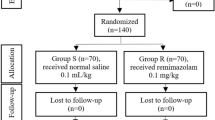
In this randomized, double-blind, and placebo-controlled study, 158 patients undergoing a diagnostic or therapeutic colonoscopy were randomly given 100 µg dose of sublingual fentanyl (Abstral 100 µg, ProStrakan) or the placebo before the procedure. The dose was based on a small pilot study of 35 patients prior to the initiation of the current study. In the pilot study, a 100 µg dose of sublingual fentanyl appeared to be a useful alternative to analgesia and sedation during an endoscopic procedure. The patients were randomized into two groups using the sealed envelope technique, and all patients, investigators, and staff members involved in the study were blinded to the treatment assignment.
Ethical considerations
This study was approved by the institutional review board (IRB) of the Hospital District of Southwest Finland (IRB number: 140/2011). The trial was registered before patient enrollment at clinicaltrials.gov (NCT01604187, Principal investigator: M.F., Date of registration: 22/05/2012) and in the EudraCT database (2011-005688-26, Principal investigator: M.F., Date of registration: 21/11/2011). All research was performed in accordance with relevant guidelines and regulations. Written informed consent was obtained from each patient.
Subject eligibility
Male and female patients between 18 and 80 years old who were scheduled for a routine diagnostic or therapeutic colonoscopy and had an American Society of Anesthesiologists (ASA) physical status classification of I–III, a body mass index (BMI) < 35, and a weight of more than 50 kg were deemed eligible for the present study. Patients who had a previous gastrointestinal surgery, sleep apnea, chronic obstructive pulmonary disease, or SpO2 < 90%, who were pregnant or nursing, or who were undergoing concomitant drug therapy known to cause significant enzyme induction or CYP3A4 inhibition were excluded from the study. Additional exclusion criteria were a history of intolerance to fentanyl or related compounds; a history of or current alcoholism or drug abuse; and a history of and psychiatric, psychological, or other emotional problems that were likely to invalidate informed consent.
Study procedures
Consecutive ambulatory colonoscopy patients were randomized to receive a sublingual fentanyl tablet (Abstral 100 µg, ProStrakan) or a matching placebo tablet 10 min before the procedure. The patients were instructed not to swallow the tablet but to allow it to completely dissolve in the sublingual cavity without chewing or sucking. The patients were not allowed to eat or drink anything until the sublingual tablet was completely dissolved. The patients did not receive any other sedative or analgesic drug during the colonoscopy.
The patients assessed their average pain intensity using a numerical rating scale (NRS 0–10) during and at the end of the colonoscopy. Adverse opioid effects and subjective effects were recorded at the end of the colonoscopy. The following adverse opioid effects were evaluated using NRS (0–10): drowsiness (alert/very drowsy), pleasantness (very unpleasant/very pleasant feeling), and nausea/vomiting (no nausea/very strong nausea). At the end of the colonoscopy, the endoscopists and the assisting nurses used NRS to evaluate whether the patient seemed to have pain or nausea and judged the level of sedation and the overall flow of the procedure. The endoscopists also estimated any technical difficulties associated with the colonoscopy. SpO2 and the respiratory rate were followed throughout the procedure. If SpO2 decreased below 90% or the respiratory rate decreased below eight per minute, additional oxygen was given. In the case of excessive opioid effects, 0.1 mg of intravenous naloxone was given.
The patients scored their overall satisfaction with the procedure prior to discharge. In addition, they were interviewed by telephone one day after the procedure. At this time, they assessed their anxiety before the procedure using a scale from 1 to 4 (1 = no anxiety, 4 = maximal anxiety) and evaluated how well they could remember events during and after the colonoscopy using a scale from 1 to 4 (1 = remember everything, 4 = cannot remember anything). Their level of abdominal pain was assessed using a scale from 1 to 4 (1 = no pain, 4 = a lot of pain). The patients were also asked whether they had any adverse effects during the day after the procedure, such as abnormal tiredness, nausea, or dizziness; they evaluated these effects using a scale from 1 to 4 (1 = no drowsiness/nausea/dizziness, 4 = a large amount of drowsiness/nausea/dizziness). Finally, patients assessed the unpleasantness of the colonoscopy from 1 to 4 (1 = not at all, 4 = very unpleasant).
