Abstract
Observational and experimental data reinforce the concept that vitamin D is associated with the pathogenesis of arterial hypertension. We investigated the effect of a single dose of 100,000 IU of cholecalciferol, in office blood pressure (BP), and 24-h ambulatory blood pressure monitoring (ABPM) in patients with type 2 diabetes mellitus (DM), hypertension, and hypovitaminosis D. Forty-three patients were randomized to a placebo or cholecalciferol group. BP was assessed by office measurements and 24-h ABPM, before and after intervention. At week 8, a greater decrease in median ABPM values was observed in cholecalciferol supplementation than in the placebo group for systolic 24-h (− 7.5 vs. − 1; P = 0.02), systolic daytime (− 7 vs. − 1; P = 0.007), systolic nighttime (− 7.0 vs. 3; P = 0.009), diastolic 24-h (− 3.5 vs. − 1; P = 0.037), and daytime DBP (− 5 vs. 0; P = 0.01). Office DBP was also reduced after vitamin D supplementation. A single dose of vitamin D3 improves BP in patients with type 2 diabetes, hypertension, and vitamin D insufficiency, regardless of vitamin D normalization. Vitamin D supplementation could be a valuable tool to treat patients with type 2 DM, hypertension, and hypovitaminosis D.
Trial registration: Clinicaltrials.gov NCT 02204527.
Similar content being viewed by others
Introduction
Observational and experimental data reinforce the concept that vitamin D is associated with the pathogenesis of arterial hypertension1,2,3,4,5. The precise mechanism by which vitamin D lowers blood pressure (BP) is still unknown. However, effects on endothelial function, inflammation and oxidative stress as well as reduced activity of the renin–angiotensin–aldosterone system (RAS)5,6 and reduced levels of parathyroid hormone have been proposed with mechanism of action of vitamin D.
In hypertensive patients with type 2 diabetes mellitus (DM) and hypovitaminosis D the potential beneficial effects of vitamin D supplementation are still scarce7,8,9. Some of the more recent Mendelian randomization studies suggest the influence of low vitamin D concentration on blood pressure10. However, previous meta-analyses showed mixed effects on BP with supplementation of vitamin D in adults7,8,9. Nevertheless, some of these meta-analyses included studies that examined the effects of different types of vitamin D (1-α-hydroxylated vitamin D derivatives or calcitriol, paricalcitol; and ergocalciferol or cholecalciferol), as well as a different access (oral, intramuscular, and parenteral vitamin D)8.
We hypothesized that the anti-hypertensive effect of vitamin D3 could be stronger in patients with vitamin D insufficiency. In fact, improved serum 25(OH) D concentrations in hypertensive individuals who had insufficient vitamin D were associated with improved control of systolic and diastolic BP and conferred a significant risk reduction for hypertension4,5. Indeed a meta-analysis that evaluates only vitamin D supplementation in vitamin D deficient subjects showed a small but significant reduction of diastolic BP11.
This study was performed in a population where effects are supposed to be maximal, namely in patients with hypertension and vitamin D insufficiency. The present trial was designed to determine the effect of a single dose of vitamin D3 compared to a placebo on BP values, independent of vitamin D3 normalization, evaluated by office and ambulatory blood pressure monitoring (ABPM), in patients with type 2 DM, hypertension, and hypovitaminosis D after 8 weeks of supplementation.
Methods
This article was designed and reported according to the Consort 2010 Statement12 providing all sections suggested to parallel-group randomized trials.
Trial design
In an 8-week, randomized double-blind, parallel, placebo-controlled clinical trial, patients were randomly assigned to a single dose of 100,000 IU of vitamin D3 or a matching placebo by an online computer-generated randomly permutated codes (www.randomization.com).
The outcome of the study was changes in office and ABPM measurements. The study was carried out between October 2015 and December 2016 and was conducted in accordance with the guidelines established in the Declaration of Helsinki13. The Hospital Ethics Committee of the Hospital de Clinicas de Porto Alegre (Porto Alegre, Brazil) approved the protocol, and all patients gave their written informed consent. This clinical trial was registered at clinicaltrials.gov as NCT02204527 in 30/07/2014.
