Abstract
Experimental evidence suggests a crucial role of the autonomic nervous system in whole body metabolism with major regulatory effects of the parasympathetic branch in postprandial adaptation. However, the relative contribution of this mechanism is still not fully clear in humans. We therefore compared the effects of transcutaneous auricular vagus nerve stimulation (taVNS, Cerbomed Nemos) with sham stimulation during an oral glucose tolerance test in a randomized, single-blind, cross-over design in 15 healthy lean men. Stimulation was performed for 150 min, 30 min before and during the entire oral glucose tolerance test with stimulation cycles of 30 s of on-phase and 30 s of off-phase and a 25 Hz impulse. Heart rate variability and plasma catecholamine levels were assessed as proxies of autonomic tone in the periphery. Neither analyzed heart rate variability parameters nor plasma catecholamine levels were significantly different between the two conditions. Plasma glucose, insulin sensitivity and insulin secretion were also comparable between conditions. Thus, the applied taVNS device or protocol was unable to achieve significant effects on autonomic innervation in peripheral organs. Accordingly, glucose metabolism remained unaltered. Therefore, alternative approaches are necessary to investigate the importance of the autonomic nervous system in postprandial human metabolism.
Similar content being viewed by others
Introduction
The autonomic nervous system modulates systemic metabolism through the innervation of peripheral organs1. This appears to be of special importance in the postprandial setting when rapid adaptations in various tissues are crucial for a healthy response to this metabolic challenge. While this is achieved predominantly via direct cellular action of postprandial factors like insulin, the autonomic nervous system appears to modulate and coordinate those effects2,3,4. More specifically, the parasympathetic nervous system is important for postprandial metabolism, as increased parasympathetic nerve activity leads to improved insulin sensitivity, insulin secretion and glucose tolerance2, 5, 6.
The major parasympathetic nerve, the vagus nerve innervates the pancreas, the hepatic portal system, and most of the gastrointestinal tract. Vagal efferents stimulate pre- and postprandial insulin secretion from the pancreas1, 7 as well as hepatic insulin sensitivity and insulin clearance8. As most of these results are derived from studies in rodents, the relative contribution of the autonomic nervous system for glucose metabolism in humans is still not fully understood.
Non-invasive transcutaneous auricular vagus nerve stimulation (taVNS) is an approach to stimulate the auricular branch of the vagus nerve through the outer ear in humans. TaVNS can potentially activate organs indirectly via vagal afferent or directly by activation of the vagal efferents from the ear to peripheral organs9.
TaVNS is successfully used for the therapy of drug-resistant epilepsy10.
TaVNS activates the vagal afferents and influences various brain functions11. Parasympathetic vagal afferent signals are integrated in the nucleus of the solitary tract and further ascend to hypothalamus12, 13 and striatum14. These brain regions also integrate and process central signals and in turn regulate the vagal efferent. Though, evidence about the effects of taVNS on the vagal efferent is sparse, taVNS may serve as an interesting non-invasive intervention that may influence vagal efferent activities.
A prior study suggested that taVNS over 15 min regulates cardiac branches of the vagal efferent. For example, heart rate variability (HRV), an indicator of the vagal efferent activity of the cardiac branches, was immediately changed by taVNS, in a direction that points to a shift from sympathetic to parasympathetic tone15. In line, Badran et al., reported decreased heart rate and attenuate heart rate rebound during taVNS compared to sham stimulation16.
Furthermore, 30 min of taVNS reduced gastric frequency, i.e. the rhythmic contractions of the stomach13.
In contrast, 14 min of taVNS did not modulate the autonomic tone to visceral organs up to 120 min post stimulation in a recent study17.
Thus, most previous studies indicated that taVNS affects vagal outflow to the periphery and might therefore be a useful tool to experimentally modulate autonomic regulations in the body.
We now aimed to study the effect of immediate modulation of parasympathetic tone by taVNS with Cerbomed NEMOS on whole-body metabolism during an oral glucose challenge and hypothesized improved insulin sensitivity and insulin secretion. Therefore, we designed a randomized, placebo-stimulation controlled, single blind, cross-over study to test effects of vagus nerve stimulation on systemic metabolism and energy expenditure.
Results
The clinical characteristics of the fifteen healthy men who were included in the current study are presented in Table 1. In a cross-over design they received taVNS versus sham stimulation (Fig. 1). Endocrine and metabolic results are shown in Fig. 2. Detailed results of the linear mixed model analyses can be found in Table 2.
