Abstract
The role of weak acids with pH values in the range of 4–7 has been implicated in the symptoms of gastroesophageal reflux disease (GERD). Prostaglandin E2 (PGE2) is associated with heartburn symptom in GERD patients; however, the precise productive mechanisms remain unclear. In this study, we revealed that exposure to weak acids increases PGE2 production with a peak at pH 4–5, slightly in human normal oesophageal cells (Het-1A), and robustly in oesophageal squamous carcinoma cells (KYSE-270). Release of PGE2 from the oesophageal mucosa was augmented by weak acid treatment in rat. Chenodeoxycholic acid (CDCA), a bile acid, upregulated cyclooxygenase-2 (COX-2) expression in Het-1A and KYSE-270 and induced PGE2 production in KYSE-270 cells. Weak acid-induced PGE2 production was significantly inhibited by cytosolic phospholipase A2 (cPLA2), ERK, and transient receptor potential cation channel subfamily V member 4 (TRPV4), a pH-sensing ion channel, inhibitors. Hangeshashinto, a potent inhibitor of COX-2, strongly decreased weak acid- and CDCA-induced PGE2 levels in KYSE-270. These results indicated that weak acids induce PGE2 production via TRPV4/ERK/cPLA2 in oesophageal epithelial cells, suggesting a role in GERD symptoms like heartburn. Interventions targeting pH values up to 5 may be necessary for the treatment of GERD.
Similar content being viewed by others
Introduction
Gastroesophageal reflux disease (GERD) is an inflammatory disease of the upper gastrointestinal tract characterised by heartburn and acid regurgitation. Damaged oesophageal mucosa caused by acid reflux may develop into Barrett’s oesophagus, which is a major risk factor for oesophageal adenocarcinoma1,2. Since the extent of mucosal damage is proportional to the acid reflux time in patients with GERD3,4,5,6, reflux with pH values < 4 is defined as “acid reflux” and is used as an index for the diagnosis and treatment of GERD. Advanced techniques for the measurement of oesophageal pH have shown that reflux events with values other than pH < 4, such as weak acids (i.e., pH 4–7), are possible responsible substances related to various GERD symptoms7. Several studies have reported that weak acids contribute to the pathogenesis of GERD symptoms in patients with proton pump inhibitor (PPI)-refractory GERD, which are considered problematic among the majority of clinicians8,9,10,11,12. Despite recent evidence for the role of weak acid reflux, the degree of risk and mechanisms by which it leads to GERD-related symptoms remain unclear.
Prostaglandin E2 (PGE2), an inflammatory mediator, is involved in various inflammatory diseases. Cyclooxygenase 2 (COX-2), a rate-limiting enzyme in PGE2 production, is overexpressed in dysplastic lesions of the oesophagus. In GERD patients, nonsteroidal anti-inflammatory drugs effectively inhibit heartburn symptoms13,14. Indeed, increased PGE2 production by acid exposure has previously been reported in healthy volunteers15. However, although bile induces PGE2 production in oesophageal epithelial cells by enhancing COX-2 expression16,17,18, few studies have examined the mechanisms of acid-stimulated PGE2 production. Especially, detailed investigations of the relationship between pH and PGE2 production in oesophageal epithelial cells are lacking.
In this study, we used several types of oesophageal epithelial cells and an animal model to investigate extracellular pH-dependent PGE2 production and the underlying mechanisms, with a focus on weak acids in comparison with bile acid stimulation. We also examined the effect of a Kampo medicine, hangeshashinto (HST), which reportedly alleviates reflux-related symptoms in patients with PPI-refractory GERD19, on PGE2 production.
Results
PGE2 production in oesophageal epithelial cells induced by acidic conditions and bile acid
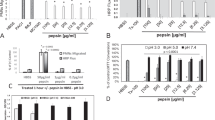
Human normal oesophageal epithelial cells, Het-1A cells, exhibited a slight increase in PGE2 production in response to pH 4.5 medium (P = 0.0031) but not acidic medium with other pH values or CDCA (200 or 400 μmol/L) (Fig. 1a). In the human oesophageal squamous cell carcinoma cell line KYSE-270, PGE2 production increased significantly after exposure to pH 4.5 (P = 0.00000014) and CDCA (CDCA [200 μmol/L]; P = 0.0090, CDCA [400 μmol/L]; P = 0.00000025) (Fig. 1b). PGE2 production increased with acidification as pH gradually decreased from 4.7 to pH 4.4 (Fig. 1c). Cell metabolic activity decreased in acidic medium at pH < 4.9, and cytotoxicity increased at pH values < 4.4 (Fig. 1c). In oesophageal adenocarcinoma cells, we observed significant but slight increase in PGE2 production by stimulation at pH 4.25–4.75 in KYAE-1 cells20, however no response was shown in FLO-1 cells (Supplementary Fig. S1).
Prostaglandin E2 (PGE2) production in oesophageal squamous cell carcinoma KYSE-270 cells and normal oesophageal epithelial Het-1A cells. (a) A slight increase in PGE2 production was observed in oesophageal epithelial Het-1A cells cultured in fresh medium (pH 7.2) for 6 h after treatment with pH 4.5 medium (n = 3) for 2 h but not with media with other pH values or Chenodeoxycholic acid (CDCA; 200 or 400 μmol/L) (n = 4). (b) In oesophageal epithelial cell carcinoma KYSE-270 cells, PGE2 production was significantly increased by culture in fresh medium with pH 7.2 for 6 h after treatment with pH 4.5 medium for 2 h and with CDCA (200 or 400 μmol/L) (n = 3). (c) In KYSE-270 cells, an increase in PGE2 production was observed in medium with pH values from 4 to 4.7, with an increase in cytotoxicity at pH < 4.4. Cell metabolic activity was decreased at pH < 4.9 (n = 3). Data are presented as means ± SD. Statistical significance was determined by Dunnett’s test; *P < .01, †P < .001, compared with vehicle (a,b) or pH 7.2 medium (c).