Participants
Outpatients with type 2 DM (HbA1c 6.5–10%), hypertension (office systolic BP ≥ 140 mm Hg or diastolic ≥ 90 mm Hg or ongoing antihypertensive treatment)14,15 and hypovitaminosis D (25(OH) D serum concentration below 20 ng/mL or 50 nmol/L) were recruited to participate in the study at the Endocrine Division of Hospital de Clinicas in Porto Alegre, Brazil. Exclusion criteria were the following: pregnant or lactating women, oral calcium or vitamin D supplementation or any medications affecting calcium or vitamin D metabolism (estrogens and calcitonin), change of antihypertensive treatment (drugs or lifestyle modifications) in the previous 4 weeks or planned changes of antihypertensive treatment during the study, any other concomitant clinical disease that could influence vitamin D metabolism (e.g. renal, hepatic, other endocrinologic disorders, and malignancies), body mass index (BMI) > 40 kg/m2, creatinine > 2 mg/dL (or > 176 mmol/L), and an inability or unwillingness to participate.
Study protocol
Figure 1 shows the flowchart of the study protocol. Of 127 screened patients, 84 were excluded: 56 without hypovitaminosis D, 23 in use of vitamin D, calcium or corticosteroids and 2 with BMI > 40 kg/m2. A total of 43 participants were randomized, of whom 100% attended the 8 weeks follow-up visits.
Selected patients were fully informed about the study, signed the consent form, and underwent a 2-week run-in period that involved two office visits. Office BP was measured at each visit. Subjects were advised not to change their lifestyle during the study. Antihypertensive drugs were also not modified during the study. Baseline laboratory, clinical, physical activity, and nutrition evaluations were performed, and all patients underwent 24-h ABPM. After the run-in period, participants were randomly assigned to one of two parallel groups: (1) a placebo group with capsules containing microcrystalline cellulose and (2) an intervention group with capsules with 100,000 IU of cholecalciferol (25(OH)D). Participants ingested the medication in the presence of the researchers to ensure 100% compliance. Office BP, ABPM measures and clinical, physical activity, and nutrition evaluations were performed at the end of the study. The intervention period was 8 weeks.
Medication (50,000 IU Cholecalciferol; Vitamin D3; Addera™, Anapólis, Goias, Brazil) and placebo were bottled and labeled by Quinta Essência pharmaceuticals, and participants and researchers were blinded until the end of the study including the statistical analyses. All concomitant medications were kept unchanged to prevent possible effects on the study parameters.
Outcomes
Blood Pressure
The changes in ABPM measurements were the primary outcome, and changes in office systolic and diastolic BP were secondary outcomes. A change in BP was the difference between BP at the baseline and at the end of the study (8 weeks).
Blood pressure was measured at the office and by 24-h ABPM. Office BP was measured twice at each visit in at least 2-min intervals, in the sitting position after 5 min of resting (on a chair with feet on the floor and the arm supported at the heart level)15, and the mean value was used for analysis (Omron Automatic BP Monitor HEM-705CP, Omron Healthcare, Inc, Lake Forest, IL). Patients were advised to avoid caffeine, exercise, and smoking 30 min before the measurements. ABPM was measured at baseline and at the end the study (week 8). ABPM was performed by an oscillometry method using a Spacelabs device (Spacelabs Healthcare, Snoqualmie, WA 90,207, serial numbers 207-054280, 207-024751, 207-054290, 207-056568, and 207038016, with calibration certification), with a 15-min interval during the day and a 20-min interval during the night14,15,16. All ABPM measurements were obtained on a normal workday. Sleep time was recorded as the period between the time when the patient went to bed and the time when the patient woke up the next morning. The means of 24-h, daytime, and night-time systolic and diastolic BP, BP loads (percentage of 24-h and daytime BP measurements ≥ 140/90 mm Hg and night-time BP measurements ≥ 120/80 mm Hg) were recorded, as well as pulse pressure (PP) (systolic BP – diastolic BP). Nondipping was defined as the failure of the BP to fall by at least 10% during sleep14,16. The night-time/daytime BP ratios for systolic and diastolic BP were calculated by dividing the night-time by the daytime BP values. The BP status of the patients was classified according to ABPM measurements as dippers: N/D BP ratio ≤ 0.90; and non-dippers: N/D BP ratio > 0.9017.
Clinical evaluation, nutritional and physical activity assessment
Clinical and demographic data were collected based on standard protocol, and medical examination procedures were performed. Briefly, we collected data about type 2 DM and hypertension duration, smoking, alcohol intake, ethnic self-classification, current medications, and usual sunlight exposure on routine days. A detailed description of the nutritional and physical activity assessment of this trial has been published elsewhere18.