(a) Overview of the course of the study. (b) Schematic overview of the experiment. Electrocardiogram (ECG) was continuously recorded from timepoint -15 min before starting transcutaneous vagus nerve stimulation (taVNS) until the end of oral glucose tolerance test (OGTT) at 150 min. taVNS and sham stimulation were performed from timepoint 0 to 150 min. Start of OGTT was 30 min after initiation of taVNS/sham stimulation with intake of 75 g glucose. The last blood sample of OGTT was taken 2 h thereafter at timepoint 150 min. Indirect calorimetry was assessed after completion of the OGTT starting at 160 min.
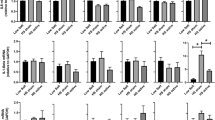
Endocrine and metabolic results. (A) Blood glucose, (B) serum insulin and (C) C-peptide increases during the oral glucose tolerance test (OGTT) that started at 0 min. Plasma adrenaline (D) and noradrenaline (E) decreased. However, transcutaneous vagus nerve stimulation (taVNS) did not significantly influence any of the hormone levels. Presented are means, error bars indicate standard errors.
Effects of taVNS on peripheral vagal activity
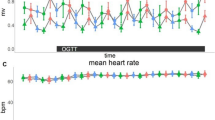
Mean heart rate decreased after the oral glucose tolerance test (OGTT) (main effect of time, p < 0.0001). Though, mean heart rate did not differ between taVNS and sham stimulation (main effect of condition, p = 0.88), nor was there a time-by-condition interaction (p = 0.22).
Likewise, root mean square of successive differences (RMSSD) as an indicator for vagal tone did not change over time (main effect of time, p = 0.15). Moreover, RMSSD did not differ between taVNS and sham conditions (main effect of condition, p = 0.39). However, there was a significant time-by-condition interaction (p = 0.025), but post hoc contrasts did not show significant differences between conditions at any time point (all pHolm > 0.1).
Low frequency to high frequency ratio (LF/HF) indicates sympathovagal balance, and higher LF/HF indicates dominance of sympathetic activity, and vice versa. We found that LF/HF increased after OGTT (main effect of time, p < 0.0001). However, LF/HF did not differ between conditions (main effect of condition, p = 0.86) and there was no time-by-condition interaction (p = 0.33).
As a second approach to address peripheral autonomic tone, we analyzed plasma catecholamines, the effectors of the sympathetic nervous system. Plasma noradrenaline levels decreased over time (p = 0.016). There was no significant time effect for adrenaline (p = 0.16). There was no statistically significant difference between taVNS and sham stimulation (adrenaline p = 0.20, noradrenaline p = 0.26) and no time-by-condition interaction was detected (adrenaline p = 0.73, noradrenaline p = 0.84).
Effects of taVNS on whole-body glucose metabolism
Glucose excursions during the OGTT where comparable between taVNS and sham stimulation (AUCglucose p = 0.1). Hence, glucose tolerance assessed as plasma glucose 2 h after initiation of OGTT, was not different between the two conditions (p = 0.4).
As readout for insulin secretion of the pancreatic beta cells, we analyzed serum insulin and C-peptide. In both conditions, there was no difference between serum insulin and C-peptide concentrations (main effect of condition, insulin p = 0.41, C-peptide p = 0.092).
Neither insulin sensitivity (ISI Matsuda p = 0.9; NEFA ISI p = 0.8) nor insulin secretion (Disposition index p = 0.2) was statistically significantly different between taVNS and sham condition.
Effects of taVNS on resting energy expenditure and post-load substrate oxidation
Resting energy expenditure was comparable between conditions (p = 0.9). In the postprandial condition 2 h after OGTT, the preferential energy source was glucose in both conditions as indicated by an RQ around 1.0 (p = 0.5).
Discussion
In the current study, we measured effects of a non-invasive vagus nerve stimulation on sympathetic and parasympathetic responses during an oral glucose tolerance test in healthy men. We found no significant influence of our stimulation approach on the tested parameters for autonomic balance. Accordingly, none of the analyzed glycemic traits were changed by the stimulation. Insulin sensitivity, insulin secretion, glucose tolerance and resting energy expenditure during the oral glucose tolerance tests did not differ between vagus stimulation and sham condition. TaVNS did not change sympathetic or parasympathetic tone to the heart as there were no detectable effects on heart rate variability. Finally, plasma catecholamines, the neurotransmitters of the sympathetic nervous system, were also unaffected by the stimulation. Thus, our data do not support major taVNS effects of taVNS on peripheral tissues.
