Abstract
Serum neurofilament light chain (sNfL) and its ability to expose axonal damage in neurologic disorders have solicited a considerable amount of attention in blood biomarker research. Hence, with the proliferation of high-throughput assay technology, there is an imminent need to study the pre-analytical stability of this biomarker. We recruited 20 patients with common neurological diagnoses and 10 controls (i.e. patients without structural neurological disease). We investigated whether a variation in pre-analytical variables (delayed freezing up to 24 h and repeated thawing/freezing for up to three cycles) affects the measured sNfL concentrations using state of the art Simoa technology. Advanced statistical methods were applied to expose any relevant changes in sNfL concentration due to different storing and processing conditions. We found that sNfL concentrations remained stable when samples were frozen within 24 h (mean absolute difference 0.2 pg/ml; intraindividual variation below 0.1%). Repeated thawing and re-freezing up to three times did not change measured sNfL concentration significantly, either (mean absolute difference 0.7 pg/ml; intraindividual variation below 0.2%). We conclude that the soluble sNfL concentration is unaffected at 4–8 °C when samples are frozen within 24 h and single aliquots can be used up to three times. These observations should be considered for planning future studies.
Similar content being viewed by others
Introduction
Neurofilaments (Nf) have spurred a compelling field of biomarker research in neurology1,2,3. Several studies underline the capacity of Nf to reliably expose the damage and loss of axons in both cerebrospinal fluid (CSF) and blood for a rising number of various neurologic diseases4,5,6,7. Historically, one has to name multiple sclerosis (MS) first, where concentrations of serum neurofilament light chains (sNfL) have shaped into a piece in the puzzle that is monitoring of disease and prediction of outcome or therapeutic response8,9,10.
Neurofilaments are part of the axon’s cytoskeleton and, as such, classified as Type IV intermediate filaments consisting of at least four subunits: a light chain (NfL), a medium chain (NfM), a heavy chain (NfH) and alpha-internexin11,12. Through the advent of fourth-generation technology, single-molecule array (Simoa)-assays have enabled a precise and more sensitive detection of sNfL in peripheral blood. Starting from a few picograms per milliliter, it is now possible to display a representative range of sNfL concentrations in disease and physiological conditions alike. This at a sensitivity which is able to illustrate and separate the degree of axonal changes that occur in normal ageing, mild head trauma, hypoxia, inflammation or neurodegeneration10,13,14,15.
Nonetheless, in order to successfully launch NfL from bench to bedside, the pre-analytical stability of this biomarker demands careful attention. It is imperative to know about ideal conditions for storage or processing and how even a slight change in these variables may potentially affect measured concentrations. For the analysis of sNfL using Simoa SR-X technology, the consequence of a deviation from standard procedure (i.e. processing blood immediately and freezing at − 70; single use of aliquots) has not been reported to this extent and in three different cohorts16,17,18. Therefore, we studied two important and mutable pre-analytic variables: the effect of delayed freezing (up to 24 h) and repeated thawing and re-freezing (up to three cycles).
Results
Patient characteristics
All 30 participants were included in both experimental set ups and the final analysis. Supplementary Table S1 reports on patient characteristics such as age, sex, disease duration, disease phenotype, the clinician reported outcome (CRO) to classify disease severity (Expanded Disability Status Scale [EDSS] for patients with MS and the Hoehn & Yahr scale for patients with Parkinson’s disease [PD]), medication relevant to disease, and baseline sNfL concentrations determined at standard conditions (i.e. processed and frozen immediately and thawed once). Overall, 13 out of 30 included patients were female (43%). Mean age across all three groups was 50.1 years [95% confidence interval (CI) 44.0–56.2]. As for the group of patients with MS, their mean age was 48.6 years (95% CI 43.6–53.6) and median EDSS was 5.0 (range: 1–6.5). Patients with PD had a mean age of 68.9 years (95% CI 63.3–74.5). Median Hoehn & Yahr scale was 2 (range: 1–3). Our controls consisting of patients without structural neurological disease were 32.8 years old (95% CI 27.6–38.0) exhibited non-acute symptoms and were diagnosed with primary headache (n = 5), unspecific dizziness (n = 3) or sinusitis (n = 2). None of the patients included were on any medication affecting blood coagulation or thrombocyte function.
