Abstract
The Sterile Insect Technique (SIT) is based on the mass release of sterilized male insects to reduce the pest population size via infertile mating. Critical for all SIT programs is a conditional sexing strain to enable the cost-effective production of male-only populations. Compared to current female-elimination strategies based on killing or sex sorting, generating male-only offspring via sex conversion would be economically beneficial by doubling the male output. Temperature-sensitive mutations known from the D. melanogaster transformer-2 gene (tra2ts) induce sex conversion at restrictive temperatures, while regular breeding of mutant strains is possible at permissive temperatures. Since tra2 is a conserved sex determination gene in many Diptera, including the major agricultural pest Ceratitis capitata, it is a promising candidate for the creation of a conditional sex conversion strategy in this Tephritid. Here, CRISPR/Cas9 homology-directed repair was used to induce the D. melanogaster-specific tra2ts SNPs in Cctra2. 100% female to male conversion was successfully achieved in flies homozygous for the tra2ts2 mutation. However, it was not possible, to identify a permissive temperature for the mutation allowing the rearing of a tra2ts2 homozygous line, as lowering the temperature below 18.5 °C interferes with regular breeding of the flies.
Similar content being viewed by others
Introduction
The production of large populations of only male pest insects is a key factor for the Sterile Insect Technique (SIT), a highly successful, environment-friendly, and species-specific method of pest control. Proposed in 1955 by Knipling1, the SIT is based on the sustained mass-release of sterile males into the existing pest population to reduce population size by infertile mating, and has been successfully applied to several pest species2,3,4,5. The release of pure male populations is important because male-only releases are more effective than bisexual ones6 by preventing the mating of sterile males with the co-released sterile females. In addition, the release of sterile females could still result in crop damage due to oviposition or, in case of vector insects, in disease transmission. Sexing, classified as the removal of females from a mass-reared insect population, can be achieved by physical sorting, female-specific lethality, or by converting females into males7. Such solutions have been developed for multiple pest species using naturally occurring or classically induced mutations8,9,10,11 or transgenesis12,13,14,15,16,17. Most of them, however, are not ready for mass-rearing yet. To allow efficient rearing of sexing strains and cost-effective operation of the program, important characteristics of sexing systems are the conditionality and early developmental time-point of the sexing, respectively. Currently, two conditional embryonically active systems exist for the devastating agricultural pest Ceratitis capitata (Wiedemann; Diptera: Tephritidae) (Mediterranean fruit fly, medfly). Medflies pose a vast economic threat to agriculture worldwide, as they feed on > 260 plants (fruits, vegetables, nuts) and are highly invasive: Native to the Afrotropical region, medfly can now be found in most tropical and temperate regions18,19.
In the successful medfly genetic sexing strains (GSS), VIENNA 7 and 8, an unknown recessive autosomal temperature-sensitive lethal (tsl) mutation eliminates all female embryos upon heat shock11. The GSS males, however, are semi-sterile due to chromosomal rearrangements necessary to rescue the WT phenotype, resulting in 50% genetically imbalanced gametes and thus non-viable zygotes. In a conditional transgenic embryonic sexing system (TESS) medfly female embryos are killed by overexpression of a pro-apoptotic gene20. The TESS can be switched off for strain maintenance by adding the antibiotic tetracycline to the fly food (Tet-off system). Compared to these systems, a sexing system based on temperature-inducible female-to-male conversion would have two advantages: (1) doubling or, compared to semi-sterile GSS, even quadrupling the number of males for the release and (2) abolishing the use of antibiotics. Both factors would considerably reduce costs and increase the efficiency of a medfly SIT program. However, population maintenance would presumably need to be done at reduced temperatures, which could decrease the productivity of the mass-rearing due to prolonged development times21. Currently, the production of one million sterile medfly pupae of the classical GSS is estimated at US$ 250–500, depending on the production level and the location of the rearing facility22.
In search of genetic elements suitable to construct sexing or sex-conversion systems, insect sex determination pathways have been studied to identify essential genes and to understand their function. The transformer-2 gene (tra2) is involved in the sex determination pathway of different insects, including C. capitata23,24. In medfly, transformer-2 is an auxiliary factor, necessary to establish and sustain the autoregulation of transformer, a gene known to be crucial for the sexual fate23,25,26. As illustrated and described in detail elsewhere23,26, maternal Cctra and Cctra2 initiate a positive feedback loop in XX fertilized eggs and control the female-specific splicing of the downstream targets doublesex and fruitless23,26. Switching off either Cctra or Cctra2 leads to male development26 and the transient knock-down of Cctra2 during embryogenesis via RNA interference (RNAi) resulted in full sex-reversal of XX-karyotype flies into phenotypic males23. In contrast to Anastrepha suspensa, where embryonic injection of dsRNA against Astra2 resulted in sex-reversed XX males, which were infertile despite testes full of sperm bundles27, medfly XX-karyotype males were fertile23,25, indicating that male-fertility is not Y-dependent in C. capitata. Sex-reversion via RNAi-mediated knock-down of tra2 was also shown in Bactrocera tau (Walker)28 and B. dorsalis (Hendel)29. However, to make use of the tra2-mediated sex-conversion for male-only production, it needs to be conditionally inducible and stable. In Drosophila melanogaster, two tra2 temperature-sensitive mutations (tra2ts1, tra2ts2) are known, supposedly causing conformational changes in the protein structure at elevated (restrictive) temperatures (29 °C). These result in a loss of protein function and therefore in sex-conversion of XX embryos (male-only offspring). At permissive temperatures (e.g. 16 °C), a functional TRA2 protein allows healthy female development and rearing of the population30,31. Due to the high conservation of TRA2 among different species23,32,33,34,35,36, gene editing techniques such as CRISPR/Cas37 can be used to exactly recreate temperature-sensitive tra2 mutations known from D. melanogaster in homologous genes of pest insects. This has been shown by Li and Handler38, who introduced the D. melanogaster tra2ts2 mutation together with a fluorescent marker into the D. suzukii tra2 gene. 16 °C and 20 °C were permissive temperatures for D. suzukii tra2ts2 mutants38, resulting in fertile and normally developed males and females. At 26 °C, all XX embryos developed as sterile intersex with sex combs and male-like genitalia, and all XY embryos showed dysmorphic testes and were sterile. However, the survival rate for both, wild-type and mutant flies was very low (5–10%) at this temperature and even lower at more elevated temperatures. While this temperature-sensitivity of D. suzukii would be problematic if the tra2ts2 mutation were to be used for sexing in an SIT application, this should not be an issue for medfly, which can be reared at 26 °C. Based on this fact and the promising results from the previous transient knock-down of tra2 in C. capitata23, Cctra2 is a good candidate for the construction of a temperature-based sex-conversion system in medfly.
Hence, we used our previously established protocol for markerless CRISPR/Cas9-HDR in medfly yielding high-efficiency mutagenesis39 to integrate the D. melanogaster tra2ts1 and tra2ts2 mutations into the Cctra2 homolog. Omitting the use of a fluorescent marker gene should facilitate the use of non-transgenic strains in SIT programs, as CRISPR/Cas9-induced single nucleotide polymorphisms (SNP) are even considered non-GMO in certain countries40.