Laboratory measurements
Blood samples were collected after at least an 8-h fast, and the season of the year was recorded. A detailed description of plasma glucose, sodium, HbA1c, total cholesterol, high-density lipoprotein cholesterol (HDL-cholesterol), low-density lipoprotein cholesterol (LDL-cholesterol), triglycerides, and urinary 24-h albumin, calcium, and sodium was determined and has been published elsewhere18. Vitamin D (25(OH)D) and the parathyroid hormone (PTH) were determined by a chemiluminescence technique.
Statistical analysis
The estimated number of included patients (N = 43) was based on a 7.3 mm Hg reduction in office systolic BP with 2 SD, a power of 80% and an α of 0.05, after a single dose of 100,000 IU of cholecalciferol in patients with type 2 DM19. Twenty-one participants would be required in each group to achieve a power of 80% and an α of 0.05.
Results were expressed as mean (SD), median (P25–P75), or number of patients with the characteristic (percentage). Student t-tests, Mann–Whitney U tests, and Pearson chi-square tests were used as appropriate. All data analyses were performed using the statistical software package IBM SPSS version 20.0 (Chicago, IL), and the type I error rate was fixed at P < 0.05 (two-tailed). The P values of less than 0.05 were assumed to be significant.
Randomization
The researchers prepared a computer-generated randomization code (1:1) stored in sealed dark envelopes until the end of the study. Each participant was given two pills of medication or placebo in sequence to preserve allocation concealment. Pills were identical in presentation and participants and researchers were blinded until the end of the study including the statistical analyses for each group to which they were allocated.
Results
Figure 1 shows the flow diagram of the study protocol. Out of 127 screened patients (between October 2015 and December 2016), 84 were excluded, mainly due to normal vitamin D (n = 56), vitamin D or calcium supplementation (n = 23), and other (n = 4). Detailed characteristics of these patients are published elsewhere18.
All patients who received the intervention completed the protocol and were included in the final analyses. Baseline demographic, clinical, anthropometric, and laboratory characteristics of the participants according to the intervention and placebo groups are shown in Table 1. Mean age was 65 ± 9 years old, diabetes duration was 12 ± 8 years, BMI was 31 ± 4 kg/m2, 25(OH)D 14 ± 5 ng/ml, and HbA1c was 7.6 ± 1.0% (60 mmol/mol). Eighteen patients (42%) were classified as having resistant hypertension.
Vitamin D and BP measurements during the study
Both treatment and placebo groups had improvements in 25(OH)D after 2 months of protocol, but the placebo group remained with hypovitaminosis at the end of the study (25(OH)D 14 ± 5 to 23 ± 7, P = 0.02 and control group 15 ± 5 to 19 ± 5, P = 0.6). At the end of the study, 11 (52.4%) subjects continued to have hypovitaminosis in the placebo group in contrast with only 4 (18%) subjects in the intervention group (P = 0.02). Fasting glucose and glycated hemoglobin did not change throughout the study (P > 0.05).
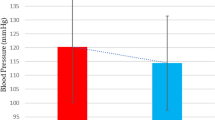
Figure 2 shows the changes in BP parameters during the study. Office diastolic BP [− 2.0 (− 4.0; 0.1) vs. 1 (− 2; 2) mm Hg; P = 0.02] decreased in the treatment group, and there was a similar trend in systolic BP [− 8 (− 10; − 2) vs. − 2 (− 5; 2) mm Hg; P = 0.07] in comparison with the placebo group. Regarding ABPM changes, there was a significant reduction in systolic 24-h [− 7.5 (− 12; − 0.5) vs. − 1 (− 6; 5) mm Hg, P = 0.02], daytime [− 7 (− 13; − 2) vs. − 1(− 5; 6) mm Hg; P = 0.007], and nighttime [− 7.0 (− 17; 1) vs. 3 (− 3; 10) mm Hg; P = 0.009] BP. Diastolic BP at 24 h [− 3.5 (− 6; − 0.8) vs. − 1 (− 3; 3.5) mm Hg; P = 0.04] and in daytime [− 5.0 (− 7.5; − 0.8) vs. 0.0 (− 4; 2) mm Hg; P = 0.01)] was also reduced in the intervention group.
Systolic and diastolic blood pressure (BP) differences for (A) office BP, (B) ABPM 24 h BP (C) ABPM daytime BP and (D) ABPM nighttime BP. The BP delta (end-of-study minus baseline) is shown as box plots (median is the line within the box, whiskers are 10th and 90th percentiles, the points above and below indicate outliers). Black identifies placebo and red identifies vitamin D group.