However, taVNS is a well validated tool for vagal afferent stimulation that affects different brain functions11, 15, 18,19,20. These functions though were not able to modulate efferent outflows. Therefore, vagal afferent/efferent interaction appears to be unaffected by our current approach. How this interaction is regulated in detail is still not fully understood and should be further investigated in mechanistic studies at the molecular level.
One reason for the ineffectiveness of taVNS with the Nemos device could be that its vagal afferent stimulation in the auricular branch did not influence efferent activity towards the body.
Furthermore, an experiment in rodents suggested that a brain-gut communication occurs by directly stimulating the right vagus nerve14 whereas in our study stimulation electrodes were placed in the left ear due to safety concerns when stimulating the right ear in humans.
The stimulation electrode can be applicated in different regions at the outer ear (tragus, concha or cymba concha), however, it is not fully known which region and which ear is the most reliable for taVNS as the innervation of the auricular branch of the vagus nerve is still not fully clear21.
As the optimal stimulation frequency, intensity and duration for the intended effects are unknown, we might not have picked optimal parameters. Furthermore, potential technical issues as well as electrode size and fit could have limited potential effects.
An increased stimulation duration could possibly show an effect of taVNS on glucose metabolism as Huang et al. showed positive effects of taVNS on glucose metabolism during a period of 12 weeks in persons with metabolic syndrome22.
Clancy et al. reported increased heart rate variability in response to taVNS, indicating a shift from sympathetic to parasympathetic predominance in a much larger cohort compared to ours15. In addition, the stimulation protocol and trial setting of our current study was different from Clancy et al., who performed taVNS with another device, different stimulation protocol and included female and male participants whereas in our study only male persons were included. Their device stimulated for 15 min continuously15, whereas we used a repetitive sequence with 30 s of stimulation followed by 30 s pause for a longer period of time. Furthermore, no metabolic challenge was applied in the study of Clancy et al.15.
In contrast to Clancy’s positive results, Borges et al. could not detect any difference between transcutaneous auricular vagus nerve stimulation and sham stimulation on cardiac vagal activity in 61 healthy men23.
Thus, possible slight effects of the stimulation might have been masked by stronger effects of the metabolic alterations in our study.
Although vagus stimulation did not affect plasma catecholamine courses, there was a time effect of adrenaline and noradrenaline in both conditions. Adrenaline and noradrenaline levels were higher at time point 0 and declined during the OGTT. This could reflect the well-known shift from sympathetic towards parasympathetic tone in the postprandial state1, 24, 25. However, as we did not study catecholamines without glucose intake, we cannot exclude other potential contributors to this response.
In conclusion, auricular vagus nerve stimulation with the Nemos device had no major acute effects on the autonomic regulation of peripheral organs and did therefore neither alter insulin sensitivity, insulin secretion, resting energy expenditure nor sympathovagal balance. The physiological significance of the autonomic nervous system for glucose metabolism in humans must therefore be investigated with alternative approaches, e.g. either by biofeedback paradigmes or pharmacologically.
Methods
Fifteen male healthy volunteers at an age between 18 and 31 years were included (further details are provided in Table 1). Body mass index (BMI) was between 19.3 and 25.2 kg/m2, body fat content was between 9.5 and 22.5% as measured by bioelectrical impedance testing (BIA 101 by Akern Srl, Florence, Italy) and estimated with Cyprus 2.7 Body Composition Analysis Software (RJL Systems, Michigan, USA). Subject characteristics are shown in Table 1.
The sample size (n = 15) provides 80% power to detect effect size f = 0.35 when setting the alpha-level to 0.05 (calculated with Gpower 3.1).
The study protocol was approved by the ethics committee of the medical faculty of the University Tübingen. Written informed consent was obtained from all study participants and all research was performed in accordance with relevant guidelines/regulations. The study was pre-registered at clinicaltrials.gov (NCT03615209; 03/08/2018).
An overview of the course of the study is given in Fig. 1.
