Abstract
Histological examination of the lymph nodes (LNs) is crucial to determine the colorectal cancer (CRC) stage. We previously reported a new fat-dissociation method (FM) to detect LNs from surgically resected mesentery. This study aimed to examine the effectiveness of FM compared with that of conventional palpation method (PM) in CRC. This single-center, open-label, randomized controlled study was performed at Osaka International Cancer Institute in Japan in 2014. Randomization was performed using a computer-generated permuted-block sequence. Patients were stratified by surgical procedures and the LN dissection area. The primary endpoint was the time required for LN identification. The secondary endpoint was the number of LNs and 5-year cancer-specific survival. The 130 enrolled patients were randomly assigned in a 1:1 ratio to the FM and the PM groups. LN identification times were 45 (range 15–80) and 15 (range 7–30) minutes in the PM and the FM groups, respectively (P < 0.001). In the PM group, body mass index and identification time were correlated (P = 0.047). The number of LN which could be examined pathologically was 16 (range 2–48) and 18 (range 4–95) in the PM and FM groups, respectively (P = 0.546). In right-sided CRC, the number of LNs was higher in the FM group than in the PM group (P = 0.031). Relapse-free survival rates and cancer-specific survival rates did not differ between the groups. In conclusion, FM reduced the time required for LN detection without reducing the number of detected LNs, making it is a useful method to detect LNs in surgical specimens.
Similar content being viewed by others
Introduction
Cancer is a leading cause of death worldwide, with approximately 17 million individuals dying of cancer annually1, and the incidence and mortality rates of cancer are still projected to increase. Colon, lung, breast, liver, and stomach cancers account for the majority of cancer-related deaths. Specifically, colorectal cancer (CRC) is the second most frequent cancer in Europe2 and the second most common cause of death in the US3. Also, in Asian countries, CRC is the leading cause of death with the number of cases identified continuing to increase4. In CRC treatment, surgical resection of the primary tumor and regional lymph nodes (LNs) is very important5, and TNM classification is determined by histological examination of a surgically resected specimen. Surgical specimens with proximal, distal, and circumferential margins, regional LNs, tumor classification, and vascular invasion are usually examined. The T factor depends on tumor invasion depth, while the N factor depends on the number of LN metastases. It is especially recommended to examine at least 12 LNs because the small number of LNs examined results in downstaging because of missing LN metastases6,7,8,9. Furthermore, the adjuvant chemotherapy regimen is defined based on the number of LN metastases10.
The detection method for LNs varies from country to country. In Japan, surgeons isolate LNs directly from the surgically resected specimen and then fix them in formalin. This procedure requires time and expertise for recognizing LNs from surgically resected specimens. In other countries, the surgical specimen is first fixed in formalin and then examined using the cross-section of the tissue or after fat clearance11,12 using Schwartz solution13 or GEWF solution14,15, or after the pathologist isolates the lymph nodes. Both solutions reduced the fat volume of the specimen to reveal LNs; however, these methods are time consuming and require from 1 to 9 days to complete. Therefore, improved methods are required for more efficient LN identification. Recently, we reported the importance of micro-metastasis as a valuable prognostic marker16. Concurrently, one-step nucleic acid amplification (OSNA) has been introduced into clinical use for the examination of micro-metastasis17,18. This method has the advantage of high precision of micrometastases, and has performed well in searching for micrometastases of sentinel lymph node in various tumors19,20,21. However, for the successful use of OSNA, it is necessary to isolate LNs directly from the specimen. The conventional method for LN isolation is the palpation method (PM). However, PM is very time consuming and depends on the experience of the physician, and in Japan, many of the physicians performing PM are surgeons. Because of the limitations associated with current methods, a universal method that is less time consuming and does not require extensive experience is needed. We previously reported a new effective method called the fat-dissociation method (FM) for LN detection22. This method enabled the dissociation of mesenteric fat and clear visualization of the LNs, making LN detection directly from the specimen easier. This new FM could be performed within 1 h, and it did not affect the histological examination.
The present study aimed to examine the clinical usefulness of FM with respect to LN detection time and the number of LNs. We hypothesized that the new FM is superior to the conventional PM method. Towards our goal, we evaluated the differences in time required for detecting LNs from surgically resected specimen and in the total number of detected LNs between the two methods.