All aliquots of serum samples analyzed in this study yielded a coefficient of variance (CV, i.e. the mean sNfL concentrations as calculated by two replicates) of below 0.2 and internal controls were within the expected range. The mean CV over the whole study was 0.09 (standard deviation: 0.06), suggesting excellent measurement accuracy. No samples were excluded from this analysis.
sNfL concentrations withstand delayed freezing up to 24 h
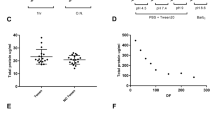
Concentrations of sNfL across the three pre-freezing intervals are given in Table 1. For the whole cohort, mean absolute differences in sNfL concentrations compared to immediate freezing were 0.3 pg/ml [intraindividual standard deviations (iSD): 1.28 pg/ml] after three hours and 0.2 pg/ml (iSD: 1.38 pg/ml) after 24 h of delayed freezing (Fig. 1a,b). Reliability of sNfL measurement after different pre-freezing intervals compared to immediate freezing are shown in Table 2. For both pre-freezing intervals, intraindividual variation (iCOV) was minimal (below 0.1%) and reproducibility nearly perfect [intraclass correlation coefficient (ICC) was 0.99]. These findings were similar an all subgroups (MS, PD, controls, lowest quartile, highest quartile).
Differences in sNfL concentrations after different freezing intervals. Bland–Altman plot showing the differences in sNfL concentration between two groups of immediate processing and delayed freezing. (a) Freezing after 10 min compared to 3 h. (b) Freezing after 10 min compared to 24 h. Single dots represent samples at two conditions with their mean concentrations on the x-axis and their difference in concentration on the y-axis. The three horizontal lines represent the mean difference (middle) and the mean difference plus 1.96xSD of that difference (upper) and mean difference minus 1.96xSD of that difference (lower).
sNfL concentrations remain stable in up to three thaw cycles
Concentrations of sNfL along three thawing cycles are given in Table 3. For the whole cohort and in comparison to its first thawing cycle, mean absolute differences in sNfL concentrations after a second thawing cycle were 1.6 pg/ml (iSD: 1.8 pg/ml) and 0.7 pg/ml (iSD: 1.8 pg/ml) after a third (Fig. 2a,b). Concerning the reliability of measured sNfL concentration after repeated thawing, intraindividual variation was minimal in the whole cohort (below 0.15%) and reproducibility nearly perfect (ICC = 0.98) (Table 4). These findings were similar in all subgroups (MS, PD, controls, lowest quartile, highest quartile).
Differences in sNfL concentrations after a different number of thawing cycles. Bland–Altman plot showing the differences in sNfL concentration between two groups of thawing cycles. (a) One versus two thawing cycles. (b) One vs. three thawing cycles. Single dots represent samples at two conditions with their mean concentrations on the x-axis and their difference in concentration on the y-axis. The three horizontal lines represent the mean difference (middle) and the mean difference plus 1.96xSD of that difference (upper) and mean difference minus 1.96xSD of that difference (lower).
Discussion
Serum NfL has turned over a new leaf in biomarker research in neurology. Concurrently, knowledge about the pre-analytical stability of this biomarker should be a prerequisite for establishing study protocols or standard operating procedures. Our study investigated the potential confounder by which measured concentrations of sNfL might change when processing and freezing of serum tubes is delayed up to 24 h in three different cohorts. Furthermore, we analyzed the stability of sNfL after three consecutive cycles of thawing and re-freezing. For both pre-analytical settings, we found that sNfL concentrations remained stable and within acceptable limits. Considered the statistical gold-standard for gauging reproducibility, the ICC takes into account the variance among subjects, the variance among measurements, and the residual error variance. An ICC of 1 represents perfect reproducibility. With an ICC of 0.99 for samples stored at 4–8 °C for 24 h (before being processed and frozen to − 70 °C) and 0.98 for sample aliquots that were thawed and re-frozen three times, we now provide solid evidence that sNfL withstands a delay in freezing for up to 24 h and at least three cycles of repeated thawing and freezing. These results are in line with another investigation looking into the stability of biomarkers in Alzheimer disease over multiple freeze–thaw cycles, including sNfL16. For our study, post-hoc power calculations revealed that a systemic measurement error of a mere 2 pg/ml would have been detected at 97% power, further validating the robustness of our results. As importantly, these findings were preserved in samples with different physiological and pathophysiological properties.