Weak acid treatment induces PGE2 production from rat oesophageal mucosa in vivo
Experiments were conducted using an animal model to examine whether weak acid also induces PGE2 production in vivo; we found that perfusion of a weak acid into the oesophageal lumen significantly increased PGE2 release in the perfusate of rats (Fig. 2a). After exposure to a weak acid, the oesophageal mucosa exhibited a marked increase in PGE2 production capacity compared with that in the PBS-treated group (Fig. 2b).
Weak acid induces PGE2 production in rat oesophageal mucosa. (a) PGE2 levels in the perfusate were increased by perfusing pH 4.5 solution to rat oesophagus at a rate of 500 μL/min for 1 h. (b) In oesophageal mucosa collected at 1 h after perfusion of a pH 4.5 solution, PGE2 production capacity significantly increased compared to that in the control group. Data are presented as means ± SD (n = 5). PGE2 prostaglandin E2. Statistical significance was determined by Student’s or Aspin-Welch’s t-test; *P < .05, †P < .001.
Involvement of cyclooxygenases in PGE2 production in oesophageal epithelial cells
KYSE-270 was used to investigate the mechanism by which PGE2 is produced by “weak acid stimulation” and pH 4.5 since there was a sufficient PGE2 production without prominent cell death. COX-2 mRNA levels in KYSE-270 cells were markedly higher than those in Het-1A cells, whereas cyclooxygenase-1 (COX-1) expression was slightly higher in KYSE-270 than in Het-1A (Fig. 3a). In a western blot analysis, COX-2 protein expression levels in KYSE-270 cells were also markedly higher than those in Het-1A cells (Fig. 3b). In KYSE-270 cells, the induction of PGE2 production by pH 4.5 medium and CDCA was inhibited by the selective COX-2 inhibitor NS-398 (Fig. 3c).
Basal expression levels of COX-1 and COX-2 in Het-1A and KYSE-270 cells. (a) The basal gene expression levels of cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) in KYSE-270 cells were higher than those in Het-1A cells. (b) The basal level of COX-2 protein expression was substantially higher in KYSE-270 cells than in Het-1A cells. The shown blots were cropped to improve the conciseness and the full-length blots are presented in Supplementary Fig. S6. (c) Treatment with the COX-2-selective inhibitor NS-398 (0.2 μmol/L) significantly inhibited PGE2 production in KYSE-270 cells treated with pH 4.5 medium or with 400 µmol/L CDCA. Data are presented as means ± SD (n = 3). PGE2 prostaglandin E2; CDCA chenodeoxycholic acid. Statistical significance was determined by Student’s or Aspin–Welch's t-test or Tukey–Kramer test; *P < .01, †P < .001.
Distinct mechanisms by which pH 4.5 and CDCA induce PGE2 production
In KYSE-270 cells, treatment with CDCA significantly increased COX-2 mRNA expression in a time-dependent manner (CDCA, [0 h]; P = 1.0, [3 h]; P = 0.00000014, [6 h]; P = 0.00000014), but treatment with pH 4.5 medium had no effect on COX-2 expression (pH 4.5, [0 h]; P = 1.0, [3 h]; P = 1.0, [6 h]; P = 0.98) (Fig. 4a). A similar phenomenon was observed in Het-1A cells (Fig. 4b). We then assessed the involvement of phospholipase A2 (PLA2), a synthetic enzyme of the cyclooxygenase substrate. The selective cytosolic PLA2 (cPLA2) inhibitor pyrrophenone inhibited PGE2 production induced by pH 4.5 medium (Pyrrophenone [0.2 μmol/L]; P = 0.98, Pyrrophenone [1 μmol/L]; P = 0.0029, Pyrrophenone [5 μmol/L]; P = 0.00065), but not by CDCA (Pyrrophenone [0.2 μmol/L]; P = 0.069, Pyrrophenone [1 μmol/L]; P = 0.99, Pyrrophenone [5 μmol/L]; P = 0.12), in KYSE-270 cells (Fig. 4c), suggesting the involvement of cPLA2 in pH 4.5-induced PGE2 production.
Different mechanisms underlying PGE2 production induced by pH 4.5 and CDCA. (a) COX-2 expression increased significantly in KYSE-270 cells cultured in fresh medium with pH 7.2 for the indicated time after treatment with CDCA (400 μmol/L) for 2 h but not with pH 4.5 medium. (b) COX-2 expression increased in fresh medium (pH 7.2) at 6 h after treatment with 400 μmol/L CDCA but not with pH 4.5 medium for 2 h in Het-1A cells. (c) Treatment with a cytosolic phospholipase A2 (cPLA2) inhibitor Pyrrophenone (Pyr; 0.2, 1, and 5 μmol/L) suppressed PGE2 production induced by pH 4.5 but not by CDCA (400 μmol/L) in KYSE-270 cells. PGE2, prostaglandin E2; CDCA, chenodeoxycholic acid; COX-2, cyclooxygenase-2. Data are presented as means ± SD (n = 3). Statistical significance was determined by Dunnett’s test or Tukey–Kramer test; *P < .01, †P < .001.
Involvement of MAPKs in PGE2 production induced by pH 4.5 medium and CDCA
Since MAPKs are involved in COX-2 expression and PLA2 activation21,22,23, we assessed their roles in PGE2 production to clarify the mechanisms underlying the observed effects of weak acids and bile acid. Among several MAPK inhibitors, we found that an ERK inhibitor (FR180204) prominently suppressed PGE2 production induced by pH 4.5 (FR180204 [0.2 μmol/L]; P = 0.00000020, FR180204 [01 μmol/L]; P = 0.00000020) but it had little effect on CDCA induced production (FR180204 [0.2 μmol/L]; P = 0.98, FR180204 [01 μmol/L]; P = 0.00044) (Fig. 5a). ERK phosphorylation level increase 5 min following treatment with pH 4.5 (Supplementary Fig. S2); simultaneously, cPLA2 phosphorylation levels were also increased (Fig. 5b). Moreover, ERK inhibitor (FR180204) suppressed cPLA2 phosphorylation under stimulation at pH 4.5 (Fig. 5c), indicating, at least in part, the involvement of ERK/cPLA2 in the PGE2 production by weak acid stimulation.