Non-invasive transcutaneous vagal stimulation was applied with the Cerbomed NEMOS device, an earpiece with titanium electrodes that is placed in the cymba conchae of the left external ear and in upside down position (ear lobe) for sham stimulation . The device stimulates the auricular branch of the nervus vagus with a mild electrical current with stimulation cycles of 30 s of on-phase and 30 s of off-phase and a 25 Hz impulse.
Auricular vagus nerve stimulation (and sham stimulation, respectively) was performed 30 min before and during the entire OGTT (150 min overall) in randomized cross-over design in the morning of two different days with 5 to 16 days washout period in a randomized single-blind design.
Stimulation procedure was done according to the protocol of Frangos et al.11. Stimulus intensity was adjusted by the participants from 0.1 mA in 0.1 mA increments until the person reported a “tingling” sensation, but no pain11. Stimulation intensity was 2.5 mA ± SD 0.9 in taVNS and 3.2 mA ± SD 1.5 in sham condition.
All persons underwent an oral glucose tolerance test. After an overnight fast, participants ingested a solution containing 75 g glucose over 5 min (Accu-Chek Dextrose OGT, Roche). Before, and 30, 60, 90 and 120 min after glucose ingestion, blood samples were obtained following standard procedures26. Oral glucose tolerance test was performed to address dynamic insulin secretion from the pancreatic beta cells and insulin sensitivity from plasma glucose, C-peptide and insulin responses after ingestion of glucose solution. OGTT is a frequently used tool to assess insulin secretion and insulin sensitivity and can furthermore assess glucose tolerance27.
HbA1c was tested at baseline, serum insulin levels, C-peptide, plasma glucose and free fatty acids were measured at all five timepoints. All measurements were performed in a routine diagnostic laboratory that is accredited with the German accredited body (DAkkS). Glucose values were measured directly using a bedside glucose analyzer (Biosen C-line, EKF-diagnostic GmbH, Barleben, Germany). Serum insulin and C-peptide levels were determined by an immunoassay with ADVIA Centaur XP Immunoassay System (Siemens Healthineers, Eschborn, Germany).
Plasma concentrations of total non-esterified fatty acids (NEFA) were measured with an enzymatic method (WAKO Chemicals, Neuss, Germany). HbA1c measurements were performed using Tosoh glycohemoglobin analyzer HLC-723G8 (Tosoh Bioscience, Tokyo, Japan).
Insulin sensitivity and insulin secretion was calculated from the OGTT as described previously28.
Plasma catecholamines adrenaline and noradrenaline were measured at baseline and 60 and 120 min after glucose ingestion. Catecholamines were analyzed with high performance liquid chromatography (HPLC) using a commercial kit (Kits No 5000, Chromsystems, Grafelfingen, Germany). Adrenaline and noradrenaline were isolated from plasma prior to chromatographic separation by solid phase extraction. After sample pre-treatment, an isocratic HPLC analysis was performed with a flow rate of 1 ml/min and a total run time of 20 min. The limit of detection (LOD) for adrenaline is 3 ng/dl, and for noradrenaline 10 ng/dl.
Electrocardiogram (ECG) recordings were performed to analyze heart rate variability as a surrogate parameter for autonomic nerve activation and parasympathetic projections to the heart. ECG was continuously recorded 15 min before starting the electric stimulation until the end of OGTT. HRV analysis was done in 10 min intervals in resting state, before the start of the stimulation and 5 min before blood extraction. ECG was recorded with Biopac MP 36 (Biopac Systems, Inc., Goleta, CA) and analyzed with Matlab (Mathworks, Inc. USA).
Standard ECG electrodes were attached to the chest wall. ECG was recorded at 2.5 kHz and transduced, amplified and filtered with a low pass filter at 25 Hz and a high pass filter at 1 Hz. The data were visually inspected for artifact correction in Artiifact29. Data with less than 2 min continuous measurement (uninterrupted by movement artifacts) were excluded. Inter-beat intervals calculated from visually inspected data were then processed in Artiifact to correct for ectopic peaks. Root mean square of successive differences (RMSSD) in inter-beat intervals was calculated in the time domain as an indicator of the vagal tone. High-frequency (0.15–0.50 Hz, HF) component and low-frequency (0.05–0.14 Hz, LF) was calculated in the frequency domain, and the low-frequency (LF/HF) ratio was calculated as an indicator of the vagal tone30, 31.
ECG was recorded for 5 min at baseline and every 15 min after glucose intake. HRV parameters collected for each 5 min time bins were then analyzed in mixed effect models.