Material and methods
Study design and participants
This was a single-center, open-label, randomized controlled study conducted at Osaka International Cancer Institute (OICI) in Japan from January to December 2014. The OICI ethics committee approved this study, and written informed consent was obtained from all patients. This study was performed in accordance to guidelines of Declaration of Helsinki. Patients who underwent surgery of primary CRC with dissection of LNs and aged 20 years or older were eligible. For rectal cancer, those whose tumor location was in the lower rectum below peritoneal reflection were excluded because given the several operative approaches23. Those whose tumor location was in the upper rectum above peritoneal reflection were included because the surgical procedure was anterior resection or low anterior resection. The enrolled patients were randomized in a 1:1 ratio for use of either the PM or the FM for detecting LNs from surgically resected specimen. Randomization was conducted using a computer-generated permuted-block sequence. The size of the blocks used for randomization was set to two. Patients were randomly assigned and balanced according to surgical procedure (ileocecal resection, right colectomy, transverse colectomy, left colectomy, sigmoid colectomy, anterior resection, and low anterior resection) and the dissection area of LNs (D2 or D3 dissection). The defined LN dissection was performed for each CRC location according to the Japanese Classification of Colorectal Carcinoma guidelines23.
Sample size
The sample size was calculated using R 3.1.3 software (CRAN, the R Foundation for Statistical Computing, Vienna, Austria). We examined 10 patients retrospectively prior to the start of this study and the time for LN detection was shorter by 30% in the FM group (data not shown). A sample size of 59 assessable patients in each group was required to achieve 90% power with 5% error (two-sided). A total of 118 patients was necessary in this study. From the number of the CRC surgery of our institute, we scheduled a 1-year study.
Outcomes and assessments
The primary efficacy endpoint was the time required to identify LNs. The secondary endpoint was the number of LNs and 5-year cancer-specific survival (CSS). After surgery, follow-up included assessments of serum concentrations of the tumor markers, carcinoembryonic antigen and carbohydrate antigen 19-9. Further examinations included imaging with abdominal ultrasonography, computed tomography, and/or positron emission tomography was conducted every 3–6 months and colonoscopy in accordance with Japanese guidelines8. Relapse-free survival (RFS) was defined as the length of time that the patients survived without any signs or symptoms of cancer after primary colorectal cancer surgery. CSS was defined as the length of time after primary colorectal cancer surgery that the patients survived.
PM and FM for LN detection
The clinical tissue samples were obtained by surgery of the primary CRC. The mesentery was carefully separated from the primary tumor lesion (Fig. 1). In the PM group, gastrointestinal surgeons isolated LNs by palpating the specimen manually. In the FM group, the mesenteric tissue was dissociated by using the fat-dissociation solution22. Next, 1 ml of the solution was added to 3 g of mesentery specimen. The samples were incubated at 40 °C between 30 and 60 min. Next, the mesentery was placed on a paper to absorb the dissociated fat solution, and then the LNs were isolated from the dissociated mesentery by gastrointestinal surgeons. Both procedures were performed by eight different gastrointestinal surgeons randomly. All isolated LNs were then treated with standard formalin for fixation and hematoxylin and eosin for staining. Several pathologists evaluated the LNs for diagnosis.
Fat-dissociation (FD) solution preparation
We previously reported the composition of FD solution as follows: 1 mg/ml collagenase (C6885; Sigma-Aldrich, St. Louis, MO, USA) and 0.25% trypsin (25200072; Life Technologies, Carlsbad, CA, USA) in Dulbecco’s phosphate-buffered saline (14190250; Life Technologies)22. Several additional conditions were examined as well (Supplementary Table S1). Finally, we modified the fat-dissociation solution as follows: 0.5 mg/ml collagenase (Sigma-Aldrich) and 5 mg/ml lipase (100817; MP Biomedicals, Santa Ana, CA, USA) in the normal saline buffer (Otsuka, Tokyo, Japan).
Statistical analysis
Data were expressed by categories and/or median value (range) for continuous variables. The clinical and surgical factors were compared between the PM and the FM groups using the Wilcoxon rank-sum and χ2 test. Kaplan–Meier survival curves were plotted and prognosis was compared by the generalized log-rank test. All data were analyzed with JMP software (version 13.0; SAS Institute, Cary, NC, USA). Differences with a P-value of < 0.05 were considered statistically significant.