Results
Cctra2 mutagenesis: gRNA and repair template design
CRISPR/Cas9 HDR gene editing was used to separately re-create the two temperature-sensitive D. melanogaster tra2 mutations (ts1, ts2) in the C. capitata homolog Cctra2 (NCBI Gene ID: 101452698). Positions of the mutations were determined by comparing amino acid sequence identity for D. melanogaster and medfly TRA2. The mutated Alanine151 in the Dmel tra2ts1 (Ala151Val)30 corresponds to Ccap Ala158, the Prolin181 of the Dmel tra2ts2 mutation (Pro181Ser) to Ccap Pro188. The ts2 mutation is located in a 19 aa linker region, which is a unique feature of TRA2 and highly conserved among species23,32,33,34,35,36 (Fig. 1a).
Strategy to re-create D. melanogaster tra2ts alleles in C. capitata tra2. (a) Amino acid alignment of C. capitata and D. melanogaster TRA2. Shown are the RNA recognition motif (RRM, black), two ribonucleoprotein identifier sequences (RNP motifs, grey), the linker region (blue), and the position of the tra2ts1 and tra2ts2 mutations (red). Consensus is shown in black, amino acids with similar characteristics in grey. (b) Overview of Cctra2 gene structure (tra2 exon structure, light grey: CDS, dark grey: UTR), primers used for genotyping (P1500/P1401) or for genomic positive control PCRs (P1500/P1532 and P1500/P1401), position of single guide RNAs (blue arrows) and mutations mediated by the HDR repair templates (ssODN). PAM sequences are marked in light yellow, position of SNPs introduced by HDR are shown and marked either in blue (silent mutation) or in red (functional mutations). Resulting amino acid exchanges are indicated.
For both mutations, a single guide RNA (gRNA) and a 140 nt single-stranded oligodeoxynucleotide (ssODN) repair template were designed to introduce the amino acid exchanges corresponding to the Dmel ts1 or ts2 mutations (ts1: 158 Ala > Val, ts2: 188 Pro > Ser), to create temperature-sensitive versions of the CcTRA2 protein. The repair template ssODN_tra2_ts1 differs from the wild-type tra2 ORF sequence by two bases, a C > T transition at position 473 of the CDS to introduce the ts1 SNP and the silent mutation 477 G > A that removes the PAM sequence to prevent re-editing. ssODN_tra2_ts2 differs by one base introducing the ts2 SNP (CDS: 562 C > T) (Fig. 1b).
Preliminary gRNA tests to confirm editing capability of tra2ts positions
To assess the functionality of the tra2ts1 and tra2ts2 gRNAs, each was injected complexed with Cas9 protein and either without (non-homologous end joining, NHEJ, knock-out) or with repair template (homology-directed repair, HDR, knock-in). G0 survivors of these injections were reared at 26 °C. 327 Egypt II wild-type (EgII WT) embryos were injected for tra2_ts1 knock-out. Ten reached adult stage (six males, four females) (Table 1a). One male was fertile. The ts1 injection with repair template (290 EgII embryos) yielded four viable but infertile adults (two males, two females), and three adults got stuck in the puparium while eclosing and died (two males, one female) (Table 1a). None of the ts1 G0 adults showed external phenotypic abnormalities. To check for editing activity of gRNA_tra2_ts1, the tra2 genotype of four randomly chosen G0 flies (two from each injection) was analysed by subcloning the tra2-specific PCR products. One of two knock-out injected G0 flies showed a 1 bp deletion in one of five sequenced clones. One of the knock-in injected G0 flies showed two independent events within five sequenced clones, the tra2ts1 HDR genotype or a 6 bp deletion (Supplementary Fig. S1a).
The tra2ts2 gRNA knock-out injection yielded six adult males from 367 injected EgII embryos (Table 1b), three of them were fertile. Additionally, three G0 flies stuck in the puparium did not survive (two males, one intersex IS1-KO). The tra2ts2 knock-in mix was injected into 244 EgII embryos (Table 1b). Eight developed to adults (six males, two intersex: IS1, IS2), and four died during eclosing (one male, three intersex: IS3-6). Intersex flies showed varying degrees of phenotypically male and female characteristics (genital terminalia apparatus and bristles) (Fig. 2a), and were sterile. In contrast, all six G0 males were fertile. The genotype of six ts2 G0 flies from the knock-out (males M5, M6, and intersex IS1-KO) and knock-in injection (IS1, IS4, IS5) was analysed. All showed NHEJ events ranging from 33 bp deletions to 4 bp insertions (Supplementary Fig. S1b). G1 offspring from both injections was not analysed.
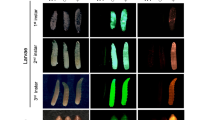
Somatic modification of tra2ts2 causes intersexuality with external and internal phenotypic abnormalities in G0. G0 survivors of tra2ts2 KI injections reared at 26 °C (a) and 19 °C (b) show intersex phenotypes with malformed external and internal reproductive organs and mixed male- and female-specific characteristics. Phenotypes included deformed ovipositors (IS1, IS2), a mixture of male- and female-specific bristles on the femur (IS3) or the head (IS8), absent genitalia (IS3), and various degrees of deformed male genitalia, combined with ovaries without spermatheca (IS6), testes-like structures (IS8, IS13) or no identifiable reproductive organs (IS11). For comparison, wild-type males have two spatulated bristles on the head, non-pigmented bristles on the femur, and male genitalia. Wild-type females have no spatulated bristles on the head, long pigmented bristles on the femur, and a prominent ovipositor. Male characteristics are highlighted by blue arrows, female characteristics by black arrows. (c) Karyotyping via Y-chromosome-specific PCR of intersex phenotype tra2ts2 KI individuals (19 °C) revealed XX-karyotype for all intersex individuals. Shown is the Y-chromosome-specific PCR (primers P1504/1505) on genomic DNA extracted from a single fly and a genomic positive control on tra2 with primers P1401/1500 using the same DNA samples. Wild-type male (WT m) and female (WT f) are shown as control. Displayed are cropped parts from different gels. Uncropped versions of the gels are provided in the supplement (Supplementary Fig. S4). L = DNA ladder; kb = kilo base pairs.
These experiments confirmed the editing activity of the ts1 and ts2 gRNAs. The lack of fertile G0 in the ts1 injections and the complete lack of females and appearance of intersexes in the ts2 injections, however, indicated that 26 °C is a restrictive temperature for the Cctra2ts mutations.