Resting energy expenditure was assessed after completion of the OGTT and the taVNS stimulation. Energy expenditure after vagus nerve and sham stimulation was calculated by indirect calorimetry measurements with Vyntus CPX (Vyaire Medical, Illinois, USA). O2 consumption and CO2 production was measured for 15 min. To correct for monitor-specific deviations and eliminate the influence of inherent variability of the device on the measurement results, individual calibration control evaluation (ICcE) was applied32.
Statistical analysis was performed using SAS 9.4 (SAS Institute, Cary, NC). All results are presented as mean ± SD. p < 0.05 was considered statistically significant.
Measurements for major outcomes were subtracted from baseline measurements and boxcox-transformed if needed to fulfill the assumption of normally distributed residuals. Mixed effect models were performed on that data, with main effects of time, taVNS, and their interaction effects as fixed effects, the subjects with random intercepts, and the visit order as a dummy variable. Denominator degrees of freedom were estimated using the default method in SAS, which is a containment method. The variance–covariance structure providing the best fit was chosen based on the minimum value of Akaike’s Information Criterion (AIC). Significant interaction effects were followed by post hoc contrasts at each time point between conditions, with stepdown Bonferroni (Holm) correction for multiple testing. Metabolic results and the resting energy expenditure and respiratory quotient were analyzed using paired t tests between conditions.
Data availability
The data are not publicly available due to them containing information that could compromise research participant privacy/consent.
References
Teff, K. L. Visceral nerves: vagal and sympathetic innervation. JPEN J. Parenter. Enteral. Nutr. 32, 569–571 (2008).
Heni, M. et al. Central insulin administration improves whole-body insulin sensitivity via hypothalamus and parasympathetic outputs in men. Diabetes 63, 4083–4088 (2014).
Begg, D. P. & Woods, S. C. Interactions between the central nervous system and pancreatic islet secretions: a historical perspective. Adv. Physiol. Educ. 37, 53–60 (2013).
Ahrén, B. & Holst, J. J. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes 50, 1030–1038 (2001).
Lindmark, S., Wiklund, U., Bjerle, P. & Eriksson, J. W. Does the autonomic nervous system play a role in the development of insulin resistance? A study on heart rate variability in first-degree relatives of Type 2 diabetes patients and control subjects. Diabet. Med. 20, 399–405 (2003).
Perin, P. C., Maule, S. & Quadri, R. Sympathetic nervous system, diabetes, and hypertension. Clin. Exp. Hypertens. 23, 45–55 (2001).
Strubbe, J. H. & Steffens, A. B. Neural control of insulin secretion. Horm. Metab. Res. 25, 507–512 (1993).
Macedo, M. P. et al. Risk of postprandial insulin resistance: the liver/vagus rapport. Rev. Endocr. Metab. Disord. 15, 67–77 (2014).
Butt, M. F., Albusoda, A., Farmer, A. D. & Aziz, Q. The anatomical basis for transcutaneous auricular vagus nerve stimulation. J. Anat. 236, 588–611 (2020).
Stefan, H. et al. Transcutaneous vagus nerve stimulation (t-VNS) in pharmacoresistant epilepsies: a proof of concept trial. Epilepsia 53, e115-118 (2012).
Frangos, E., Ellrich, J. & Komisaruk, B. R. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. 8, 624–636 (2015).
Berthoud, H.-R. Vagal and hormonal gut–brain communication: from satiation to satisfaction. Neurogastroenterol. Motil. 20, 64–72 (2008).
Teckentrup, V. et al. Non-invasive stimulation of vagal afferents reduces gastric frequency. Brain Stimul. 13, 470–473 (2020).
Han, W. et al. A neural circuit for gut-induced reward. Cell 175, 665-678.e23 (2018).
Clancy, J. A. et al. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul. 7, 871–877 (2014).
Badran, B. W. et al. Short trains of transcutaneous auricular vagus nerve stimulation (taVNS) have parameter-specific effects on heart rate. Brain Stimul. 11, 699–708 (2018).
Gancheva, S. et al. Constant hepatic ATP concentrations during prolonged fasting and absence of effects of Cerbomed Nemos® on parasympathetic tone and hepatic energy metabolism. Mol. Metab. 7, 71–79 (2017).
Marshall, L., Mölle, M., Hallschmid, M. & Born, J. Transcranial direct current stimulation during sleep improves declarative memory. J. Neurosci. 24, 9985–9992 (2004).