Different effects of pH 4.5 medium and CDCA on mitogen-activated protein kinase (MAPK) activation. (a) Treatment with the extracellular signal-regulated kinase (ERK) inhibitor FR180204 (FR; 0.2 and 1 μmol/L) inhibited PGE2 production induced by pH 4.5 medium but it had little or no effect on CDCA (400 μmol/L)-induced production in KYSE-270 cells. (b) ERK and cPLA2 phosphorylation were increased after pH 4.5 stimulation for 5 min. (c) pH 4.5-induced cPLA2 phosphorylation was inhibited by ERK inhibitor FR180204 (FR; 0.2 μmol/L) treatment. These shown blots were cropped to improve the conciseness and the full-length blots were presented in Supplementary Fig. S7. PGE2 prostaglandin E2; CDCA chenodeoxycholic acid; cPLA2 cytosolic phospholipase A2. Data are presented as means ± SD (n = 3). Statistical significance was determined by Tukey–Kramer test; *P < .001.
Extracellular acid-sensing mechanism in oesophageal epithelial cells
In addition to the activation of ERK, an increase in intracellular calcium is essential for cPLA2 activation24. We confirmed that intracellular calcium increased in response to weak acid but not CDCA (Supplementary Fig. S3). We also investigated the expression levels of ion channels associated with acid-sensing and the regulation of calcium influx in normal cells and carcinoma cells. We found that TRPV4 expression was substantially higher in KYSE-270 cells than in Het-1A cells (P = 0.0012) (Fig. 6a). Moreover, two TRPV4 inhibitors, RN-1734 and HC067047, suppressed PGE2 production induced by pH 4.5 (RN-1734 [0.2 μmol/L]; P = 0.12, RN-1734 [1 μmol/L]; P = 0.028, RN-1734 [5 μmol/L]; P = 0.00019) (HC067047 [2 μmol/L]; P = 0.16, HC067047 [10 μmol/L]; P = 0.0020, HC067047 [50 μmol/L], P = 0.00012) (Fig. 6b). ERK phosphorylation was slightly increased by TRPV4 agonist treatment (Supplementary Fig. S4), indicating the involvement of TRPV4/ERK/cPLA2 pathway in the weak acid-induced PGE2 production, at least in part.
Involvement of transient receptor potential vanilloid 4 (TRPV4) in pH 4.5-induced PGE2 production in KYSE-270 cells. (a) Expression of TRPV4 mRNA was higher in KYSE-270 cells than in Het-1A cells. (b) Treatment with the TRPV4 inhibitors RN-1734 (RN; 0.2, 1, 5 μmol/L) and HC067047 (HC; 2, 10, 50 μmol/L) significantly inhibited PGE2 production in KYSE-270 cells treated with pH 4.5 medium. PGE2 prostaglandin E2. Data are presented as means ± SD (n = 3). Statistical significance was determined by Student’s or Aspin–Welch's t-test or Tukey–Kramer test; *P < .05, †P < .01, ‡P < .001.
On the contrary, TRPV1 expression was lower in KYSE-270 cells than in Het-1A cells (Fig. 6a), while TRPV1 antagonist did not suppress PGE2 production induced by weak acid stimulation in KYSE-270 cells (Supplementary Fig. S5).
Effect of HST on PGE2 production by pH 4.5 medium and CDCA
In KYSE-270 cells, HST treatment inhibited both pH 4.5- and CDCA- induced PGE2 production (pH 4.5; HST [1 μg/mL]; P = 0.74, HST [10 μg/mL]; P = 0.17, HST [100 μg/mL]; P = 0.0029) (CDCA; HST [1 μg/mL]; P = 0.73, HST [10 μg/mL]; P = 0.07, HST [100 μg/mL]; P = 0.0000077) (Fig. 7a) but did not affect cell viability (Fig. 7b). To assess the effect of HST on PGE2 synthesis, we measured the PGE2 production induced by AA. In KYSE-270 cells, the increase in PGE2 production by the addition of AA was suppressed by HST in a dose-dependent manner (HST [1 μg/mL]; P = 0.96, HST [10 μg/mL]; P = 0.00000048, HST [100 μg/mL]; P = 0.00000033) and was also suppressed by a COX-2 inhibitor (NS-398) (Fig. 7c). AA-induced PGE2 production was minimally affected by HST (HST [1 μg/mL]; P = 0.95, HST [10 μg/mL]; P = 1.0, HST [100 μg/mL]; P = 0.95) and NS-398 in Het-1A cells (Fig. 7c).
Effects of Hangeshashinto (HST) on PGE2 production and viability in oesophageal epithelial cells. (a) Treatment with HST (1, 10, and 100 μg/mL) significantly inhibited PGE2 production in KYSE-270 cells treated with pH 4.5 medium or with 400 μmol/L CDCA. (b) HST (1, 10, and 100 μg/mL) had no effect on viability in KYSE-270 cells. (c) HST (1, 10, and 100 μg/mL) and NS-398 (0.2 μmol/L) inhibited PGE2 production induced by arachidonic acid (AA) supplementation (3 μmol/L, 15 min) in KYSE-270 cells but not in Het-1A cells. PGE2 prostaglandin E2; CDCA chenodeoxycholic acid. Data are presented as means ± SD (n = 3). Statistical significance was determined by Dunnett's or Tukey–Kramer test; *P < .01, †P < .001.