Evaluation of medfly rearing at low temperatures
To evaluate if D. melanogaster and D. suzukii tra2ts2 permissive temperatures, 16 °C or 16 to 20 °C, respectively30,31,38, are applicable to medfly, newly eclosed WT EgII (60–160 adults per experiment) were transferred from 26 °C to 16, 18, or 19.5 °C and eggs of these crosses were collected for seven days (for temperature profiles and egg collection timepoints see Supplementary Table S1 and Fig. S2a–c). At 19.5 °C, the number of adult offspring was reduced to about 40%, compared to 26 °C, and at 18 °C to about 1%. At 16 °C, no larvae hatched from more than 2,000 collected eggs. Hence, 19 °C was chosen as a possible rearing and potential permissive temperature for the subsequent Cctra2ts injections.
CRISPR/Cas9-HDR injections at 19 °C do not produce stable Cctra2ts1 lines
Rearing of ts1-injected G0 at 19 °C increased the number of adult G0 survivors to 19.5% compared to 3% and 2.4% for the ts1 injections at 26 °C (Table 1a). None of them showed external phenotypic abnormalities. Twelve G0 flies were backcrossed individually to EgII (M5-M10 and F4-F9), remaining flies were backcrossed in three groups (M-group I, F-group I, II). After allowing sufficient time for mating and egg laying, all individually crossed G0 flies were dissected to examine their reproductive organs. Phenotypes included females without ovaries (F4), only one ovary (F7), or normal ovaries (F6, F8, F9). Males showed normal reproductive organs, except for M7, which had no testes (Supplementary Fig. S3a, b). F5 and M10 died and could not be dissected. Overall, 47 G1 flies eclosed from eight fertile families (F6, F8, M6, M8, M9, M10, group M_I and F_II). Since no phenotypic marker was inserted to track successful mutagenesis in G1, non-lethal genotyping was used to analyse G1 offspring reared at 19 °C for the presence of the tra2ts1 mutation. DNA was extracted from a single leg, and the ts1 target site region was PCR-amplified and sequenced. 38 of 47 G1 flies provided sufficient quality sequence information. All showed WT genotype.
CRISPR/Cas9-HDR successfully creates inheritable Cctra2ts2 mutation at 19 °C
Rearing of ts2 HDR-injected G0 at 19 °C yielded lower survival numbers than the ts1 HDR injection, but still about twice as high as the experiments at 26 °C (7.1% compared to 2.4% and 4.9%; Table 1b). Injection of 181 EgII embryos resulted in five viable males and six intersex. Additionally, two intersex flies (IS9, IS10) died during eclosing. Males and intersex were individually backcrossed to WT virgin females. Eggs were collected every second day for 10 consecutive days (for temperature profile during egg collection see Supplementary Table S1, Fig. S2b). Two of the eleven crosses (M8, M11) produced G1 offspring (Supplementary Table S2). After mating, all alive G0 were dissected. Males M8, M9, and M11 showed normal testes, while M10 did not have testes (Supplementary Fig. S3c). Flies with intersex phenotype showed apparently normal ovaries but no spermathecae (IS6), hypertrophic testes (IS8), miniaturized testes (IS13), or no identifiable reproductive organs (IS7, IS11, IS12; Fig. 2b). To assess the karyotype of all 13 G0 flies, PCR on Y-chromosome-specific repetitive elements was performed, whereby absence of a PCR signal implies a XX-karyotype. None of the intersex phenotype G0 flies was positive for the Y-chromosome-specific PCR (Fig. 2c), indicating that all XX (female) karyotype G0 embryos were transformed to intersex flies. The absence of phenotypically female G0 in all three tra2ts2 injections indicates a high efficiency of gRNA_tra2_ts2 and the importance of the targeted position for proper TRA2 function in female sex development.
The tra2 genotype of the G1 flies was analysed via non-lethal genotyping. For family M8, ten of twelve analysed G1 (83%) were heterozygous for the knock-in genotype (tra2ts2), and two (17%) carried NHEJ events. The remaining individuals were not analysed due to low DNA quality. From the G1 offspring of family M11, 60 flies were randomly chosen for genotyping. The heterozygous tra2ts2 genotype was found in 45 flies (75%). This percentage was similar in males (26 of 33) and females (19 of 27). Nine flies (15%) were WT and six flies could not be analysed (low DNA quality).
Inbreeding of the ts2 mutation at 19 °C does not produce phenotypic females homozygous for tra2ts2
Heterozygous tra2ts2 mutant G1 flies were either inbred or backcrossed to EgII to ensure the propagation of the line if inbreeding should turn out to be sterile. Details on crosses, egg collection numbers, and temperature profiles are shown in Supplementary Tables S1, S3, and Fig. S2. Inbreeding of heterozygous M8 offspring produced 121 G2 flies with a 1:2 female to male ratio (Supplementary Table S3). 27 of 78 phenotypic G2 males were homozygous for the ts2 mutation (tra2ts2|ts2), 38 were heterozygous (tra2ts2|WT), and 13 were WT (tra2WT|WT, Fig. 3a). In contrast, none of the 38 phenotypic females were homozygous for tra2ts2, 24 were heterozygous, and 14 had two WT tra2 alleles (Fig. 3a). Inter se crosses of M11 offspring resulted in a similar phenotypic female to male ratio as M8 inbreeding (26 and 42, respectively). Non-lethal genotyping showed that also M11 inbreeding produced phenotypic tra2ts2-homozygous males (21%), but no phenotypic females with two tra2ts2 alleles (Fig. 3a). Backcross of tra2ts2 heterozygous M11 offspring produced a 1:1 phenotypic sex ratio (Supplementary Table S3), which was not further analysed molecularly.
Analysis of tra2ts2 genotypes and phenotypes in G2. (a) Shown are frequencies of tra2 genotypes (homozygous for the WT or the tra2ts2 allele, or heterozygous tra2ts2 mutants) within the number of analyzed individuals (n), found in phenotypic male or female G2 offspring of family M8 and M11 inbreeding (ib). Both families are lacking homozygous tra2ts2 mutants with a female phenotype. (b) Karyotyping of phenotypic G2 males via Y-chromosome specific PCR (primers P1504/1505) on genomic DNA extracted from a single leg of family M8 and M11 offspring. A positive control PCR was performed on tra2 with primers P1532/P1500 using the same DNA samples as in the Y-specific PCR, to exclude lack of PCR product due to DNA quality. Individuals lacking a signal in the Y-chromosome-specific PCRs but not in the genomic control PCR are marked in bold letters to indicate the XX-karyotype. M11ib_m29 was excluded from the analysis, due to low DNA quality. One phenotypic male (WT m) and female (WT f) from family M8 with WT tra2 genotype are shown as controls. Displayed are cropped parts from different gels. Uncropped versions of the gels are provided in the supplement (Supplementary Fig. S5a and b). L = DNA ladder; kb = kilo base pairs. (c) Phenotypic male flies carrying the tra2ts2 mutation were dissected and compared to WT EgII flies to assess external and internal sexual organ formation. Shown are representative tra2ts2 homozygous XX (M8ib_m14, M8ib_m65) or XY (M8ib_m30) individuals as well as one XY male heterozygous for tra2ts2 (M8ib_m75). Black, dark and light grey boxes indicate the tra2 genotype, with colors following the legend in (a). Mutants were not able to coil and store their distiphallus. Testes were normal or decolorized (M8ib_m75).
tra2ts2 homozygous XX embryos are transformed into phenotypic males at 19 °C
The absence of phenotypic females homozygous for the tra2ts2 mutation in G2 implied that XX embryos homozygous for tra2ts2 are either not viable or transformed into phenotypic males at 19 °C. Y-specific primers were used to assess the karyotype of 35 G2 tra2ts2-homozygous and 60 heterozygous male G2 flies by PCR. In family M8, nine of 27 phenotypic males homozygous for tra2ts2 showed a signal in the control genomic PCR but not in the Y-chromosome-specific PCR, confirming the transformation of tra2ts2-homozygous XX flies into phenotypic males. This also applied to two out of eight phenotypic males in family M11 (Fig. 3b). For one M11 offspring, M11ib_m29, no statement can be made as the control PCR failed to produce a signal. In contrast, all tra2ts2-heterozygous males were positive for the Y-chromosome-specific PCR (Supplementary Fig. 6), excluding sex conversion as reason for the male-biased sex ratio in the G2 heterozygotes.