Results
In total, 130 patients were registered and assessed between January and December 2014. The number of patients exceeded the planned sample size. Overall, 65 patients were included in the PM group, and another 65 patients were included in the FM group. No patient dropped out from this study for evaluation of the primary endpoint. There were 35 (54%) and 36 (55%) male patients in the PM and the FM groups, respectively. The median BMI was 21.0 (range 14.4–29.8) and 21.0 (range 14.6–28.3) kg/m2 in the PM and the FM groups, respectively. LN dissection was D2 (51%) and D3 (49%) in the PM group and D2 (48%) and D3 (52%) in the FM group. There were no significant differences in the patients’ characteristics between the two groups (Table 1). The mesentery after FM is shown in Fig. 2. Most fat was dissociated, and the vascular structure together with LNs were clearly visible. LNs were located around vessels, and the LN region was easily identified. The median searching time was 45 min (range 15–80) in the PM group and 15 min (range 7–30) in the FM group. The time required for LN identification was significantly shorter in the FM group than in the PM group (P < 0.001). The median number of detected LNs that could be examined pathologically was 16 (range 2–48) in the PM group and 18 (range 4–95) in the FM group. The number of LN did not differ significantly between the two groups (P = 0.546). These results were summarized in Table 2.
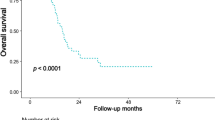
For right-sided CRC, the number of LNs was higher in the FM group than in the PM group (P = 0.031), but the number of LNs did not differ significantly between the two groups for left-sided CRC (P = 0.407) (Fig. 3A). Moreover, the LN identification time correlated with BMI in the PM group (R2 = 0.061, P = 0.047), while it did not correlate with BMI in the FM group (R2 = 0.003, P = 0.674) (Fig. 3B). These results suggest that it is more difficult to identify LNs in fat-enriched rich specimens. Lastly, prognosis was examined. Postoperative RFS was examined in patients without stage IV. 5-year RFS rate was 85% in the PM group and 85% in the FM group. They did not differ between the two groups (P = 0.877) (Fig. 4). Postoperative CSS was examined in all patients. 5-year CSS rate was 93% in the PM group and 88% in the FM group. They also did not differ between the two groups (P = 0.344).
Lymph node quantification according to tumor location and the relationship between LN identification time and BMI. (A) In right-side colorectal cancer, the median number of lymph nodes was 20 (range 7–46) in the PM group and 27 (range 11–95) in the FM group (*P = 0.031). In left-side colorectal cancer, the median number of lymph nodes was 16 (range 2–48) in the PM group and 15 (range 4–33) in the FM group (P = 0.408). (B) LN identification time increased with BMI in the PM group (R2 = 0.061), but there was no correlation in the FM group (R2 = 0.003).
Relapse-free survival curves and cancer-specific survival curves. (A) 5-year postoperative relapse-free survival rate, except in those with stage IV disease was 85% in the PM group and 85% in the FM group. (B) 5-year postoperative cancer-specific survival rate in all patients was 93% in the PM group and 88% in the FM group.
Additionally, LNs were examined pathologically, and there was no difficulty in diagnosing cancer after applying FM (Supplementary Fig. S1A). Protein expression for molecules such as epithelial cell adhesion molecule and CK20 could also be detected in LNs with metastasis. mRNA presence, that is more susceptible to degradation as compared with DNA, was also examined. KRT19 mRNA expression was significantly higher in samples with metastasis even after FM (Supplementary Fig. S1B).
Discussion
In cancer, proper LN detection is crucial for staging. However, the current methods for LN detection are time consuming and rely on extensive experience of the surgeons and pathologist. In this study, we examined the clinical usefulness of FM with respect to LN detection time and the number of LNs in CRC. The results showed that by dissociating fat tissue, FM improved clearing of the mesenteric structure, therefore making the LNs visible. FM allowed easier identification of LNs around vessels, thus reducing the time required for LN detection. In addition, a higher number of LNs were identified in samples obtained from right-sided CRC. Identifying LNs directly from surgically resected specimen is technically challenging because the color of LNs is similar to that of fat. Such identification mostly depends on the experience of the researcher. In the cases of right-sided CRC, the range of mesenteric size is large, and identification took time. Therefore, if the amount of lymph nodes searched is wrongly identified to be sufficient, it may result in downstaging because of the lower number of lymph nodes detected. The fat-dissociation method that we developed can be used even by inexperienced researchers as it will enable to easily recognize LNs, and it seems to be a useful method for direct procedures such as OSNA18 which requires LNs freshly isolated from surgically resected specimens. In our protocol for preparation of the fat-dissociation solution, the incubation time was set from 30 to 60 min for safety reasons. However, if a higher concentration solution is used, the incubation time could be shorter. Therefore, high concentrations and short periods of time may be desirable, such as when searching for sentinel lymph nodes for OSNA during surgery. In this study, we set the low concentration for safety. Because if we missed the time, the LN will be damaged in a high-concentration solution. However, if there is a dedicated person performing the work, the time will be shorter with higher concentration. The concentration of this solution could be modified and would be applicable in several institutions in several situations. In addition, our fat-dissociation solution at low concentration was commercialized as Imofully (Sysmex, Kobe, Japan). Similar to our study, it was reported that the results were excellent in short-term results such as the number of lymph nodes and the search time by using Imofully (Sysmex)24,25.