Discussion
The development of an excellent surgical animal model for GERD has contributed to the elucidation of the pathophysiological mechanisms underlying GERD25, but many unresolved issues remain. In particular, there has been little progress in research on the effect of reflux materials with a range of pH values on GERD symptoms owing to the limitations of animal models for such detailed investigations. In the present study, we demonstrated for the first time that PGE2 production increases in oesophageal epithelial cells in response to a narrow pH range of pH 4–5 via TRPV4/ERK/cPLA2 (Fig. 8). These results indicate that weak acids (i.e. pH 4–5) could contribute to GERD symptoms like heartburn via PGE2 production.
A graphical hypothesis of this study. Weak acid exposure at pH 4–5 induces PGE2 production via TRPV4/ERK/cPLA2. Under normal conditions, oesophageal epithelial cells produce only a small amount of PGE2 in response to weak acid stimulation, which may contribute to biological protection. Meanwhile, in cases of high expression of COX-2 due to stimulations, such as CDCA, weak acid exposure at pH 4–5 produces excess PGE2, which may trigger various symptoms including heartburn and oesophageal cancer development. HST is effective in treating various GERD symptoms due to excessive PGE2 production by suppressing COX-2-dependent production. PGE2 prostaglandin E2; TRPV4 transient receptor potential vanilloid 4; ERK extracellular signal-regulated kinase; cPLA2 cytosolic phospholipase A2; COX-2 cyclooxygenase-2; CDCA chenodeoxycholic acid; HST hangeshashinto.
PGE2 is involved in the induction of heartburn symptoms14,26. Interestingly, heartburn symptoms were most frequently reported when weak acid was refluxed, especially at pH 5, in patients with PPI-refractory GERD10,11. Moreover, the administration of weak acids (pH 4–5) induced heartburn symptoms in nearly 50% of patients with GERD symptoms27. Although increase in PGE2 production by oesophagus acid exposure is reported in healthy volunteers, the relation between PGE2 production and precise extracellular pH has not been fully investigated15. In the present study, we demonstrated that weak acids, at pH 4–5, significantly induced the production of PGE2 in human oesophageal squamous epithelial cell carcinoma (KYSE-270). Similar results were obtained in normal oesophageal epithelial squamous cells (Het-1A) and normal rat oesophageal mucosa (in vivo), suggesting a widely applicable phenomenon in oesophagus epithelial cells. Our results indicate that excessive PGE2 production by oesophageal epithelial cells induced by weak acids (pH 4–5) may explain heartburn symptoms observed in patients with PPI-refractory GERD. Moreover, we found that PGE2 production increased as pH decreased from 4.7, peaked at pH 4.4, and gradually reduced thereafter due to increased cytotoxicity in KYSE-270 cells. Until now, acid reflux in the oesophagus with pH values < 4 has been a focus of GERD diagnosis, and reducing the reflux time with pH < 4 has been considered important in PPI therapy28. However, our data suggest that careful attention should be paid not only to acid reflux with pH < 4 but also to weak acid reflux with pH 4–5. PGE2 is suggested to be involved in the exacerbation of various gastrointestinal cancers, including oesophageal cancer29,30. However, there are no reports regarding the possible involvement of weak acid reflux in oesophageal cancer. In this study, weak acid stimulation significantly induced PGE2 production in human oesophageal squamous epithelial cell carcinoma (KYSE-270) but not oesophagus adenocarcinoma cells (FLO-1 and KYAE-1). Although weak acid reflux may play a role in exacerbating oesophageal cancer through PGE2 production in the oesophageal mucosa, further in vivo investigations are required to verify the involvement of weak acids in oesophageal cancer.
In this study, we showed that PGE2 production in response to pH 4.5 is mediated by cPLA2 activation, since its inhibitor suppressed PGE2 production induced by a weak acid in KYSE-270 cells. ERK activation and calcium influx are considered important for cPLA2 activation24,31; thus, we also confirmed that intracellular calcium levels were increased by weak acidification of the medium, and weak acid-induced cPLA2 phosphorylation was suppressed by ERK inhibitor treatment. Furthermore, ERK phosphorylation was increased by TRPV4 agonist treatment. Our data indicated that TRPV4/ERK/cPLA2 are involved in weak acid-induced PGE2 production. Recently, not only acid reflux but also bile acid reflux has been attracting attention in GERD pathogenesis32. Interestingly, bile acid-induced PGE2 production was not affected by cPLA2 and ERK inhibitors despite the ERK activation, and bile acid stimulation had no effect on calcium influx. Bile acids strongly induce COX-2 expression and PGE2 production in oesophageal epithelial cells18, which is consistent with our results in KYSE-270 cells. In Het-1A cells, PGE2 production was not elevated by CDCA stimulation, although COX-2 expression was significantly increased. This might be attributed to the significantly lower basal and induced expression levels of COX-2 compared to those in KYSE-270 cells. Interestingly, pH 4.5 stimulation induced PGE2 production without the induction of COX-2 expression in both Het-1A cells and KYSE-270 cells, indicating that the mechanisms underlying PGE2 production differ between weak acid and bile acid stimulation.
Previous studies have shown that TRPV4 is involved in the regulation of intracellular calcium in oesophagus epithelial cells, although its physiological role remains unclear33,34. TRPV4 is reportedly activated by changes in not only temperature and osmotic pressure but also extracellular pH35. Particularly, TRPV4 begins to be activated below pH 6 and most potent activation were observed at around pH 436. In the present study, we showed that TRPV4 is more highly expressed in oesophageal epithelial squamous cell carcinoma cells (KYSE-270) than in normal oesophageal cells. Two kinds of TRPV4 inhibitors significantly suppressed PGE2 production induced by pH 4.5. We also confirmed that TRPV4 agonist increased ERK phosphorylation, which is essential for cPLA2 activation, suggesting a novel physiological role of TRPV4 in oesophageal epithelial cells. TRPV1, which may be a crucial factor involved in oesophageal hyperesthesia, plays a role in pH sensing of weak acid37,38. However, TRPV1 inhibitor did not suppress weak acid-induced PGE2 production in our study. The suppressive effect of the TRPV4 inhibitor on PGE2 production was limited and further investigations of other acid-sensing mechanisms are warranted.