At the end of study, 65% of patients in the intervention group reached values of 24-h BP < 130/80 mm Hg as compared with 35% in the control group (P = 0.047). At the beginning of the study, only 26% of patients were dipping; however, at the end of the study, the intervention group dipped more [10 (46%) vs. 3 (14%); P = 0.03] than the control group. There was a significant reduction in 24 h pulse pressure in the vitamin D group (60 ± 9 to 57 ± 9 mmHg; P = 0.046). No serious adverse events were reported during the trial. Twenty-one percent reported a muscle and joint improvement in the intervention group as compared with 4.7% in the control group (P = 0.04).
Discussion
We demonstrate that a single dose of cholecalciferol improves BP in a short period of supplementation in patients with type 2 DM with hypertension and 25(OH)D < 20 ng/ml, regardless of vitamin D3 normalization.
In this sample of patients with type 2 DM, hypertension, and 25(OH)D < 20 ng/ml, the administration of a single dose of cholecalciferol resulted in clinically significant decreases in BP. The most relevant effects were observed in ABPM measurements, and decreases were observed in 24-h systolic (− 7.5 mm Hg), daytime systolic (7 mm Hg), and nighttime systolic (− 7 mm Hg) BP. The magnitude of observed BP reduction seems to be clinically relevant, and this reduction is similar to that observed with antihypertensive medications20. Furthermore, at the end of study, 65% of the patients in the intervention group reached values lower than 130/80 mm Hg in daytime ABPM, and nocturnal dipping was also more frequent in the intervention group.
As far as we know, this is the first study to evaluate ABPM readings after vitamin D supplementation in patients with type 2 DM, hypertension, and hypovitaminosis D. ABPM has been considered to be the reference standard for the diagnosis of hypertension, allowing a complete assessment of BP parameters: 24hour, daytime, and nighttime BP means and loads; nocturnal dipping patterns; and presence of masked and white-coat hypertension. ABPM is a better predictor of future cardiovascular events as compared with conventional office-based BP measurements21,22,23. A recent study confirmed that 24-h systolic ABPM was more strongly associated with all-cause and cardiovascular mortality than the office systolic BP24.
Nondipping is associated with microvascular complications in type 2 DM patients, like an increase in albuminuria and more rapid progression of diabetic kidney disease25,26. After vitamin D supplementation, more patients had dipping patterns than in the control group. Nondipping of BP is common in patients with DM26, and improvement in the dipping pattern with vitamin D supplementation seems to be a promising intervention in this population, in the same way as other strategies have already proven to be effective, such as diuretics and reduction in salt intake27.
The exact mechanism by which vitamin D lowers BP is still unknown. Vitamin D may be an inverse endocrine regulator of the renin-angiotensin system (RAS). Experimental studies suggest that cholecalciferol suppresses the renin system5,6; consequently, the inappropriate activation of the RAS could increase BP and the risk of cardiovascular diseases28. Based on that, some authors attribute the failure of previous trials to show antihypertensive effects of vitamin D to the concomitant use of renin-angiotensin inhibitors19,29. However, in our trial, 48.8% of the patients were using this class of medication, and despite that, 65% of the patients in the intervention group reached the recommended goals for daytime BP30.
It is possible that different populations have diverse responses to the anti-hypertensive effect of vitamin D. Most studies were performed in selected white populations19,29 or cultures that are not regularly exposed to sunlight31,32. Phenotypic analyses of a Mendelian randomization study demonstrated that people with genetic variants associated with low endogenous production of 25(OH)D have an increased risk of hypertension10. Brazilians have a mixed ethnic background that may be favorable to the antihypertensive response of vitamin D, but genetic tests were not performed in our sample.
Vitamin D directly regulates PTH hormone secretion, since this vitamin controls dietary calcium absorption. Even a slight vitamin D insufficiency is compensated by an increase in serum PTH. A meta-analysis that evaluated vitamin D3 supplementation according to subgroups of remeasured serum 25(OH)D on cardiovascular and glucometabolic surrogate markers suggested that vitamin D supplementation has a beneficial effect on PTH. On the other hand, a recent systematic review that evaluated patients with hyperparathyroidism and hypovitaminosis D, observed that vitamin D replacement did not modify PTH levels while serum 25(OH)D levels were improved33. In our study, baseline PTH was similar in both groups, and we believe that eight weeks is a short period of time to change this hormone. In fact, in patients with secondary hyperparathyroidism from severe vitamin D deficiency, parathyroid hyperplasia may take months to over a year to reverse to normal34. Our study was not designed to evaluate the independent effects of PTH on BP or any mechanism of action of vitamin D3. We believe this a pioneering study that evaluated the effect of vitamin D3 only in insufficient patients, thus it is a proof of concept study of the action of this vitamin in this population.