Regarding generalizability of our data, there are some limitations. This investigation was performed in a specialized biomarker lab with a protocol specifically evolving around two predetermined hypotheses. Furthermore, our results only apply to the requirements set by our biobanking protocol for handling serum samples19. It is worth mentioning in this context that plasma samples reportedly yield about 75% of serum NfL20. Also, the range of sNfL concentrations we investigated was 2.7–60.8 pg/ml. Therefore, pre-analytical stability may not be assumed for diseases with higher suspected sNfL concentrations. Additionally, the results from this study are derived from only one site with a single person performing sNfL analyses. It does not take into account certain effects or circumstances such as interrater variability or protocol deviations stemming from the busy environment of a routine lab where, usually, various analyses are performed simultaneously and for different analytes. Thus, variance in sNfL levels may be increased in real-world settings. Even so, interrater variability for varying sNfL measurements is considered low and has been reported on already21. Besides, our laboratory itself is temperature-controlled, operates at 22–23 °C and does not have any outside-facing windows that could cause disturbances. Interestingly, we noticed a rise in sNfL concentrations over multiple thawing cycles in some cases. While this rise was not statistically significant, it is tempting to speculate that this might be attributed to sample vials being opened and exposed to air more frequently which might cause evaporation resulting in relatively higher protein concentration. This hypothesis might be intriguing to investigate in the future. Ultimately, our study confirms the results of another study published just recently. In this reference sample study, the authors evaluated a subgroup of twelve serum samples for pre-analytical properties and found a stability of 95% for three-day storage at room temperature and 92% after three freeze–thaw cycles compared to standardized conditions22.
It is exciting to notice an increase in research invested in the study of intrinsic factors influencing sNfL concentrations in health and disease. A recent cohort study, for example, revealed that healthy individuals exhibit a linear rise in their fourth and fifth decade of life, shifting to a non-linear rise after the sixth. These observations were shown to be related to continuing brain volume loss and were irrespective of sex23. Another investigation found a negative correlation between NfL plasma levels and body mass index or blood volume24. These are just two examples showing the necessity to understand the multitude of potential influences and confounders in a specific biomarker, in our case, sNfL. This is even more essential as sNfL concentrations (and even more changes in sNfL levels) may occur over the range of some picograms per milliliter.
In summary, we showed that sNfL concentrations remained stable in both our experimental set ups of delayed freezing for up to 24 h and three cycles of repeated thawing and freezing. Therefore, we conclude that serum tubes can be stored up to 24 h in a refrigerator before being processed and thawed/frozen repeatedly for at least three cycles while still yielding reliable results for sNfL.
Materials and methods
Ethics and consent
This study was approved by the ethics committee at the Medical University of Vienna (EK2100/2019). Written informed consent was obtained from each patient according to our local biobanking protocol (EK2195/2016). The authors have complied with the World Medical Association Declaration of Helsinki regarding ethical conduct of research involving human subjects.
Study population
From March 2019 through July 2019, we recruited a total of 30 patients from the outpatient clinic at the Department of Neurology, Medical University of Vienna. They were divided into three predetermined groups: Ten patients diagnosed with MS, ten with PD, and ten controls. These controls were classified as such after their neurologic routine diagnostic workup was unremarkable. This group was comprised of mostly young patients with symptoms such as primary headache or dizziness. Controls’ status was confirmed by two independent reviewers at our department.
Blood sampling
Peripheral venous blood was collected in Greiner Bio-One Vacuette serum tubes (GBO, Kremsmuenster, Austria) and sent to the local biobank where the blood sample was processed according to standard operating procedures in an ISO 9001-certified environment as described previously19. In brief, tubes were centrifuged at 1.884 × g for 10 min at room temperature after clotting had completed. Serum was then transferred to 500µL-virgin polypropylene tubes and subsequently stored as 400µL aliquots at − 70 °C (temperature controlled) until analysis.