Dissection of six XX- and four XY-karyotype males homozygous for tra2ts2, and two XY tra2ts2-heterozygous males (all G2) showed that all tra2ts2-homozygous males (XX and XY) had apparently normal or slightly decolorized testes. The two tra2ts2-heterozygous males, in contrast, showed severely decolorized testes (Fig. 3c). In addition, across the G2 offspring of both families, M8 and M11, 81.8% of the tra2ts2 homozygous XX males, 4.3% of the tra2ts2 homozygous XY males, and 16.6% of the tra2ts2 heterozygous XY males were not capable to coil and store their distiphallus (Fig. 3c). This phenotype was also observed in random samples of WT flies of different ages; while its penetrance in WT is higher at 19 °C (24.8%, n = 161) than at 26 °C (6.9%, n = 174), it is still markedly lower than observed in the tra2ts2 homozygous XX males (81.8%, n = 11) and might, therefore, be also an effect of the ts2 mutation.
Rearing at lower temperature leads to low fertility rates
Based on the karyotyping experiments, 19 °C still is a restrictive temperature for the ts2 mutation in Cctra2, contrary to D. suzukii tra2ts2 where 20 °C was permissive38. Data from D. melanogaster suggests 16 °C as permissive temperature30,31. However, medflies do not breed at such low temperatures, as the small-scale fertility tests at 16 °C had shown. To attain a permissive temperature for the medfly tra2ts2 mutation that does not affect breeding, the temperature was lowered to 18.5 °C, the mating threshold temperature41, for G2 crossing and egg laying (Supplementary Table S1, Fig. S2c). ts2-homozygous XX and XY G2 males were backcrossed to EgII females individually (13 crosses) or in groups (two crosses). ts2-heterozygous males and females were inbred (three crosses) or backcrossed (one group). Overall, during 13 days and 81 egg collections, more than 8,000 eggs were collected from these 19 crosses (Supplementary Table S4). A total of five larvae hatched from two egg collections of homozygous tra2ts2 XY male group-backcrosses, and only one survived to adulthood (M11ib_m1-het, Supplementary Table S4). Noteworthy, due to technical restrictions the temperature could not be kept constantly at 18.5 °C during the experiment, and these larvae hatched from a late egg collection (383 h; Supplementary Fig. S2c), prior to which the temperature had been above 18.5 °C for about two days. The male (G3) was crossed to 40 EgII females but did not reproduce. Therefore, maintaining the ts2 mutant strain by lowering the temperature to a permissive range was not possible.
Discussion
CRISPR/Cas9-HDR gene editing was used to create temperature-sensitive mutations in the C. capitata sex-determination gene transformer-2, equivalent to the two chemically induced point mutations in D. melanogaster30,31. The D. melanogaster tra2ts temperature-dependent sex-conversion phenotype promises great advantages for creating male-only populations needed for SIT programs, as it doubles the amount of male offspring per parental egg capacity, and only heat is needed for induction. Some countries do not regulate the use of organisms carrying CRISPR-induced SNPs as they could have also occurred by natural mutagenesis and selective breeding40,42. Hence, only the trats SNPs, but no exogenous DNA was inserted, to help facilitate a potential field release of Cctra2ts strains. This was possible due to the high mutagenesis rate achieved with our previously published CRISPR/Cas9-HDR protocol39, which we now successfully applied for the first time without using a visible phenotype.
The injections aiming at creating the tra2ts1 allele did not result in any mutant G1 offspring at 19 °C, despite promising prerequisites; ts1 gRNA and ssODN were functional in the preliminary tests at 26 °C, and the high number of G0 adult survivors in the 19 °C injection increased the chance to obtain mutant offspring. Moreover, G0 flies showed deformities of internal reproductive organs (Supplementary Fig. 3b). It can’t be excluded, however, that these are the result of physical damage to the embryo caused by the injection. Possible reasons for the poor efficiency of the ts1 knock-in could be the low on-target activity score of the ts1 gRNA (0.045), or a stronger phenotypic impact of the tra2ts1 mutation compared to tra2ts2 as observed in D. melanogaster30, which could reduce the chance to obtain viable ts1 mutant flies. Testing of other ts1 gRNAs could shed more light on possible reasons for the failure to create a stable ts1 line; but considering the decreased viability in D. melanogaster and the permissive temperature issues in medfly, these experiments have little prospect for success.
In contrast, the tra2ts2 mutation could be introduced with high efficiency, detectable already from the absence of phenotypic females and the appearance of intersexes in G0, in the frequency of HDR-positive fertile G0 (100% at 19 °C), as well as in the high penetrance of the mutant genotype within their G1 offspring (83% and 75% knock-in for family M8 and M11, respectively). This matches the higher on-target activity score of the ts2 gRNA (0.140).
The observed overall higher survival rate of injected G0 at 19 °C compared to 26 °C might be the result of a lower Cas9 editing activity43 and a potentially associated off-target rate, but could also be connected to the reduced speed of embryonic development allowing more time for repair mechanisms to fix injection-induced damage to the embryo44, which is unrelated to Cas9 editing. Extensive comparative injections would be needed to answer this question.
The lack of phenotypic females homozygous for tra2ts2 and the conversion of XX embryos into phenotypic males at 19 °C suggests that this is still a restrictive temperature for the Cctra2ts2 mutation, which does not allow correct protein folding, and indicates the importance of this position in the highly conserved TRA2 linker region for correct protein conformation. This observation is in line with the results obtained for the D. melanogaster tra2ts2 mutation, where the temperature had to be lowered to 16 °C to generate fertile males and females, while 18 °C produced sterile males and females, and 29 °C resulted in sterile males and pseudomale-like intersexes30. A further reduction of the temperature to an average of 18.3 °C, however, resulted in a loss of the strain due to mainly unviable eggs deposited by the G2 generation. This was not unexpected since our small-scale tests with WT at 18 °C and 16 °C produced very little or no viable offspring, respectively. Furthermore, some males were not capable of coiling and storing their distiphallus. While this phenotype was also observed in WT males at low temperatures, it seems to be enhanced by the tra2 mutant allele. However, the numbers are too small for a robust statement. Fertility and mating behaviour of this phenotype have not been assessed.