There are important strengths in our study. The first one is the design that guarantees 100% compliance, as medication was taken in the presence of the investigator, and the second is the full scope of BP evaluation. Furthermore, there was no change in the office BP during the run-in period, excluding a possible Hawthorne effect in our sample, and no antihypertensive medication was changed during the study, avoiding other medication bias.
A possible limitation of this study is the short follow-up period: although we demonstrated an important improvement in BP after vitamin D supplementation, the beneficial effects of this intervention were only evaluated in an 8-week period. It is unknown if this effect would endure for longer periods and if it will be sustained after reaching vitamin D sufficiency for longer periods. Longer and larger clinical trials properly addressing this question in patients with type 2 DM and hypertension, as well as the capability of vitamin D to reduce hard cardiovascular outcomes, are needed.
In conclusion, a single dose of vitamin D3 improves BP in patients with type 2 DM, hypertension, and vitamin D insufficiency, regardless of vitamin D normalization. Since the values of vitamin D are usually low in such patients18,19, the supplementation of vitamin D may be part of the therapeutic arsenal in this scenario.
References
McGreevy, C. & Williams, D. New insights about vitamin D and cardiovascular disease. Ann. Intern. Med. 155, 820–882 (2011).
Grossman, H. Ambulatory blood pressure monitoring in the diagnosis and management of hypertension. Diabetes Care 36(suppl 2), 307–331 (2013).
Ke, L., Mason, R. S., Kariuki, M., Mpofu, E. & Brock, K. E. Vitamin D status and hypertension: a review. Integr. Blood Press Control 8, 13–35 (2015).
Mirhosseini, N., Vatanparast, H. & Kimball, S. M. The Association between Serum 25(OH)D status and blood pressure in participants of a community-based prog. Nutrients 9(11), E1244 (2017).
Li, Y. C. Vitamin D: roles in renal and cardiovascular protection. Curr. Opin. Nephrol. Hypertens. 21, 72–79 (2012).
Zhang, W. et al. Administration of exogenous 1,25(OH)2D3 normalizes overactivation of the central renin-angiotensin system in 1α(OH)ase knockout mice. Neurosci. Lett. 19(588), 184–189 (2015).
Witham, M., Adnan Nadir, M. & Struthers, A. D. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J. Hypertens. 27, 1948–1954 (2009).
Beveridge, L. A. et al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA 175(5), 745–754 (2015).
Paula, T. P., Kramer, C. K., Viana, L. V. & Azevedo, M. J. Effects of individual micronutrients on blood pressure in patients with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. Sci. Rep. 7, 40 (2017).
Vimaleswaran, K. S. et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2(9), 719–729 (2014).
Shu, L. & Huang, K. Effect of vitamin D supplementation on blood pressure parameters in patients with vitamin deficiency: a systematic review and meta-analysis. J. Am. Soc. Hypertens. 12(7), 488–496 (2018).
Schulz, K. F., Altman, D. G., Moher, D. & CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann. Intern. Med. 152(11), 726–732 (2010).
World Medical Association. Ethical principles for medical research involving human subjects, 64th WMA General Assembly, Fortaleza, Brazil. JAMA 310(20), 2191–2194 (2013).
Chobanian, A. V. et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 42, 1206–1252 (2003).
O’Brien, E. et al. Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ 322, 531–536 (2001).
Staessen, J. A. et al. Nocturnal blood pressure fall on ambulatory monitoring in a large international database. The “Ad Hoc” Working Group. Hypertension 29, 30–39 (1997).
Fagard, R. H. Dipping pattern of nocturnal blood pressure in patients with hypertension. Expert Rev. Cardiovasc. Ther. 7(6), 599–605 (2009).
Moreira, J. S. R. et al. Association of plasma vitamin D status with lifestyle patterns and ambulatory blood pressure monitoring parameters in patients with type 2 diabetes and hypertension. Diabetes Res Clin. Pract. 139, 139–146 (2018).