Handling of samples for the experimental set up of delayed freezing
To investigate the effect of delayed freezing on sNfL concentrations, three serum tubes from each patient were used. For the first tube, clotting was allowed for 10 min at room temperature, before samples were processed further (i.e. centrifuged and stored in aliquots) in our local biobank. The second tube was placed in a refrigerator (temperature controlled at 4–8 °C) for 3 h before it was sent to the local biobank for processing. The third tube was stored in that same refrigerator for 24 h before being delivered to the biobank.
Handling of samples for the experimental set up of repeated thawing and re-freezing
The effect of repeated thawing and re-freezing on sNfL levels was studied using aliquots that were handled according to standard procedure (i.e. processed to the biobank without delay). We determined sNfL concentrations three times. For the first thaw cycle, sNfL was measured as described below. After a thawing period of a cumulative three hours, the remaining samples were put back and stored at − 70 °C for two days. For the second thaw cycle, the exact same samples were re-thawed, sNfL concentrations were measured and the remaining samples were again re-frozen at − 70 °C for two days. This process was repeated for the third thaw cycle accordingly.
Analysis of serum neurofilament light chain concentrations
For sNfL measures, samples were thawed for 60 min at room temperature and were analyzed by an investigator blinded to clinical data using the Simoa Nf-light kits and provided consumables in the Simoa SR-X Analyzer (Quanterix, Lexington, MA, USA)15. The sNfL assay was performed according to the manufacturer’s instructions and protocol. Briefly, thawed samples and calibrators were equilibrated to room temperature, diluted in sample diluent (1:4) and dispensed in 96-well plates as duplicates. 20 µl of detector and 25 µl of paramagnetic beads were consecutively dispensed in each well and plates were incubated and shaken (Simoa microplate incubator, 30 °C, 800RPM for 30 min). After pre-set washing steps (Simoa microplate washer), 100 μl streptavidin β-galactosidase was added to each well and plates were incubated (30 °C, 800RPM for 10 min) and washed. After a final washing step, plates were dried for 10 min before being transferred to the Quanterix SR-X analyzer for reading. For the experimental set up of delayed freezing, all samples from one participant were measured on the same plate to avoid inter-assay variability. All assay kits and consumables used in this study were derived from the same kit lot.
Statistical analyses
Statistical analysis was performed using SPSS 25.0 (SPSS Inc, Chicago, IL, USA). Categorical variables were expressed in frequencies and percentages, continuous variables were tested for normal-distribution by the Shapiro–Wilk test and displayed as mean and 95% CI or median and range as appropriate. Differences between sNfL values after different freezing intervals and thawing cycles were analyzed using repeated measurement ANOVA. A two-sided p value of 0.05 was considered the level of significance. Reproducibility of sNfL measurement across all three freezing intervals and thawing cycles was assessed by calculating iSD and iCOV, plotting Bland–Altman plots to analyze the agreement between two conditions for the same sample (these show the mean difference of a sample’s concentration at two conditions on the x-axis and the absolute difference between two samples measured at two conditions on the y-axis) and the applying the formula CV = standard deviation/mean × 100%25. In addition, ICC were calculated. ICC calculation is based on a repeated measure ANOVA model using the variance among subjects, the variance among measurements, and the residual error variance25. An ICC of 1 represents perfect reproducibility, while an ICC above 0.9 is rated excellent for laboratory tests. ICC was calculated from the sNfL levels after immediate freezing or thawing cycle 1, respectively.
Abbreviations
- sNfL:
-
Serum neurofilament light chain
- Nf:
-
Neurofilament
- CSF:
-
Cerebrospinal fluid
- MS:
-
Multiple sclerosis
- NfL:
-
Neurofilament light chain
- NfM:
-
Neurofilament medium chain
- NfH:
-
Neurofilament heavy chain
- PD:
-
Parkinson’s disease
- CI:
-
Confidence interval
- iSD:
-
Intraindividual standard deviation
- iCOV:
-
Intraindividual coefficient of viariation
- ICC:
-
Intraclass correlation coefficient
- CRO:
-
Clinician reported outcome
- EDSS:
-
Expanded disability status scale
References
Gaetani, L. et al. Neurofilament light chain as a biomarker in neurological disorders. J. Neurol. Neurosurg. Psychiatry 90, 870–881. https://doi.org/10.1136/jnnp-2018-320106 (2019).