Statistics
Based on previous study18, it was calculated that 87 patients would be needed per group to demonstrate a 30% decrease in the worst experienced pain at a level of significance 0.05 and power of 90%. The standard deviation of the worst experienced pain was assumed to be 50% of the mean pain score in the placebo group. Because of possible dropouts, 100 patients were planned to be recruited to both groups. However, because it was unexpectedly difficult to recruit suitable patients, we decided to settle for a smaller power. We estimated that we needed 73 patients each in the fentanyl and placebo groups to demonstrate, using NRS, a 25% difference in the worst experienced pain at a significance level of 0.05 and a power of 85%. Because of possible dropouts, 75 patients were planned to be recruited to both groups. The primary efficacy analysis was summarized descriptively for overall success evaluated by the patients, endoscopists and nurses and by treatment group. We performed the chi-square test or the Fisher’s exact test where appropriate and used the analysis of variance model with treatment as the main effect for overall and pairwise comparisons.
Results
A total of 158 patients at Turku University Hospital were recruited between April 2012 and December 2018. Fourteen patients were excluded from the study because of missing data. As such, there were 72 patients in each group. Descriptive data are summarized in Table 1.
There were no differences in pain intensity or degree of sedation between the fentanyl and placebo groups. The maximum pain experienced by patients was 7 [4.75–8] in the fentanyl group and 85,6,7,8,9 in the placebo group, respectively. The fentanyl group had a median pain intensity value of 4.5, and the placebo group had a median pain intensity value of 5. The female patients had a median pain intensity value of 5 in both groups, and the male patients of 3 and 4 in the fentanyl and placebo groups, respectively. There was no statistical difference between the groups in male and female patients (p = 0.124). Both groups had a median self-reported degree of sedation of 0. The results are summarized in Table 2.
Three procedures (one in the fentanyl group and two in the placebo group) were interrupted due to excessive pain. Both groups had a median time interval between the end of the colonoscopy and hospital discharge of 25 min (fentanyl group: range = 9–60 min, placebo group: range = 5–90 min) (Table 1).
Oxygen saturation was in the normal range in both groups, and there were no desaturation events. Respiratory rates were above eight per minute, and naloxone was not needed in any of the patients. Post-procedural interviews the day after the colonoscopy showed no differences in patient experience reminiscence between the fentanyl and placebo groups (Table 3).
Discussion
The study findings indicate that a 100 µg dose of sublingual fentanyl did not affect colonoscopy-related pain intensity compared to the placebo. The sublingual fentanyl did not cause significant adverse effects or sedation, and patient hospital stays were not prolonged. Although patients reported moderate pain, nearly all of the procedures were completed successfully. The fentanyl did not have a significant effect on the satisfaction of the endoscopists. Finally, there were no differences between the fentanyl and placebo groups on the day after the procedure when the patients were asked to assess their anxiety level before the procedure.
The primary aim of this study was to evaluate the efficacy of a single sublingual dose of fentanyl in relieving pain during a colonoscopy. Sublingual administration was chosen to avoid the need for intravenous access. Although Singh et al. showed that a 200 µg dose of transmucosal fentanyl as a premedication in surgical patients undergoing general anesthesia caused effective anxiolysis with minimal adverse effects17, we found no difference between the fentanyl and placebo groups when pain during the colonoscopy was assessed using NRS. Similarly, a previous study evaluating the effect of a 200 µg dose of sublingual fentanyl as a premedication before bone marrow aspiration and biopsy19 found the fentanyl to be inadequate in relieving patients’ pain.