While the mean survivorship of medfly egg and larval stages at 15, 20, 25 and 30 °C has been reported to not differ significantly21, and the described threshold for ovarian maturation with 8.1 °C to 16.6 °C21,41,45 is also below the tested Cctra2ts2 permissive temperature of 18.3 °C, Prokopy and Hendrichs41 showed that 18.5 °C is the temperature threshold for mating in medfly. During the cross of tra2ts2 G2 flies, temperatures were above the threshold mainly during the first days (1–72 h) and last days (337–517 h) of the crossing (Supplementary Table S1, Fig. S2c). As ovarian maturation takes up to 10 days at this temperature, and crosses have been set up with 3–5 d old flies, no successful mating could have been achieved during the first period above 18.5 °C. During the main egg collection period (72–336 h), temperature was mainly below 18.5 °C (Supplementary Fig. S2c). The successful mating appeared within the second period of exceeded temperature. A possible explanation for the loss of the tra2ts2 strain therefore is that the low temperatures prevented mating and eggs have not been fertilized until temperatures had exceeded 18.5 °C for at least 2 d. On the other hand, control crosses of EgII flies managed to produce a small amount of offspring at temperatures mainly lower than or equal to 18.5 °C (2,796 collected eggs, 16 larvae, 8 adults; Supplementary Fig. S2c), showing that low mating activity is taking place at or below the threshold. Therefore, it is possible that the ts2 mutation, even in the heterozygous state, affects the fertility of the flies at temperatures lower than 18.5 °C. However, as numbers are very small, no robust statement is possible. Overall, using the EgII background for the tra2ts experiments, it could not be determined if the permissive temperature for the medfly tra2ts2 mutation is lower than 18.5 °C or if the ts2 mutant phenotype in medfly is not temperature-dependent at all.
As strains with different genetic backgrounds can have markedly different sensitivities for elevated or low temperatures due to adaptation mechanisms, using another medfly WT background might allow to investigate lower permissive temperatures for ts2. It might also be possible to induce cold acclimation in a WT strain by successively reducing the rearing temperature over several generations before generating the tra2ts2 mutation. This strategy would fail, however, if there is no acclimation with respect to the mating threshold, as shown for B. tryoni46.
Moreover, with regard to the use of the tra2ts2 mutation for medfly sexing in a mass-rearing facility, the presumably low (< 18.5 °C) permissive temperature of the medfly ts2 mutation would be problematic, as temperature and development time show a linear relation. At 19 °C, for example, the development from egg to adults takes about 32.7 d plus 9 d for ovarian maturation, compared to 17.4 d plus 5.3 d at 26 °C21. The even longer development times at < 18 °C would thus be problematic for the production scale and the cost-effectiveness of a mass-rearing program and investigations into lower temperatures would thus certainly not be relevant for insect pest control applications in medfly.
In conclusion, we demonstrated the successful creation of the D. melanogaster tra2ts2 point mutation in C. capitata via markerless CRISPR/Cas9-HDR gene editing and the importance of the respective amino acid for the correct function of TRA2 in the female sex-determination. The previously shown high HDR efficiency in medfly using a ssODN repair template to convert the marker gene eGFP (enhanced green fluorescent protein) into BFP (blue), could be confirmed in this study, where we achieved 100% knock-in efficiency (2 out of 2 fertile G0) compared to 86% (6 out of 7 fertile G0) in the previous study39. Also, the high penetrance of mutant offspring within the G1 with 75–83% in this study is similar compared to 90% in the previous one. It was not possible, however, to identify a permissive temperature at which the tra2ts2 mutation does not affect female development, as it would be located below the mating threshold of medfly. Therefore, it could not be determined if we hadn’t reached the permissive temperature yet, or if the tra2ts2 phenotype in medfly, in contrast to Drosophila, is not temperature dependent. Based on the data presented here, a medfly sexing strain built solely on tra2ts2 would be unsuitable for an SIT program and mass-rearing, either because the rearing would be too slow to be productive on a large scale, or because the sex conversion could not be switched off for strain maintenance. Other possibilities to create a sex-conversion system in medfly could be to target other sex-determination genes, like transformer27,29,47, or to force (over)expression of the maleness factor MoY, which induces masculinization in XX embryos48. However, conditionality would need to be engineered for both options.
Material and methods
Rearing conditions
Ceratitis capitata wild-type Egypt-II (EgII) flies were received from the FAO/IAEA Agriculture and Biotechnology Laboratory, Austria, and kept at 26 °C, 48% RH and 14/10 h light/dark cycle. For fertility tests, freshly eclosed EgII adult flies were transferred from 26 °C to 19.5 °C, 60% RH, 24 h light or 16 or 18 °C, 46–48% RH, 24 h light, where egg collections and subsequent rearing took place. tra2ts mutants were kept at 19 °C or 18.5 °C, 60% RH, and 24 h light. Temperature and humidity were measured every five minutes of the experiment using an EL-USB-2 data logger (Lascar electronics, measurement precision for temperature ± 1 °C, for humidity ± 3%). Readout of the data logger showed that during the rearing of the tra2ts2 mutants, short-term variations of the temperature (+ 3 °C/− 1 °C) occurred (see Supplementary Table S1 and Fig. S2). These could not be avoided due to technical restrictions of the experimental setup. Furthermore, the targeted temperature (19 °C) was once exceeded for 3.5 h up to a temperature of 25 °C during an outage of the air conditioning system. This occurred during the late larval or pupal stage of tra2ts2 G1. Feeding and screening conditions were as described in Aumann et al.39.
CRISPR/Cas9 gene editing
Design of gRNAs targeting tra2 (gRNA_tra2_ts1 and gRNA_tra2_ts2) and assessment of potential off-target effects was performed using the C. capitata genome version Ccap 2.1 (GCF_000347755.3, NCBI)49 and the Software Package Geneious Prime50. On-target activity score was 0.045 for gRNA_tra2_ts1, and 0.140 for gRNA_tra2_ts2 (scores are between 0 and 1; 1 = highest expected activity50). Both gRNAs showed zero off-targets sites in the medfly genome. gRNA synthesis, in vitro transcription and purification was performed as described before39, using primers P_1439 (GAAATTAATACGACTCACTATAGGTGATGATATAGCTGATGCTAGTTTTAGAGCTAGAAATAGC) and P_369 (GCACCGACTCGGTGCCACTTTTTCAAGTTGATAACGGACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAAC) for gRNA_tra2_ts1 and primers P_1440 (GAAATTAATACGACTCACTATAGGCCCATATAAACGCCAGGTGTGTTTTAGAGCTAGAAATAGC) and P_369 for gRNA_tra2_ts2. The sequences of the 140 bp single-stranded HDR templates ‘ssODN_tra2_ts1′ and ‘ssODN_tra2_ts2′ (EXTREMer oligo, Eurofins Genomics) were: ssODN_tra2_ts1 (sense): TGAGTAATCTACGCGTATGCGTCGATCATCGATTTCCATGCCGGAACATGCGTCCTTGGCTGCTTTAACATCAGCTATATCATCATAATAGATAAAGCAAAAGCCACGAGATCGGCCAGTCTGAAAAAAGAAAAAAATAG; ssODN_tra2_ts2 (antisense): AAACGATTTAAATCACATGCACATGCGAAGTATACCTTGTGTGTCGTCCCATATAAACGCCAGGTGTGGAAGTGTGTGGTCTCTGTGTAGTTGAGTAATCTACGCGTATGCGTCGATCATCGATTTCCATGCCGGAACAT; Base changes introducing the ts1 or ts2 mutation are shown in bold. Purified Cas9 protein (PNA Bio Inc.) was reconstituted to 1 µg/µl in 20 mM Hepes, 150 mM KCl, 2% sucrose and 1 mM DTT (pH 7.5).