Zetterberg, H. Neurofilament light: a dynamic cross-disease fluid biomarker for neurodegeneration. Neuron 91, 1–3. https://doi.org/10.1016/j.neuron.2016.06.030 (2016).
Julien, J.-P. Neurofilament functions in health and disease. Curr. Opin. Neurobiol. 9, 554–560. https://doi.org/10.1016/S0959-4388(99)00004-5 (1999).
Bridel, C. et al. Diagnostic value of cerebrospinal fluid neurofilament light protein in neurology: a systematic review and meta-analysis. JAMA Neurol. 76, 1035–1048. https://doi.org/10.1001/jamaneurol.2019.1534 (2019).
Khalil, M. et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 14, 577–589. https://doi.org/10.1038/s41582-018-0058-z (2018).
Mariotto, S. et al. Serum and CSF neurofilament light chain levels in antibody-mediated encephalitis. J. Neurol. 266, 1643–1648. https://doi.org/10.1007/s00415-019-09306-z (2019).
Altmann, P. et al. Increased serum neurofilament light chain concentration indicates poor outcome in Guillain-Barré syndrome. J. Neuroinflamm. 17, 86. https://doi.org/10.1186/s12974-020-01737-0 (2020).
Bsteh, G. et al. Serum neurofilament light levels correlate with change of olfactory function in multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 5, 2055217319885987. https://doi.org/10.1177/2055217319885987 (2019).
Bsteh, G. et al. Serum neurofilament levels correlate with retinal nerve fiber layer thinning in multiple sclerosis. Mult. Scler. J. https://doi.org/10.1177/1352458519882279 (2019).
Disanto, G. et al. Serum neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann. Neurol. 81, 857–870. https://doi.org/10.1002/ana.24954 (2017).
Lee, M. K., Xu, Z., Wong, P. C. & Cleveland, D. W. Neurofilaments are obligate heteropolymers in vivo. J. Cell Biol. 122, 1337–1350. https://doi.org/10.1083/jcb.122.6.1337 (1993).
Petzold, A. Neurofilament phosphoforms: surrogate markers for axonal injury, degeneration and loss. J. Neurol. Sci. 233, 183–198. https://doi.org/10.1016/j.jns.2005.03.015 (2005).
Gisslén, M. et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine 3, 135–140. https://doi.org/10.1016/j.ebiom.2015.11.036 (2016).
Kuhle, J. et al. in Clinical Chemistry and Laboratory Medicine (CCLM) Vol. 54 1655 (2016).
Rissin, D. M. et al. Single-molecule enzyme-linked immunosorbent assay detects serum proteins at subfemtomolar concentrations. Nat. Biotechnol. 28, 595–599. https://doi.org/10.1038/nbt.1641 (2010).
Keshavan, A., Heslegrave, A., Zetterberg, H. & Schott, J. M. Stability of blood-based biomarkers of Alzheimer’s disease over multiple freeze-thaw cycles. Alzheimers Dement (Amst) 10, 448–451. https://doi.org/10.1016/j.dadm.2018.06.001 (2018).
Koel-Simmelink, M. J. A., Teunissen, C. E., Behradkia, P., Blankenstein, M. A. & Petzold, A. The neurofilament light chain is not stable in vitro. Ann. Neurol. 69, 1065–1066. https://doi.org/10.1002/ana.22438 (2011).
Petzold, A. et al. Protein aggregate formation permits millennium-old brain preservation. J. R. Soc. Interface 17, 20190775. https://doi.org/10.1098/rsif.2019.0775 (2020).
Haslacher, H. et al. Usage data and scientific impact of the prospectively established fluid bioresources at the hospital-based meduni wien biobank. Biopreserv. Biobank. 16, 477–482. https://doi.org/10.1089/bio.2018.0032 (2018).
Sejbaek, T. et al. Dimethyl fumarate decreases neurofilament light chain in CSF and blood of treatment naïve relapsing MS patients. J. Neurol. Neurosurg. Psychiatry 90, 1324. https://doi.org/10.1136/jnnp-2019-321321 (2019).