In the present study, the median NRS pain values were 4.5 in the fentanyl group and 5 in the placebo group, which is commonly considered moderate pain. In a study by Lazaraki et al., patients received a small dose of intravenous fentanyl or midazolam, and the mean pain scores (on a scale of 0–10) were 2.59 in the fentanyl group and 4.43 in the midazolam group14. Meanwhile, when the effects of administering 1,000 mg of intravenous paracetamol and 0.5–1 µg/kg of intravenous fentanyl to colonoscopy patients were compared, the mean pain assessments were 4.00 and 3.77, respectively20. Thus, in our study, the median pain scores were similar to previous findings.
The bioavailability of sublingual fentanyl is approximately 70%, and a 100 µg dose of sublingual fentanyl—as used in the present study—has been shown to be effective and safe in average-sized adults and in elderly patients with comorbidities21,22,23. Regarding the intravenous route, a single 36 µg dose of intravenous fentanyl has been shown to be sufficient prior to colonoscopy14. In the pilot study, the 100 µg dose of sublingual fentanyl did not delay home readiness following the endoscopic procedure. For safety and ethical reasons, we wanted to keep the dose of fentanyl relatively low, especially since most patients were opioid naïve. Furthermore, although, according to its summary of product characteristics, Abstral is contraindicated for opioid intolerant patients and the treatment of acute pain, we considered its off-label use in procedural sedation under the supervision of a senior anesthesiologist trained to use fentanyl to be acceptable for the present study purposes.
Our results indicate that a single sublingual low-dose opioid is not an ideal premedication for patients having a colonoscopy. Furthermore, we hypothesize that pain is seldom the only limiting factor for success in a colonoscopy; discomfort and anxiety might also be of importance. Although pain is a subjective experience, we evaluated analgesia by recording self-reported pain ratings using NRS, which is a reliable and validated method for quantifying pain24. The patients in either group did not feel sedated, indicating that pain is probably the worst part of a colonoscopy and that pain, anxiety, and discomfort should be treated together.
Moderate or conscious sedation is safer and more cost efficient than deep sedation25. However, since there are very few studies in which analgesics alone have been used to treat pain during a colonoscopy, comparing sedation levels to those in other studies is difficult. Most studies used a combination of an opioid and midazolam to achieve sedation26,27. In the present study, the endoscopists and the assisting nurses assessed the patients’ sedation levels using NRS and determined the median level of sedation to be 0 in both groups. The median time between the end of the colonoscopy and hospital checkout was very short in both groups: 25 (range 5–90) minutes in the placebo group and 25 (range 9–60) minutes in the fentanyl group. To ensure the smooth operation of a colonoscopy unit, it is important that patients recover rapidly from sedation. The time required to reach home discharge readiness was found to be lower in patients who received only intravenous fentanyl for sedation than those who received only midazolam14. Routinely given conscious sedation does not benefit patients or make a colonoscopy technically easier4, and premedication or sedation should only be offered to very anxious patients.
Our study has limitations, one of which is the long recruitment period. The main reasons for this were patients’ unwillingness to undergo a colonoscopy without sedation and the proscription of driving a car after the administration of the study drugs. Another limitation was the timing of the fentanyl dose. Patients received the drug 10 min before the procedure, which coincides with the first detectable concentrations and reported onset of effect28. However, the peak concentration of sublingual fentanyl in the blood was reached 39.7 min after the 100 µg dose of sublingual fentanyl was administered. Since the median length of the colonoscopy in the current study was short (15 min in the placebo group and 20 min in the fentanyl group), the patients could have had better pain relief during the procedure if the time interval between the administration of the drug and the beginning of colonoscopy had been longer. Meanwhile, although the 100 µg dose of sublingual fentanyl was considered adequate based on previous reports14, the lack of difference in the efficacy of the fentanyl and the placebo is a strong indication that the dose of fentanyl was probably insufficient.