It has been reported recently that molecular characterization of cancer based on gene expression is important for proper disease stratification26, and the appropriate drug treatment is selected according to specific mutations2,9. We examined the KRT19 mRNA expression via RT-PCR analysis. The KRT19 mRNA levels serve as useful marker to detect the metastasis of CRC to the LN27. Our results suggested that FM did not interfere with the detection of mRNA expression and would be a useful pre-method in the diagnosis of LN metastasis using RT-PCR system such as OSNA in the future. Furthermore, it may be possible to automate the LN diagnosis by combining FM with the OSNA method.
However, this study has several limitations. One is that this is a single-center study. Second, the study needs to be verified not only in surgeons, but also in other personnel including laboratory technicians. Moreover, the primary endpoint was not prognostic in this study. We did not specify the tumor stage in this study; there were more patients with advanced stage IIIb and IV in the FM group. Focusing on stage II and IIIa, where more accurate lymph node identification is reflected in the stage, the 5-year RFS was 77% and 87% and the 5-year CSS was 93% and 100% in the PM and the FM groups, respectively. However, the difference was not significant, and additional study is required to verify the effect of FM on prognosis. In addition, postoperative adjuvant chemotherapy was not specified, and the number of cases was not sufficient for prognostic analysis. Therefore, we could not mention that FM was an essential method to determine the N factor that reflect prognosis. More patients with stage II–III need to be evaluated to determine whether FM is a truly useful method for determining the N factor. Nevertheless, FM can help visualize LNs in surgical specimens and reduce the time required for identification and diagnosis of LNs in cancer tissue.
In conclusion, FM reduced the time required for LN identification by allowing better and more efficient visualization of LNs in surgically resected mesentery. This is an innovative useful method to detect LNs from surgical specimens.
Data availability
All data of our results was included.
Abbreviations
- CRC:
-
Colorectal cancer
- LN:
-
Lymph nodes
- FM:
-
Fat-dissociation method
- PM:
-
Palpation method
- OSNA:
-
One-step nucleic acid amplification
- OICI:
-
Osaka International Cancer Institute
- KRT19:
-
Cytokeratin 19
- CK20:
-
Cytokeratin 20
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- RT-PCR:
-
Real-time polymerase chain reaction
References
Global Burden of Disease Cancer, C et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol. 4, 1553–1568. https://doi.org/10.1001/jamaoncol.2018.2706 (2018).
Van Cutsem, E., Cervantes, A., Nordlinger, B., Arnold, D. & Group, E. G. W. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 25(Suppl 3), iii1–iii9. https://doi.org/10.1093/annonc/mdu260 (2014).
Bray, F. G. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. https://doi.org/10.3322/caac.21492 (2018).
Park, H. M. et al. Colorectal cancer incidence in 5 Asian countries by subsite: An analysis of Cancer Incidence in Five Continents (1998–2007). Cancer Epidemiol. 45, 65–70. https://doi.org/10.1016/j.canep.2016.09.012 (2016).
Schmoll, H. J. et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann. Oncol. 23, 2479–2516. https://doi.org/10.1093/annonc/mds236 (2012).
Glimelius, B., Tiret, E., Cervantes, A., Arnold, D. & Group, E. G. W. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 24(Suppl 6), vi81–vi88. https://doi.org/10.1093/annonc/mdt240 (2013).
Labianca, R. et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 24(Suppl 6), vi64–vi72. https://doi.org/10.1093/annonc/mdt354 (2013).
Network NCC NCCN guidlines for treatment of cancer by site. Colon/Rectal Cancer. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp Accessed 24 Apr 2019.
Watanabe, T. et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014 for treatment of colorectal cancer. Int. J. Clin. Oncol. 20, 207–239. https://doi.org/10.1007/s10147-015-0801-z (2015).
Watanabe, T. et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 23, 1–34. https://doi.org/10.1007/s10147-017-1101-6 (2018).
Hida, J. et al. Metastases from carcinoma of the colon and rectum detected in small lymph nodes by the clearing method. J. Am. Coll. Surg. 178, 223–228 (1994).
Morikawa, E. et al. Distribution of metastatic lymph nodes in colorectal cancer by the modified clearing method. Dis. Colon Rectum 37, 219–223 (1994).