Microinjection of embryos: 10 µl injection mix for knock-out experiments contained 360 ng/µl Cas9 protein and 200 ng/µl gRNA_tra2_ts1 or gRNA_tra2_ts2 in 300 mM KCl39,51. For knock-in experiments, 200 ng/µl ssODN_tra2_ts1 or ssODN_tra2_ts2 were added to the mix. The mixes were freshly prepared on ice, incubated at 37 °C for 10 min to allow pre-assembly of gRNA-Cas9 ribonucleoprotein complexes and stored on ice prior to injections. For microinjection of WT C. capitata embryos, eggs were collected over a 30–50 min period, prepared for injection and handled afterwards as previously described39. Injections were performed using siliconized quartz glass needles (Q100-70–7.5; LOT171381; Science Products, Hofheim, Germany), drawn out on a Sutter P-2000 laser-based micropipette puller. Injection equipment consisted of a manual micromanipulator (MN-151, Narishige), an Eppendorf FemtoJet 4i microinjector, and an Olympus SZX12-TTR microscope (SDF PLAPO 1xPF objective). Injection survivors were numbered successively across ts1 injections and ts2 injections, respectively.
Crossing strategies and dissection of internal reproductive organs
Crossing of G0: Each G0 adult injection survivor was individually crossed to three EgII WT males or virgin females, except for the 19 °C ts1 knock-in injection. Here, six males and six females were individually backcrossed, the remaining flies were group-backcrossed (five G0 males to 15 females, ten G0 females to ten WT males, and six G0 females to nine WT males). Eggs were collected three to five times, with an interval of one to two days. For the 19 °C knock-in experiments, G1 and G2 flies (if applicable) were kept individually until their genotype was assessed via non-lethal genotyping.
Crossing of tra2ts2 G1: males and females heterozygous for tra2ts2 were inbred. Additionally, heterozygous males were backcrossed (Supplementary Table S3). Eggs were collected six times, with an interval of one to two days.
Crossing of tra2ts2 G2: phenotypic males and females heterozygous for tra2ts2 were inbred (Supplementary Table S4). Additionally, four tra2ts2 heterozygous XY males, not capable of coiling and storing their distiphallus, were group backcrossed. tra2ts2 homozygous XY males were either backcrossed or crossed with heterozygous tra2ts2 females (Supplementary Table S4). Nine males homozygous for tra2ts2 with XX-karyotype, all not able to coil and store their distiphallus, were individually backcrossed to four females each. Eggs of the G2 crosses were collected four to seven times over seven to 13 days (Supplementary Table S4).
Dissections: G0 flies and single crossed G2 flies were allowed to mate for 5–10 days (G0) or 7–13 days (G2) days. If still alive, they were then dissected to examine their internal reproductive organs.
Molecular analyses of G0 mosaics
To analyse the mosaic genotype of G0 flies, DNA was extracted from single flies according to a standard protocol. The target region encompassing the ts1 and ts2 mutant sites (1213 bp) was amplified using the tra2-specific primers P1401 (TGCTTGGTGGTCCGCAAATA) and P1500 (TGTGCATATACTAAAGGCTCTCCC), 50–100 ng DNA, and the Q5 High-fidelity DNA polymerase (New England Biolabs) according to the manufacturer’s protocol in a Bio-Rad C1000 Touch thermal cycler [98 °C, 1 min; 35 cycles of (98 °C, 15 s; 56 °C, 30 s; 72 °C, 45 s); 72 °C, 2 min]. PCR fragments were purified using the Zymo Research DNA Clean & Concentrator-5 kit and subcloned into the pCR4-blunt TOPO vector (Invitrogen) for sequencing. Three to five clones were sequenced using primer mfs13 (TGTAAAACGACGGCCAGT) (Macrogen Europe, Amsterdam) for each analysed fly. Verification of CRISPR-induced mutations from the sequencing results was performed using the Software Package Geneious Prime50 by mapping the sequencing results to the tra2 reference sequence (Gene ID: 101,452,698).
Non-lethal genotyping of G1 and G2 flies
To identify the tra2 genotype of G1 and G2 flies, non-lethal genotyping was performed using an adapted version of the protocol established by Carvalho et al.52. A single leg of an anesthetized fly was cut at the proximal femur using scissors and homogenized in 50 µl buffer (10 mM Tris–Cl pH 8.2, 1 mM EDTA, 25 mM NaCl) for 15 s (6 m/s) using ceramic beads and a FastPrep-24 5G homogenizer (M.P. Biomedicals). 28.3 µl buffer mixed with 1.7 µl proteinase-K (2.5 U/mg) were added and incubated for 1 h at 37 °C. The reaction was stopped 4 min at 98 °C. The solution was cooled down on ice and directly used as PCR template to amplify the region surrounding the tra2 target site. A 25 µl PCR reaction contained DreamTaq polymerase and buffer (Life Technologies), dNTPs and the tra2-specific primers P1401 and P1500 according to the manufacturer’s instructions, and 3.75 µl template solution in a Bio-Rad C1000 Touch thermal cycler [95 °C, 3 min; 35 cycles of (95 °C, 30 s; 56 °C, 30 s; 72 °C, 1 min); 72 °C, 5 min]. The size of the PCR product (1,213 bp) was verified on an agarose gel. PCR products were purified using the Zymo Research DNA Clean & Concentrator-5 kit, sequenced using primer P1500, and subsequently analysed using Software Package Geneious Prime50.
Molecular karyotyping-Y chromosome specific PCR
Y-specific repetitive elements were amplified from genomic DNA extracted either from a single fly (G0) or a single leg (G2) using the published Y-specific oligonucleotides P1504_Y-spec1 (TACGCTACGAATAACGAATTGG) and P1505_Y-spec2 (GCGTTTAAATATACAAATGTGTG)53. 10 µl PCR reactions contained either 50 ng DNA (single fly) or 3.75 µl single-leg DNA template solution, and the Y-specific primers and DreamTaq PCR components as described above. PCR cycling conditions (Bio-Rad C1000 Touch) were [95 °C, 3 min; 35 cycles of (95 °C, 30 s; 58 °C, 30 s; 72 °C, 1 min); 72 °C, 5 min]. Absence of a PCR product was interpreted as absence of the Y chromosome (XX-karyotype). The same PCR conditions with primers P1532 (AGTGAAAACGATTTAAATCACATGCAC) and P1500 for genomic DNA extracted from a single-leg, or P1401 and P1500 for DNA extracted from a single fly were used to amplify 328 bp or 1,213 bp fragments, respectively of tra2 as a positive control PCR to confirm sufficient quality of extracted genomic DNA.