Quanterix. Data Sheets and Technical Notes for NF-light. https://www.quanterix.com/products-technology/assays/nf-lightr-sr-x-version (Accessed March 2020).
Hviid, C. V. B., Knudsen, C. S. & Parkner, T. Reference interval and preanalytical properties of serum neurofilament light chain in Scandinavian adults. Scand. J. Clin. Lab. Investig. 80, 291–295. https://doi.org/10.1080/00365513.2020.1730434 (2020).
Khalil, M. et al. Serum neurofilament light levels in normal aging and their association with morphologic brain changes. Nat. Commun. 11, 812. https://doi.org/10.1038/s41467-020-14612-6 (2020).
Manouchehrinia, A. et al. Confounding effect of blood volume and body mass index on blood neurofilament light chain levels. Ann. Clin. Transl. Neurol. 7, 139–143. https://doi.org/10.1002/acn3.50972 (2020).
Kramer, M. S. & Feinstein, A. R. Clinical biostatistics: LIV. The biostatistics of concordance. Clin. Pharmacol. Ther. 29, 111–123. https://doi.org/10.1038/clpt.1981.18 (1981).
Acknowledgements
The authors would like to thank their nursing staff Ms. Sylvia Baeumel, Mr. Martin Krutzler and Ms. Elke Hohenbichler (outpatient clinic, Department of Neurology, Medical University of Vienna, Austria) for helping with this study.
Funding
The NF-light kits and consumables used in this study were sponsored by Quanterix International. The study was supported by the Medical University of Vienna.
Author information
Authors and Affiliations
Contributions
Conceptualization, P.A., F.L., P.R., A.P., G.B.; Data curation: P.A., F.L., H.Za., M.P.; Methodology, P.A., A.P., H.Ze.; Formal Analysis, P.A., M.S., H.Ze, G.B., R.W.; Writing – Original Draft Preparation, P.A., G.B., P.R.; Writing – Review and Editing: T.B., H.Ze., A.P., P.R., G.B; Supervision, P.R.; Funding Acquisition, P.A. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The sponsoring organization played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication. PA is the recipient of the sponsorhip by Quanterix International mentioned above. HZe has served at scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed and CogRx, has given lectures in symposia sponsored by Fujirebio, Alzecure and Biogen, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg, all unrelated to the work presented in this paper. TB has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS: Allergan, Bayer, Biogen, Bionorica, Celgene, MedDay, Merck, Novartis, Octapharma, Roche, Sanofi-Genzyme, Teva. His institution has received financial support in the past 12 months by unrestricted research grants (Biogen, Bayer, Merck, Novartis, Sanofi Aventis, Teva and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Octapharma, Roche, Sanofi-Genzyme, Teva.GB has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Celgene, Merck, Novartis, Sanofi-Genzyme and Teva, and received honoraria for consulting Biogen, Roche and Teva. The other authors report no competing interests or any financial or personal relationships with organizations that could potentially be perceived as influencing the described research.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Altmann, P., Leutmezer, F., Zach, H. et al. Serum neurofilament light chain withstands delayed freezing and repeated thawing. Sci Rep 10, 19982 (2020). https://doi.org/10.1038/s41598-020-77098-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-77098-8
This article is cited by
-
Matching proposed clinical and MRI criteria of aggressive multiple sclerosis to serum and cerebrospinal fluid markers of neuroaxonal and glial injury
Journal of Neurology (2024)
-
Glial fibrillary acidic protein is a robust biomarker in cerebrospinal fluid and peripheral blood after traumatic spinal cord injury: a prospective pilot study
Acta Neurochirurgica (2023)
-
Validity of serum neurofilament light chain as a prognostic biomarker of disease activity in multiple sclerosis
Journal of Neurology (2023)
-
Die subjektive Verarbeitung chronischer Symptome und Schmerz bei Menschen mit Multipler Sklerose: Visualisierung und Externalisierung als Ausdruck der Lebensqualität
psychopraxis. neuropraxis (2022)
-
Seven day pre-analytical stability of serum and plasma neurofilament light chain
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.