Neuser, M. P. et al. Vagus nerve stimulation increases vigor to work for rewards. bioRxiv 789982 (2019). https://doi.org/10.1101/789982.
Dietrich, S. et al. A novel transcutaneous vagus nerve stimulation leads to brainstem and cerebral activations measured by functional MRI. Biomed. Tech. (Berl) 53, 104–111 (2008).
Badran, B. W. et al. Tragus or cymba conchae? Investigating the anatomical foundation of transcutaneous auricular vagus nerve stimulation (taVNS). Brain Stimul. 11, 947–948 (2018).
Huang, F. et al. Effect of transcutaneous auricular vagus nerve stimulation on impaired glucose tolerance: a pilot randomized study. BMC Complement. Altern. Med. 14, 203 (2014).
Borges, U., Laborde, S. & Raab, M. Influence of transcutaneous vagus nerve stimulation on cardiac vagal activity: not different from sham stimulation and no effect of stimulation intensity. PLoS ONE 14, e0223848 (2019).
Lu, C.-L., Zou, X., Orr, W. C. & Chen, J. D. Z. Postprandial changes of sympathovagal balance measured by heart rate variability. Dig. Dis. Sci. 44, 857–861 (1999).
Strubbe, J. H. Parasympathetic involvement in rapid meal-associated conditioned insulin secretion in the rat. Am. J. Physiol. 263, R615-618 (1992).
Pacini, G. & Mari, A. Methods for clinical assessment of insulin sensitivity and β-cell function. Best Pract. Res. Clin. Endocrinol. Metab. 17, 305–322 (2003).
Stumvoll, M. et al. Use of the oral glucose tolerance test to assess insulin release and insulin sensitivity. Diabetes Care 23, 295–301 (2000).
Wagner, R. et al. A novel insulin sensitivity index particularly suitable to measure insulin sensitivity during gestation. Acta Diabetol. 53, 1037–1044 (2016).
Kaufmann, T., Sütterlin, S., Schulz, S. M. & Vögele, C. ARTiiFACT: a tool for heart rate artifact processing and heart rate variability analysis. Behav. Res. Methods 43, 1161–1170 (2011).
Appelhans, B. M. & Luecken, L. J. Heart rate variability as an index of regulated emotional responding. Rev. Gen. Psychol. 10, 229–240 (2006).
Quintana, D. S. et al. Reduced heart rate variability in schizophrenia and bipolar disorder compared to healthy controls. Acta Psychiatr. Scand. 133, 44–52 (2016).
Schadewaldt, P., Nowotny, B., Strassburger, K., Kotzka, J. & Roden, M. Indirect calorimetry in humans: a postcalorimetric evaluation procedure for correction of metabolic monitor variability. Am. J. Clin. Nutr. 97, 763–773 (2013).
Acknowledgements
We thank all the research volunteers for their participation. We gratefully acknowledge the excellent technical assistance of A. Eberle, I. Wagener, E. Metzinger, D. Neuscheler, I. Fink and H. Runge. We appreciate the helpful discussions and comments by Dr. Nils Kroemer (University of Tübingen) during preparation of this manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study supported in parts by a Grant (01GI0925) from the Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD e.V.). We acknowledge support by the Deutsche Forschungsgemeinschaft and the Open Access Publishing Fund of the University of Tübingen.
Author information
Authors and Affiliations
Contributions
A.V. researched and analyzed data and drafted the manuscript. D.Z. analyzed data and contributed to discussion. L.F., R.L., K.K. and A.P. researched data and contributed to discussion. H.U.H., A.B., A.F., R.W., H.P., S.K. and D.M.S. contributed to discussion. M.H. contributed to analyses, supervised the project and contributed to discussion. All authors approved the final version of the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vosseler, A., Zhao, D., Fritsche, L. et al. No modulation of postprandial metabolism by transcutaneous auricular vagus nerve stimulation: a cross-over study in 15 healthy men. Sci Rep 10, 20466 (2020). https://doi.org/10.1038/s41598-020-77430-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-77430-2
This article is cited by
-
A systematic review of the effects of transcutaneous auricular vagus nerve stimulation on baroreflex sensitivity and heart rate variability in healthy subjects
Clinical Autonomic Research (2023)
-
Slow deep breathing modulates cardiac vagal activity but does not affect peripheral glucose metabolism in healthy men
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.