Chapman, B. et al. Impact of Schwartz enhanced visualization solution on staging colorectal cancer and clinicopathological features associated with lymph node count. Dis. Colon Rectum 56, 1028–1035. https://doi.org/10.1097/DCR.0b013e31829c41ba (2013).
Gregurek, S. F. & Wu, H. H. Can GEWF solution improve the retrieval of lymph nodes from colorectal cancer resections?. Arch. Pathol. Lab. Med. 133, 83–86. https://doi.org/10.1043/1543-2165-133.1.83 (2009).
Newell, K. J., Sawka, B. W., Rudrick, B. F. & Driman, D. K. GEWF solution. Arch. Pathol. Lab. Med. 125, 642–645. https://doi.org/10.1043/0003-9985(2001)125%3c0642:GS%3e2.0.CO;2 (2001).
Yamamoto, H. et al. Micrometastasis volume in lymph nodes determines disease recurrence rate of stage II colorectal cancer: A prospective multicenter trial. Clin. Cancer Res. 22, 3201–3208. https://doi.org/10.1158/1078-0432.CCR-15-2199 (2016).
Nakagawa, K. et al. The novel one-step nucleic acid amplification (OSNA) assay for the diagnosis of lymph node metastasis in patients with non-small cell lung cancer (NSCLC): Results of a multicenter prospective study. Lung Cancer 97, 1–7. https://doi.org/10.1016/j.lungcan.2016.03.015 (2016).
Tamaki, Y. One-step nucleic acid amplification (OSNA): Where do we go with it?. Int. J. Clin. Oncol. 22, 3–10. https://doi.org/10.1007/s10147-016-1030-9 (2017).
Osako, T. et al. Intraoperative molecular assay for sentinel lymph node metastases in early stage breast cancer: A comparative analysis between one-step nucleic acid amplification whole node assay and routine frozen section histology. Cancer 117, 4365–4374. https://doi.org/10.1002/cncr.26060 (2011).
Matsuzuka, T. et al. Intraoperative molecular assessment for lymph node metastasis in head and neck squamous cell carcinoma using one-step nucleic acid amplification (OSNA) assay. Ann. Surg. Oncol. 19, 3865–3870. https://doi.org/10.1245/s10434-012-2409-0 (2012).
Lopez-Ruiz, M. E. et al. One-step nucleic acid amplification (OSNA) for the detection of sentinel lymph node metastasis in endometrial cancer. Gynecol. Oncol. 143, 54–59. https://doi.org/10.1016/j.ygyno.2016.07.106 (2016).
Fujino, S. et al. New enhanced and effective method for staging cancer to detect lymph nodes after fat-dissociation. Oncol. Rep. https://doi.org/10.3892/or.2014.3315 (2014).
Watanabe, T. et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 17, 1–29. https://doi.org/10.1007/s10147-011-0315-2 (2012).
Kinami, S. et al. Efficacy of the fat-dissociation method for nodal harvesting in gastric cancer. World J. Gastrointest. Surg. 12, 277–286. https://doi.org/10.4240/wjgs.v12.i6.277 (2020).
Fujieda, Y. et al. Lymph node retrieval after colorectal cancer surgery: A comparative study of the efficacy between the conventional manual method and a new fat dissolution method. Surg. Today 50, 726–733. https://doi.org/10.1007/s00595-019-01944-0 (2020).
Guinney, J. et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 21, 1350–1356. https://doi.org/10.1038/nm.3967 (2015).
Yamamoto, H. et al. OSNA-based novel molecular testing for lymph node metastases in colorectal cancer patients: Results from a multicenter clinical performance study in Japan. Ann. Surg. Oncol. 18, 1891–1898. https://doi.org/10.1245/s10434-010-1539-5 (2011).
Acknowledgements
We would like to thank Drs. T. Fukata, T. Umeda, T. Hara, Y. Shishido, and Y. Wada for searching for lymph nodes in the surgically resected mesentery. This work was partly supported by Nakatani Foundation Grant Program.
Author information
Authors and Affiliations
Contributions
S.F. wrote the main manuscript text and prepared all figures. N.M. supervised them. A.I. performed basic examination. M.O., M.Y., T.O., H.T., M.U., C.M., H.Y., T.M., Y.D., and H.E. performed surgery and followed up patients. S.F., N.M. and N.M. planed this prospective study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fujino, S., Miyoshi, N., Ohue, M. et al. A new fat-dissociation method to detect lymph nodes in colorectal cancer: a prospective randomized study. Sci Rep 10, 20205 (2020). https://doi.org/10.1038/s41598-020-77195-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-77195-8
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.