HST, a Japanese traditional medicine (Kampo medicine), contains the extracts of seven medicinal herbs and has been approved by Japanese Ministry of Health, Labour and Welfare for clinical use39. HST is used for the treatment of inflammatory diarrhoea, gastritis, and heartburn and is effective for the treatment of stomatitis and diarrhoea via the reduction of PGE2 production40,41,42,43,44. Combined treatment with PPI and HST is effective for alleviating heartburn symptoms in patients with PPI-refractory GERD to the same extent as a double dose of PPI19. Furthermore, HST significantly inhibited carcinogenesis in a surgical rat reflux model45. In this study, we showed that HST suppressed both weak acid- and bile acid-induced PGE2 production in oesophagus epithelial cells. These findings suggest that oesophageal PGE2 suppression could relieve the clinical symptoms of PPI-refractory GERD in patients exhibiting weak acid reflux. HST suppresses PGE2 production by inhibiting COX-2 activity, not COX-1 activity, as confirmed using recombinant proteins46. Moreover, components of HST, especially ginger-derived, are reported to suppress COX-2 activity in several studies42,47,48. Additionally, we showed that HST prevented AA-induced PGE2 production only in KYSE-270 cells, but not in Het-1A cells with low COX-2 expression. The suppressive effect of the COX-2 inhibitor NS-398 on PGE2 production was stronger in KYSE-270 cells than in Het-1A cells, suggesting that HST supressed COX-2-dependent PGE2 production. Excessive PGE2 production is involved in pain and tumour progression, while a small amount of PGE2 derived from COX-1 is important in tissue repair and gastrointestinal mucosal protection30,49. Thus, the selective effect of HST on COX-2 may contribute to the alleviation of GERD without disrupting mucosal protection. In the future, we hope to further examine the safety of HST and to clarify the efficacy of HST in patients with GERD in greater detail.
In summary, we demonstrated that weak acid reflux induced PGE2 production by oesophagus epithelial cells via a unique mechanism, which may be involved in the pathogenesis of refractory GERD. Therefore, pH correction up to 5 in patients with GERD may prevent the heartburn symptoms.
Methods
Reagents
HST was obtained by spray-drying a hot water extract of a mixture of seven crude drugs: Pinellia tuber (Pinelliae tuber) 5.0 g, Scutellaria root (Scutellariae radix) 2.5 g, Glycyrrhiza (Glycyrrhizae radix) 2.5 g, Jujube (Zizyphi fructus) 2.5 g, Ginseng (Ginseng radix) 2.5 g, Processed ginger (Zingiberis processum rhizome) 2.5 g, and Coptis rhizome (Coptidis rhizome) 1.0 g. HST was suspended in phosphate-buffered saline (PBS) at 100 mg/mL and added to the culture medium at final concentrations of 1–100 µg/mL.
Cell culture
Human oesophageal squamous cell carcinoma KYSE-270 cells (ECACC, Salisbury, UK, RRID:CVCL 1350) were cultured in F12/RPMI-1640 medium (1:1) supplemented with 100 units/mL penicillin G and 0.1 mg/mL streptomycin containing 2% foetal bovine serum and 2 mmol/L glutamine at 37 °C and 5% CO2. Human normal oesophageal epithelial cells Het-1A (ATCC, Manassas, VA, USA, RRID: CVCL_3702) were cultured in Bronchial Epithelial Cell Growth Medium (BEGM) supplemented with 100 units/mL penicillin G and 0.1 mg/mL streptomycin at 37 °C and 5% CO2. Human oesophageal adenocarcinoma KYAE-1 cells (JCRB cell bank, Osaka, Japan, RRID: CVCL_1825) were cultured in F12/RPMI-1640 medium (1:1) supplemented with 100 units/mL penicillin G and 0.1 mg/mL streptomycin containing 5% foetal bovine serum and 2 mmol/L glutamine at 37 °C and 5% CO2. Human oesophageal adenocarcinoma FLO-1 cells (ECACC, Salisbury, UK, RRID: CVCL_2045) were cultured in DMEM supplemented with 100 units/mL penicillin G and 0.1 mg/mL streptomycin containing 10% foetal bovine serum and 2 mmol/L glutamine at 37 °C and 5% CO2.
Cell treatment
Cells were seeded in 96-well plates (Het-1A cells; 5.0 × 104/well, KYSE-270 cells; 2.5 × 104/well) or 24-well plate (Het-1A cells; 5.0 × 105/well, KYSE-270 cells; 3.0 × 105/well) or 12-well plates (KYSE-270 cells; 5.0 × 106/well) and incubated overnight. The cells were treated for 2 h in acidic culture conditions (pH 6.5 to pH 3.5) or with chenodeoxycholic acid (CDCA; 200 or 400 μmol/L) (Wako Chemical, Osaka, Japan). The cells were then washed and cultured in fresh pH 7.2 medium for an additional 6 h. HST (1, 10, and 100 μg/mL) or inhibitors [NS-398 (Wako Chemical), pyrrophenone (Merck & Co., Kenilworth, NJ, USA), FR180204 (Merck & Co.), RN-1734 (Wako Chemical), HC067047 (Wako Chemical), AMG9810 (Tocris Bioscience, Bristol, UK), A784168 (Tocris Bioscience), GSK1016790A (Sigma-Aldrich, St. Louis, MO, USA)] were added to both acidic and pH 7.2 culture medium for 8 h. Finally, the culture medium was collected to estimate PGE2 concentrations using the PGE2 Enzyme Immunoassay Kit (Cayman Chemical Co., Ann Arbor, MI, USA).