Equipment and settings for image acquisition
For bright field image acquisition of flies (either dead or anesthetized with CO2 and placed on a 4 °C cooler) was carried out using a fully automated Leica M205FC stereo microscope with a PLANAPO 1.0 × objective, a Leica DFC7000 T camera and the Leica LAS X 3.4.2.18368 software. To enhance screen and print display of the pictures the image processing software Fiji ImageJ Version 2.0.054 was used to apply moderate changes to image brightness and contrast. Changes were applied equally throughout the entire image and across all images.
Data availability
All data generated or analysed is included in this article or the supplement.
References
Knipling, E. F. Possibilities of insect control or eradication through the use of sexually sterile males. J. Econ. Entomol. 48, 459–462 (1955).
Hendrichs, J., Franz, G. & Rendon, P. Increased effectiveness and applicability of the sterile insect technique through male-only releases for control of Mediterranean fruit-flies during fruiting seasons. J. Appl. Entomol. 119, 371–377 (1995).
Vreysen, M. J. Principles of area-wide integrated tsetse fly control using the sterile insect technique. Med. Trop. (Mars) 61, 397–411 (2001).
Ito, Y., Kakinohana, H., Yamagishi, M. & Kohama, T. Eradication of the Melon Fly, Bactrocera cucurbitae, from Okinawa, Japan, by means of the sterile insect technique, with special emphasis on the role of basic studies. J. Asia-Pacif. Entomol. 6, 119–129 (2003).
Gilles, J. R. et al. Towards mosquito sterile insect technique programmes: exploring genetic, molecular, mechanical and behavioural methods of sex separation in mosquitoes. Acta Trop. 132(Suppl), S178-187 (2014).
Rendon, P., McInnis, D., Lance, D. & Stewart, J. Medfly (Diptera: Tephritidae) genetic sexing: large-scale field comparison of males-only and bisexual sterile fly releases in Guatemala. J. Econ. Entomol. 97, 1547–1553 (2004).
Lutrat, C. et al. Sex sorting for pest control: It’s raining men!. Trends Parasitol. 35, 649–662 (2019).
Yamada, H. et al. Genetic sex separation of the malaria vector, Anopheles arabiensis, by exposing eggs to dieldrin. Malar. J. 11, 208 (2012).
Orozco, D., Meza, J. S., Zepeda, S., Solis, E. & Quintero-Fong, J. L. Tapachula-7, a new genetic sexing strain of the Mexican fruit fly (Diptera: Tephritidae): sexual compatibility and competitiveness. J. Econ. Entomol. 106, 735–741 (2013).
McInnis, D. O. et al. Development of a pupal color-based genetic sexing strain of the Melon fly, Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 97, 1026–1033 (2004).
Franz, G. Genetic sexing strains in Mediterranean fruit fly, an example for other species amenable to large-scale rearing for the Sterile Insect Technique. In Sterile Insect Technique—Principles And Practice In Area-Wide Integrated Pest Management (eds Dyck, V. A. et al.) 427–451 (Springer, Berlin, 2005).
Schetelig, M. F. & Handler, A. M. A transgenic embryonic sexing system for Anastrepha suspensa (Diptera: Tephritidae). Insect. Biochem. Mol. Biol. 42, 790–795 (2012).
Fu, G. et al. Female-specific insect lethality engineered using alternative splicing. Nat. Biotechnol. 25, 353–357 (2007).
Concha, C. et al. A transgenic male-only strain of the New World screwworm for an improved control program using the sterile insect technique. BMC Biol. 14, 72 (2016).
Schetelig, M. F., Targovska, A., Meza, J. S., Bourtzis, K. & Handler, A. M. Tetracycline-suppressible female lethality and sterility in the Mexican fruit fly, Anastrepha ludens. Insect. Mol. Biol. 25, 500–508 (2016).
Ant, T. et al. Control of the olive fruit fly using genetics-enhanced sterile insect technique. BMC Biol. 10, 51 (2012).
Jin, L. et al. Engineered female-specific lethality for control of pest Lepidoptera. ACS Synth. Biol. 2, 160–166 (2013).
Malacrida, A. R. et al. Globalization and fruitfly invasion and expansion: the medfly paradigm. Genetica 131, 1–9 (2007).
Arias, M. B., Elfekih, S. & Vogler, A. P. Population genetics and migration pathways of the Mediterranean fruit fly Ceratitis capitata inferred with coalescent methods. PeerJ 6, e5340–e5340 (2018).
Ogaugwu, C. E., Schetelig, M. F. & Wimmer, E. A. Transgenic sexing system for Ceratitis capitata (Diptera: Tephritidae) based on female-specific embryonic lethality. Insect. Biochem. Mol. Biol. 43, 1–8 (2013).
Duyck, P. F. & Quilici, S. Survival and development of different life stages of three Ceratitis spp. (Diptera: Tephritidae) reared at five constant temperatures. Bull. Entomol. Res. 92, 461–469 (2007).
Mumford, J. D. Application of benefit/cost analysis to insect pest control using the Sterile Insect Technique. In Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management (eds Dyck, V. A. et al.) 481–498 (Springer, Berlin, 2005).
Salvemini, M. et al. Ceratitis capitata transformer-2 gene is required to establish and maintain the autoregulation of Cctra, the master gene for female sex determination. Int. J. Dev. Biol. 53, 109–120 (2009).
Gomulski, L. M. et al. Gene discovery in an invasive tephritid model pest species, the Mediterranean fruit fly Ceratitis capitata. BMC Genom. 9, 243 (2008).
Pane, A., Salvemini, M., Delli Bovi, P., Polito, C. & Saccone, G. The transformer gene in Ceratitis capitata provides a genetic basis for selecting and remembering the sexual fate. Development 129, 3715–3725 (2002).
Saccone, G., Salvemini, M. & Polito, L. C. The transformer gene of Ceratitis capitata: a paradigm for a conserved epigenetic master regulator of sex determination in insects. Genetica 139, 99–111 (2011).
Schetelig, M. F., Milano, A., Saccone, G. & Handler, A. M. Male only progeny in Anastrepha suspensa by RNAi-induced sex reversion of chromosomal females. Insect. Biochem. Mol. Biol. 42, 51–57 (2012).
Thongsaiklaing, T., Nipitwattanaphon, M. & Ngernsiri, L. The transformer2 gene of the Pumpkin fruit fly, Bactrocera tau (Walker), functions in sex determination, male fertility and testis development. Insect. Mol. Biol. 27, 766–779 (2018).