Sugden, J. A., Davies, J. I., Witham, M. D., Morris, A. D. & Struthers, A. D. Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabet. Med. 25, 320–325 (2008).
Psaty, B. M. et al. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA 289(19), 2534–2544 (2003).
Fan, H. Q. et al. International database on ambulatory blood pressure in relation to cardiovascular outcomes investigators: prognostic value of isolated nocturnal hypertension on ambulatory measurement in 8711 individuals from 10 populations. J. Hypertens. 28(10), 2036–2045 (2010).
Piper, M. A. et al. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 162(3), 192–204 (2015).
Clement, D. L. et al. Prognostic value of ambulatory blood pressure recordings in patients with treated hypertension. N. Engl. J. Med. 348(24), 2407–2415 (2003).
Banegas, J. R. et al. Relationship between clinic and ambulatory blood-pressure measurements and mortality. N. Engl. J. Med. 378, 1509–1520 (2018).
Lurbe, E. et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N. Engl. J. Med. 347(11), 797–805 (2002).
Leitao, C. B. et al. Blood pressure means rather than nocturnal dipping pattern are related to complications in type 2 diabetic patients. Diabet. Med. 25, 308–313 (2008).
Mehta, R. & Drawz, P. E. Is nocturnal blood pressure reduction the secret to reducing the rate of progression of hypertensive chronic kidney disease?. Curr. Hypertens. Rep. 13(5), 378–385 (2011).
Mehrotra, R. et al. Chronic kidney disease, hypovitaminosis D, and mortality in the United States. Kidney Int. 76, 977–983 (2009).
Witham, M. D. et al. The effect of different doses of vitamin D3 on markers of vascular health in patients with type 2 diabetes: a randomized controlled trial. Diabetologia 53, 2112–2119 (2010).
Whelton, P. K. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension 71(6), e13–e115 (2018).
Nasri, H., Behradmanesh, S., Ahmadi, A. & Rafieian-Kopaeiet, M. Impact of oral vitamin D (cholecalciferol) replacement therapy on blood pressure in type 2 diabetes patients: a randomized, double-blind, placebo controlled clinical trial. J. Nephropathol. 3, 29–33 (2014).
Shab-Bidar, S. et al. Regular consumption of vitamin D-fortified yogurt drink (Doogh) improved endothelial biomarkers in subjects with type 2 diabetes: a randomized double-blind clinical trial. BMC Med. 9, 125–135 (2009).
Loh, H. H., Lim, L. L., Yee, A. & Loh, H. S. Vethakkan SR Effect of vitamin D replacement in primary hyperparathyroidism with concurrent vitamin D deficiency: a systematic review and meta-analysis. Minerva Endocrinol. 44(2), 221 (2019).
Al-Ali, H. & Fuleihan, G. E. Nutritional osteomalacia: substantial clinical improvement and gain in bone density posttherapy. J. Clin. Densitom. 3(1), 97 (2000).
Acknowledgements
The authors thank all participants with type 2 DM, hypertension and hypovitaminosis D who participated. The authors also acknowledge the invaluable support from the medical student and nutritional student teams. Our thanks to Projeto Nacional de Pós-Doutorado (PNPD/CAPES), and FIPEHospital de Clínicas de Porto Alegre. The medication in this study was provided by Brainfarma Indústria Química e Farmaceutica S.A. Finally, this manuscript was dedicated to the memory of our dear friend, colleague, mentor, and coauthor MJA, who died in May 2017.
Funding
This study was supported by FAPERGS/CNPQ – Edital 12/2014, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundo de Incentivo à Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE). TPP received grants from Projeto Nacional de Pós-Doutorado (PNPD/CAPES).
Author information
Authors and Affiliations
Contributions
T.P.P., and L.V.V. designed the research; T.P.P., L.F.S., M.E.P.M., J.M., and L.V.V. conducted the research; and T.P.P. and L.V.V. analyzed the data; T.P.P. and L.V.V. wrote the article; and T.S. and J.M. contributed to the discussion and reviewed/edited the article. T.P.P. had primary responsibility for the final content, and all authors read and approved the final manuscript draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Paula, T.P., Moreira, J.S.R., Sperb, L.F. et al. Efficacy of single-dose cholecalciferol in the blood pressure of patients with type 2 diabetes, hypertension and hypovitaminoses D. Sci Rep 10, 19611 (2020). https://doi.org/10.1038/s41598-020-76646-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-76646-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.