Measurement of weak acid-induced PGE2 production in rats (in vivo)
Animal experimental procedures were performed according to the Guidelines for the Care and Use of Laboratory Animals and approved by the Laboratory Animal Committee (permit no. 20–018) of Tsumura & Co. (Tokyo, Japan).
Six-week-old male Sprague–Dawley rats weighing 180–200 g were purchased from Charles River Laboratories Japan, Inc. (Kanagawa, Japan). All animals were housed in a room with controlled ambient temperature (23 ± 3 °C), humidity (50 ± 20%), and lighting (12 h light–dark cycle) conditions. The animals were provided with ad libitum water and a standard laboratory animal diet (MF; Oriental Yeast Co., Ltd., Tokyo, Japan).
Rats (n = 10) were anesthetized with urethane (Sigma-Aldrich) and α-chloralose (Wako Chemical) and injected with the analgesic agent Vetorphale (Meiji Seika Pharma, Tokyo, Japan). The oesophagus was orally cannulated with airway management, after which warmed weak acid solution (pH 4.5) or PBS was perfused at approximately 500 μL/min for 1 h using a perfusion pump. The perfusate was drained outside from the cannula inserted into the oesophagus and emerged from the stomach and collected for the last 5 min. The samples were concentrated using a Sep-Pak C18 cartridge (Waters, Milford, MA, USA) to assess PGE2 concentration.
To measure the capacity of PGE2 production by the rat oesophageal mucosa, rats were euthanized after perfusion and the oesophageal mucosa was collected. The mucosa was divided into proximal, intermediate, and distal regions and incubated in F12/RPMI-1640 medium (1:1) for 2 h at 37 °C. PGE2 concentration in the medium was then measured; PGE2 production capacity was calculated by dividing the total amount of PGE2 in the medium by the amount of total protein in each mucosal tissue. The data were shown as the average values of the three regions (proximal, intermediate, and distal) per hour.
Measurement of PGE2 synthetic capacity using intact cells
Enzymatic activity related to PGE2 synthesis was determined by measuring the accumulation of PGE2 induced by arachidonic acid (AA; Wako Chemical) in the culture fluids. Briefly, Het-1A cells (5.0 × 104/well) and KYSE-270 cells (2.5 × 104/well) were cultured overnight in 96-well plates. The culture fluids were replaced with the same fresh medium containing HST or the COX-2 inhibitor NS-398 for 15 min, and AA was added to the culture medium at a final concentration of 3 μmol/L. PGE2 concentrations were measured as described above after further incubation for 15 min.
Gene expression analysis
To measure mRNA expression, real-time qRT-PCR with TaqMan technology (Applied Biosystems, Warrington, UK) was used. Cells were lysed in QIAzol Lysis Reagent (Qiagen, Valencia, CA, USA), and total RNA was isolated using an RNeasy Kit (Qiagen) according to the manufacturer’s instructions. The cDNA was prepared using a High-capacity RT Kit (Applied Biosystems). PCR was performed using the ABI Prism 7900 sequence detector (Applied Biosystems) with default parameters. Sample-to sample variation in RNA loading was controlled by comparison with ACTB or GAPDH. The primer/probe sets were as follows: COX-1 (Hs00377726_m1), COX-2 (Hs00153133_m1), TRPA1 (Hs00175798_m1), TRPV1 (Hs00218912_m1), TRPV4 (Hs01099348_m1), ASIC1 (Hs00952807_m1), ACTB (Hs01060665_g1), and GAPDH (Hs02786624_g1).
Cytotoxicity assay
Cytotoxicity was assayed using the LDH-Cytotoxic Test Kit (Wako Chemical). The accurate estimation of LDH activity in acidic conditions is challenging; accordingly, cytotoxicity was evaluated by examining the amount of LDH remaining in living cells. After stimulation, the cells were solubilised by cell lysis buffer (Cell Signaling Technology, Danvers, MA, USA) containing 1% Triton X-100, and the supernatant was used for the LDH assay after centrifugation. Cytotoxicity was calculated using the following formula for relative LDH activity: Cell death (%) = 100 × [(b − a)/b], where a = absorbance at 560 nm for the test sample, b = control.
Cell viability after HST treatment was evaluated by the amount of LDH released in the medium. As a positive control, cells were treated with 0.2% Tween 20 for 15 min and the medium was collected. Cell viability was calculated using the following formula for relative LDH activity: viability (%) = 100 × [(c − a)/(c − b)], where a = test sample, b = blank well, and c = positive control. Absorbance was measured using a microplate reader SpectraMax Plus 384 (Molecular Devices, San Jose, CA, USA).
Cell metabolic activity assay
Cell metabolic activity was evaluated using a Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan). After stimulation, 8 µL of CCK-8 reagent was added to 100 µL of culture medium, and the plates were incubated at 37 °C in an atmosphere of 5% CO2 for 1 h. Cell metabolic activity is presented as the change in absorbance at 450 nm, as determined using a microplate reader (SpectraMax Plus 384).
Immunoblotting analysis
Cells were lysed with a cell lysis buffer (Cell Signaling Technology) containing 1% Triton X-100, Protease Inhibitor Cocktail (Sigma-Aldrich), and Phosphatase Inhibitor Cocktail 3 (Sigma-Aldrich). After centrifugation at 10,000×g for 15 min, the supernatants were collected. The cell lysates were then fractionated by SDS–polyacrylamide gel electrophoresis and electroblotted onto PVDF membranes. The membranes were probed with primary antibodies and HRP-conjugated secondary antibodies. Protein was detected using the ECL system and analysed using a ChemiDoc system (Bio-Rad Laboratories, Hercules, CA, USA). The following primary antibodies were used: anti-phospho-ERK (Thr202/tyr204; #4377, RRID: AB_331775), anti-ERK (#4695, RRID: AB_390779), anti-COX2 (#4842, RRID: AB_2084968), anti-cPLA2 (#2832S, RRID: AB_2164442), anti-phospoh-cPLA2 (#2831S, RRID: AB_2164445) and anti-GAPDH (#2118, RRID: AB_561053), all from Cell Signaling Technology. HRP-conjugated anti-rabbit IgG (NA934; GE Healthcare, Chicago, IL, USA) was used as the secondary antibody.