Liu, G., Wu, Q., Li, J., Zhang, G. & Wan, F. RNAi-mediated knock-down of transformer and transformer 2 to generate male-only progeny in the Oriental fruit fly, Bactrocera dorsalis (Hendel). PLoS ONE 10, e0128892 (2015).
Amrein, H., Maniatis, T. & Nöthiger, R. Alternatively spliced transcripts of the sex-determining gene tra-2 of Drosophila encode functional proteins of different size. EMBO J. 9, 3619–3629 (1990).
Belote, J. M. & Baker, B. S. Sex determination in Drosophila melanogaster: analysis of transformer-2, a sex-transforming locus. Proc. Natl. Acad. Sci. U.S.A. 79, 1568–1572 (1982).
Chandler, D. et al. Evolutionary conservation of regulatory strategies for the sex determination factor transformer-2. Mol. Cell. Biol. 17, 2908–2919 (1997).
Wang, Y. et al. Molecular cloning, expression pattern analysis, and in situ hybridization of a transformer-2 gene in the oriental freshwater prawn, Macrobrachium nipponense (de Haan, 1849). 3 Biotech 9, 205 (2019).
Martin, I., Ruiz, M. F. & Sanchez, L. The gene transformer-2 of Sciara (Diptera, Nematocera) and its effect on Drosophila sexual development. BMC Dev. Biol. 11, 19 (2011).
Li, X. et al. Two of the three transformer-2 genes are required for ovarian development in Aedes albopictus. Insect. Biochem. Mol. Biol. 109, 92–105 (2019).
Dauwalder, B., Amaya-Manzanares, F. & Mattox, W. A human homologue of the Drosophila sex determination factor transformer-2 has conserved splicing regulatory functions. Proc. Natl. Acad. Sci. U.S.A. 93, 9004–9009 (1996).
Doudna, J. A. & Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096 (2014).
Li, J. & Handler, A. M. Temperature-dependent sex-reversal by a transformer-2 gene-edited mutation in the spotted wing drosophila, Drosophila suzukii. Sci. Rep. 7, 12363 (2017).
Aumann, R. A., Schetelig, M. F. & Häcker, I. Highly efficient genome editing by homology-directed repair using Cas9 protein in Ceratitis capitata. Insect. Biochem. Mol. Biol. 101, 85–93 (2018).
Waltz, E. CRISPR-edited crops free to enter market, skip regulation. Nat. Biotechnol. 34, 582 (2016).
Prokopy, R. J. & Hendrichs, J. Mating behavior of Ceratitis capitata on a field-caged host tree. Ann. Entomol. Soc. Am. 72, 642–648 (1979).
Hamburger, D. Comparative analysis: the regulation of plants derived from Genome Editing in Argentina, Australia, Canada, the European Union, Japan and the United States. In Regulation of Genome Editing in Plant Biotechnology: A Comparative Analysis of Regulatory Frameworks of Selected Countries and the EU (eds Dederer, H.-G. & Hamburger, D.) 313–363 (Springer, Berlin, 2019).
Xiang, G., Zhang, X., An, C., Cheng, C. & Wang, H. Temperature effect on CRISPR-Cas9 mediated genome editing. J. Genet. Genom. 44, 199–205 (2017).
Handler, A. M. & O’Brochta, D. A. Ch. 10: Transposable elements for insect transformation. In Insect Development: Morphogenesis, Molting and Metamorphosis (ed. Gilbert, L. I.) 459–496 (Elsevier BV, Amsterdam, 2009).
Tassan, R. L. et al. Mediterranean fruit fly life cycle estimations for the California eradication program. In Fruit Flies of Economic Importance (ed. Cavalloro, R.) 564–570 (Balkema, Amsterdam, 1983).
Meats, A. & Fay, H. A. C. The effect of acclimation on mating frequency and mating competitiveness in the Queensland fruit fly, Dacus tryoni, in optimal and cool mating regimes. Physiol. Entomol. 1, 207–212 (1976).
Shukla, J. N. & Palli, S. R. Sex determination in beetles: Production of all male progeny by parental RNAi knockdown of transformer. Sci. Rep. 2, 602 (2012).
Meccariello, A. et al. Maleness-on-the-Y (MoY) orchestrates male sex determination in major agricultural fruit fly pests. Science 365, 1457–1460 (2019).
Papanicolaou, A. et al. The whole genome sequence of the Mediterranean fruit fly, Ceratitis capitata (Wiedemann), reveals insights into the biology and adaptive evolution of a highly invasive pest species. Genome Biol. 17, 192 (2016).
Kearse, M. et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 28, 1647–1649 (2012).
Burger, A. et al. Maximizing mutagenesis with solubilized CRISPR-Cas9 ribonucleoprotein complexes. Development 143, 2025–2037 (2016).
Carvalho, G. B., Ja, W. W. & Benzer, S. Non-lethal genotyping of single Drosophila. Biotechniques 46, 312–314 (2009).
Anleitner, J. E. & Haymer, D. S. Y enriched and Y specific DNA sequences from the genome of the Mediterranean fruit fly Ceratitis capitata. Chromosoma 101, 271–278 (1992).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Acknowledgements
We wish to thank Jakob Martin, Tanja Rehling, Johanna Rühl, and Julia Hehner for technical assistance and help with insect rearing.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study benefitted from discussions at meetings for the Coordinated Research Project, “Comparing Rearing Efficiency and Competitiveness of Sterile Male Strains Produced by Genetic, Transgenic or Symbiont-based Technologies”, funded by the International Atomic Energy Agency (IAEA). This work was supported by projects of the LOEWE Centre for Insect Biotechnology & Bioresources and the LOEWE Centre DRUID of the Hessian Ministry of Science and Arts (MFS), and has also been funded by the Hort Frontiers Fruit Fly Fund part of the Hort Frontiers strategic partnership initiative developed by Hort Innovation, with co-investment from Macquarie University, USDA, and JLU Gießen and contributions from the Australian Government (FF17000 to MFS). The funding sources were not involved in any of the following: study design, collection, analysis and interpretation of data, writing of the report, and the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
R.A.A. performed research; R.A.A., M.F.S. and I.H. designed research; R.A.A., M.F.S. and I.H. analysed data; and R.A.A., I.H. and M.F.S. wrote the paper. All authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Aumann, R.A., Häcker, I. & Schetelig, M.F. Female-to-male sex conversion in Ceratitis capitata by CRISPR/Cas9 HDR-induced point mutations in the sex determination gene transformer-2. Sci Rep 10, 18611 (2020). https://doi.org/10.1038/s41598-020-75572-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-75572-x
This article is cited by
-
Deep orange gene editing triggers temperature-sensitive lethal phenotypes in Ceratitis capitata
BMC Biotechnology (2024)
-
Next-generation genetic sexing strain establishment in the agricultural pest Ceratitis capitata
Scientific Reports (2023)
-
Engineered sex ratio distortion by X-shredding in the global agricultural pest Ceratitis capitata
BMC Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.