In conclusion, a single 100 µg dose of sublingual fentanyl as a monotherapy administered 10 min before the start of an endoscopic procedure was not found to be beneficial compared to the placebo in the treatment of procedural pain during a colonoscopy. Current ambulatory practice requires fast patient turnover, an efficient and short-acting analgesic, and the rapid discharge of patients. It remains to be elucidated whether a combination of fentanyl and oral benzodiazepine would be more effective, equally safe, and able to eliminate the need for deeper intravenous sedation during a colonoscopy.
References
Subramanian, S., Liangpunsakul, S. & Rex, D. K. Preprocedure patient values regarding sedation for colonoscopy. J. Clin. Gastroenterol. 39(6), 516–519 (2005).
Leung, F. W. Is there a place for sedationless colonoscopy?. J. Interv. Gastroenterol. 1(1), 19–22 (2011).
Leung, F. W. Methods of reducing discomfort during colonoscopy. Dig. Dis. Sci. 53(6), 1462–1467 (2008).
Ristikankare, M., Hartikainen, J., Heikkinen, M., Janatuinen, E. & Julkunen, R. Is routinely given conscious sedation of benefit during colonoscopy?. Gastrointest. Endosc. 49(5), 566–572 (1999).
Porostocky, P., Chiba, N., Colacino, P., Sadowski, D. & Singh, H. A survey of sedation practices for colonoscopy in Canada. Can. J. Gastroenterol. 25, 255–260 (2011).
Froehlich, F., Harris, J. K., Wietlisbach, V., Burnand, B., Vader, J. P., & Gonvers, J. J. Current sedation and monitoring practice for colonoscopy: an International Observational Study (EPAGE) Endoscopy. 38, 461–469 (2006).
Cooper, G. S., Kou, T. D. & Rex, D. K. Complications following colonoscopy with anesthesia assistance: a population-based analysis. JAMA Intern. Med. 173(7), 551–556 (2013).
Lin, O. S. Sedation for routine gastrointestinal endoscopic procedures: a review on efficacy, safety, efficiency, cost and satisfaction. Intest. Res. 15(4), 456–466 (2017).
Robin, C. & Trieger, N. Paradoxical reactions to benzodiazepines in intravenous sedation: a report of 2 cases and review of the literature. Anesth. Prog. 49(4), 128–132 (2002).
Elphick, D. A., Donnelly, M. T., Smith, K. S. & Riley, S. A. Factors associated with abdominal discomfort during colonoscopy: a prospective analysis. Eur. J. Gastroenterol. Hepatol. 21(9), 1076–1082 (2009).
Zhang, W., Zhu, Z. & Zheng, Y. Effect and safety of propofol for sedation during colonoscopy: a meta-analysis. J. Clin. Anesth. 51, 10–18 (2018).
Lovett, P., Gómez, V., Hodge, D. O. & Ladlie, B. Propofol versus midazolam/fentanyl sedation for colonoscopy in the elderly patient population. J. Perianesth. Nurs. 32(3), 210–214 (2017).
Ball, A. J., Rees, C. J., Corfe, B. M. & Riley, S. A. Sedation practice and comfort during colonoscopy: lessons learnt from a national screening programme. Eur. J. Gastroenterol. Hepatol. 27(6), 741–746 (2015).
Lazaraki, G. et al. Single use of fentanyl in colonoscopy is safe and effective and significantly shortens recovery time. Surg. Endosc. 21(9), 1631–1636 (2007).
DiPalma, J. A., Herrera, J. L., Weis, F. R., Dark-Mezick, D. L. & Brown, R. S. Alfentanil for conscious sedation during colonoscopy. South Med. J. 88(6), 630–634 (1995).
Deng, C. et al. Comparison of nalbuphine and sufentanil for colonoscopy: a randomized controlled trial. PLoS ONE 12(12), e0188901 (2017).