Ca2+ measurements
KYSE-270 cells were loaded with 5 μM Fura-2-AM (Dojindo Laboratories) in HBSS buffer for 60 min at 37 °C. Fura-2 fluorescence intensity was measured using a fluorescence microplate reader (FlexStation 3, Molecular Devices, ex: 340 or 380 nm, em: 510 nm). Intracellular Ca2+ concentration was evaluated as the change in ratio of fluorescence intensity exited at 340 to that at 380 nm.
Statistical analyses
Data are reported as means ± standard deviation. Student’s or Aspin-Welch’s t-test was performed for two-group comparisons, and the Tukey–Kramer or Dunnett test for multiple-group comparisons. Statistical differences were analysed using StatLight (Yukms Co. Ltd., Kawasaki, Japan). P < 0.05 was considered statistically significant.
Data availability
All data generated or analysed during this study are included in this published article.
References
Bujanda, D. E. & Hachem, C. Barrett’s esophagus. Mol. Medl 115, 211–213 (2018).
Que, J., Garman, K. S., Souza, R. F. & Spechler, S. J. Pathogenesis and cells of origin of Barrett’s esophagus. Gastroenterology 157, 349–364 (2019).
Johnsson, F., Joelsson, B. & Isberg, P. E. Ambulatory 24 hour intraesophageal pH-monitoring in the diagnosis of gastroesophageal reflux disease. Gut 28, 1145–1150 (1987).
Kahrilas, P. J. & Quigley, E. M. Clinical esophageal pH recording: a technical review for practice guideline development. Gastroenterology 110, 1982–1996 (1996).
Sifrim, D., Castell, D., Dent, J. & Kahrilas, P. J. Gastro-oesophageal reflux monitoring: review and consensus report on detection and definitions of acid, non-acid, and gas reflux. Gut 53, 1024–1031 (2004).
Johnson, L. F. & Demeester, T. R. Twenty-four-hour pH monitoring of the distal esophagus. A quantitative measure of gastroesophageal reflux. Am. J. Gastroenterol. 62, 325–332 (1974).
Namasivayam, V., Arora, A. S. & Murray, J. A. Weakly acidic reflux. Dis. Esophagus 24, 56–62 (2011).
de Bortoli, N. et al. Between GERD and NERD: the relevance of weakly acidic reflux. Ann. N. Y. Acad. Sci. 1380, 218–229 (2016).
Zerbib, F., Duriez, A., Roman, S., Capdepont, M. & Mion, F. Determinants of gastro-oesophageal reflux perception in patients with persistent symptoms despite proton pump inhibitors. Gut 57, 156–160 (2008).
Abe, Y. et al. Influence of the pH value of refluxate and proximal extent on heartburn perception in patients with proton pump inhibitor-refractory non-erosive reflux disease. Digestion https://doi.org/10.1159/000500133 (2019).
de Bortoli, N. et al. Lower pH values of weakly acidic refluxes as determinants of heartburn perception in gastroesophageal reflux disease patients with normal esophageal acid exposure. Dis. Esophagus 29, 3–9 (2016).
Scarpellini, E. et al. Management of refractory typical GERD symptoms. Nat. Rev. Gastroenterol. Hepatol. 13, 281–294 (2016).
Jankowski, J. A. Z. et al. Esomeprazole and aspirin in Barrett’s oesophagus (AspECT): a randomised factorial trial. The Lancet 392, 400–408 (2018).
Kondo, T. et al. The nonsteroidal anti-inflammatory drug diclofenac reduces acid-induced heartburn symptoms in healthy volunteers. Clin. Gastroenterol. Hepatol. 13, 1249–1255 (2015).
Kondo, T. et al. Prostaglandin E(2) mediates acid-induced heartburn in healthy volunteers. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G568-573 (2013).
Soma, T. et al. Chenodeoxycholic acid stimulates the progression of human esophageal cancer cells: a possible mechanism of angiogenesis in patients with esophageal cancer. Int. J. Cancer 119, 771–782 (2006).
Looby, E. et al. Deoxycholate induces COX-2 expression via Erk1/2-, p38-MAPK and AP-1-dependent mechanisms in esophageal cancer cells. BMC Cancer 9, 190 (2009).
Zhang, F., Subbaramaiah, K., Altorki, N. & Dannenberg, A. J. Dihydroxy bile acids activate the transcription of cyclooxygenase-2. J. Biol. Chem. 273, 2424–2428 (1998).
Takeuchi, T. et al. Efficacy and safety of hangeshashinto for treatment of GERD refractory to proton pump inhibitors : Usual dose proton pump inhibitors plus hangeshashinto versus double-dose proton pump inhibitors: randomized, multicenter open label exploratory study. J. Gastroenterol. https://doi.org/10.1007/s00535-019-01588-4 (2019).
Kan, T. et al. Gene expression profiling in human esophageal cancers using cDNA microarray. Biochem. Biophys. Res. Commun. 286, 792–801 (2001).
Berenbaum, F., Humbert, L., Bereziat, G. & Thirion, S. Concomitant recruitment of ERK1/2 and p38 MAPK signalling pathway is required for activation of cytoplasmic phospholipase A2 via ATP in articular chondrocytes. J. Biol. Chem. 278, 13680–13687 (2003).
Hirabayashi, T., Murayama, T. & Shimizu, T. Regulatory mechanism and physiological role of cytosolic phospholipase A2. Biol. Pharm. Bull. 27, 1168–1173 (2004).
Chun, K. S. & Surh, Y. J. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem. Pharmacol. 68, 1089–1100 (2004).
Clark, J. D. et al. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell 65, 1043–1051 (1991).
Miyashita, T. et al. Impact of inflammation-metaplasia-adenocarcinoma sequence and prevention in surgical rat models. Digestion 87, 6–11 (2013).
Kondo, T. et al. A novel prostanoid EP1 receptor antagonist, ONO-8539, reduces acid-induced heartburn symptoms in healthy male volunteers: a randomized clinical trial. J. Gastroenterol. 52, 1081–1089 (2017).
Smith, J. L., Opekun, A. R., Larkai, E. & Graham, D. Y. Sensitivity of the esophageal mucosa to pH in gastroesophageal reflux disease. Gastroenterology 96, 683–689 (1989).
Fossmark, R., Brenna, E. & Waldum, H. L. pH 4.0. Scand. J. Gastroenterol. 42, 297–298 (2007).
Greenhough, A. et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 30, 377–386 (2009).
Nagaraju, G. P. & El-Rayes, B. F. Cyclooxygenase-2 in gastrointestinal malignancies. Cancer 125, 1221–1227 (2019).
Lin, L.-L. et al. cPLA2 is phosphorylated and activated by MAP kinase. Cell 72, 269–278 (1993).
Mermelstein, J., Chait Mermelstein, A. & Chait, M. M. Proton pump inhibitor-refractory gastroesophageal reflux disease: challenges and solutions. Clin. Exp. Gastroenterol. 11, 119–134 (2018).
Mihara, H., Boudaka, A., Sugiyama, T., Moriyama, Y. & Tominaga, M. Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophageal keratinocytes. J. Physiol. 589, 3471–3482 (2011).
Ueda, T., Shikano, M., Kamiya, T., Joh, T. & Ugawa, S. The TRPV4 channel is a novel regulator of intracellular Ca2+ in human esophageal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G138-147 (2011).
Glitsch, M. Mechano- and pH-sensing convergence on Ca(2+)-mobilising proteins: a recipe for cancer?. Cell Calcium 80, 38–45 (2019).
Suzuki, M., Mizuno, A., Kodaira, K. & Imai, M. Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 278, 22664–22668 (2003).
Guarino, M. P. et al. Increased TRPV1 gene expression in esophageal mucosa of patients with non-erosive and erosive reflux disease. Neurogastroenterol. Motil. 22, 746–751 (2010).
Wu, L. et al. PAR-2 activation enhances weak acid-induced ATP release through TRPV1 and ASIC sensitization in human esophageal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G695-702 (2015).
Uezono, Y. et al. A review of traditional Japanese medicines and their potential mechanism of action. Curr. Pharm. Des. 18, 4839–4853 (2012).
Nishikawa, K. et al. The clinical impact of Hangeshashinto (TJ-14) in the treatment of chemotherapy-induced oral mucositis in gastric cancer and colorectal cancer: Analyses of pooled data from two phase II randomized clinical trials (HANGESHA-G and HANGESHA-C). J. Cancer 9, 1725–1730 (2018).
Matsuda, C. et al. Double-blind, placebo-controlled, randomized phase II study of TJ-14 (Hangeshashinto) for infusional fluorinated-pyrimidine-based colorectal cancer chemotherapy-induced oral mucositis. Cancer Chemother. Pharmacol. 76, 97–103 (2015).
Kono, T. et al. Multitargeted effects of hangeshashinto for treatment of chemotherapy-induced oral mucositis on inducible prostaglandin E2 production in human oral keratinocytes. Integr. Cancer Ther. 13, 435–445 (2014).
Kono, T. et al. Topical application of hangeshashinto (TJ-14) in the treatment of chemotherapy-induced oral mucositis. World J. Oncol. 1, 232–235 (2010).
Kase, Y., Hayakawa, T., Ishige, A., Aburada, M. & Komatsu, Y. The effects of Hange-shashin-to on the content of prostaglandin E2 and water absorption in the large intestine of rats. Biol. Pharm. Bull. 20, 954–957 (1997).
Miyashita, T. et al. Preventive effect of oral hangeshashinto (TJ-14) on the development of reflux-induced esophageal cancer. Surgery https://doi.org/10.1016/j.surg.2018.02.003 (2018).
Kase, Y., Saitoh, K., Ishige, A. & Komatsu, Y. Mechanisms by which Hange-shashin-to reduces prostaglandin E2 levels. Biol. Pharm. Bull. 21, 1277–1281 (1998).
van Breemen, R. B., Tao, Y. & Li, W. Cyclooxygenase-2 inhibitors in ginger (Zingiber officinale). Fitoterapia 82, 38–43 (2011).
Tjendraputra, E., Tran, V. H., Liu-Brennan, D., Roufogalis, B. D. & Duke, C. C. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg. Chem. 29, 156–163 (2001).
Takeuchi, K. & Nagahama, K. Animal model of acid-reflux esophagitis: pathogenic roles of acid/pepsin, prostaglandins, and amino acids. Biomed. Res. Int. 2014, 532594 (2014).
Acknowledgements
The manuscript has been carefully reviewed by Mizukami Y. and Fichera A.
Author information
Authors and Affiliations
Contributions
D.S. and N.F. designed and performed the experiments. All authors interpreted the data. N.F., and T.K. contributed to the conception and design of the study. D.S., S.M., and N.F. drafted the manuscript. T.K. revised the manuscript and supervised. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
This work was supported by Tsumura & Co. D.S., S.M., and N.F. are employees of Tsumura & Co.; T.K. received grant support from Tsumura & Co.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sadatomi, D., Kono, T., Mogami, S. et al. Weak acids induce PGE2 production in human oesophageal cells: novel mechanisms underlying GERD symptoms. Sci Rep 10, 20775 (2020). https://doi.org/10.1038/s41598-020-77495-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-77495-z
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.