Singh, R. B., Choubey, S. & Mehra, R. Efficacy of oral transmucosal fentanyl citrate for premedication in patients for surgery under general anesthesia. Anesth. Essays Res. 11(4), 854–858 (2017).
Amer-Cuenca, J. J. et al. Pain relief by applying transcutaneous electrical nerve stimulation (TENS) during unsedated colonoscopy: a randomized double-blind placebo-controlled trial. Eur. J. Pain 15(1), 29–35 (2010).
Kuivalainen, A. M., Ebeling, F. & Rosenberg, P. H. Pre-medication with sublingual fentanyl did not relieve pain associated with bone marrow aspiration and biopsy: a randomized feasibility trial. Eur. J. Pain. 17(9), 1357–1364 (2013).
Ahmadi, A., Amri, P., Shokri, J. & Hajian, K. Comparison of the analgesic effect of intravenous paracetamol/midazolam and fentanyl in preparation of patients for colonoscopy: A double blind randomized clinical trial. Caspian J. Intern. Med. 6(2), 87–92 (2015).
Rauck, R. et al. Vetticaden S Pharmacokinetics and safety of fentanyl sublingual spray and fentanyl citrate intravenous: a single ascending dose study in opioid-naïve healthy volunteers. Curr. Med. Res. Opin. 33(11), 1915–1920 (2017).
Rauck, R. et al. Efficacy and safety of fentanyl sublingual spray for the treatment of breakthrough cancer pain: a randomized, double-blind, placebo-controlled study. Curr. Med. Res. Opin. 28(5), 859–870 (2012).
Price, D. D., Harkins, S. W., Rafii, A., & Price, C. A simultaneous comparison of fentanyl's analgesic effects on experimental and clinical pain. Pain 24, 197–203 (1986).
Ferreira-Valente, M. A., Pais-Ribeiro, J. L. & Jensen, M. P. Validity of four pain intensity rating scales. Pain 152(10), 2399–2404 (2011).
Lim, S., Lee, O. H., Yoon, I. J. & Choi, G. J. Kang H Moderate versus deep sedation in adults undergoing colonoscopy: systematic review and meta-analysis. Curr. Med. Res. Opin. 35(5), 879–885 (2019).
Holloway, A. M. & Logan, D. A. Pain relief for outpatient colonoscopy: a comparison of alfentanil with fentanyl. Anaesth. Intensive Care. 18(2), 210–213 (1990).
Usta, B. et al. Patient-controlled analgesia and sedation with alfentanyl versus fentanyl for colonoscopy: a randomized double blind study. J. Clin. Gastroenterol. 45(7), e72–e75 (2011).
Lennernäs, B., Hedner, T., Holmberg, M., Bredenberg, S. & Nyström, C. Lennernäs H Pharmacokinetics and tolerability of different doses of fentanyl following sublingual administration of a rapidly dissolving tablet to cancer patients: a new approach to treatment of incident pain. Br. J. Clin. Pharmacol. 59(2), 249–253 (2005).
Acknowledgements
We want to thank the skillful staff of the Department of Endoscopies at the Turku University Hospital for their assistance and participation in the practical implementation of the present study.
Funding
This work was supported by governmental research grant number 13821 from the Hospital District of Southwest Finland (T.I.S.). ProStrakan, the manufacturer of Abstral 100 µg, provided identical placebo tablets but played no other role in the study.
Author information
Authors and Affiliations
Contributions
The authors have been equally involved in composing and writing the manuscript. Pirita Varpe and Heikki Huhtinen performed the colonoscopies. Mari Fihlman and Emmi Karru gathered information from the assessment forms.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fihlman, M., Karru, E., Varpe, P. et al. Feasibility of a transmucosal sublingual fentanyl tablet as a procedural pain treatment in colonoscopy patients: a prospective placebo-controlled randomized study. Sci Rep 10, 20897 (2020). https://doi.org/10.1038/s41598-020-78002-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-78002-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.